Abstract
Tumor necrosis factor-α (TNF-α)-induced protein 8-like-2 (TNFAIP8L2 or TIPE2), a member of the tumor necrosis TNFAIP8 family, was found to be involved in the development and progression of several tumors. However, to date, the role of TIPE2 in breast cancer is still unclear. Thus, the aim of this study is to explore the role of TIPE2 in breast cancer. Our results indicated that TIPE2 expression was significantly decreased in human breast cancer tissue and cell lines. Overexpression of TIPE2 inhibited the proliferation in vitro and tumor xenograft growth in vivo. TIPE2 also inhibited the migration/invasion of breast cancer cells through preventing the epithelial-to-mesenchymal transition (EMT) phenotype. Mechanically, TIPE2 inhibited the expression of β-catenin, cyclin D1, and c-Myc in breast cancer cells. In conclusion, our findings show that TIPE2 may play an important role in breast cancer cell proliferation, invasion, and tumorigenesis in vivo. Therefore, TIPE2 may be a potential molecular target for the treatment of breast cancer.
Key words: Tumor necrosis factor (TNF)-α-induced protein 8-like-2 (TIPE2), Breast cancer, Invasion, Wnt/β-catenin pathway
INTRODUCTION
Breast cancer is the leading malignancy in women worldwide. The percentage of female breast cancer found among all female cancers is 27% in developed countries (1,2). Although a wide array of therapeutic options including chemotherapy, radiation, and hormone therapy is available for treating breast cancer, the 5-year survival rate of patients with breast cancer is very low because of distant metastases (3–5). To date, the mechanisms of breast cancer oncogenesis remain unclear. Thus, dissecting the molecular mechanisms that regulate breast cancer progression may facilitate the advancement of clinical treatment.
Tumor necrosis factor-α (TNF-α)-induced protein 8-like-2 (TNFAIP8L2 or TIPE2) is a member of the tumor necrosis TNFAIP8 family that is an essential negative regulator of both innate and adaptive immunity (6). TIPE2 is constitutively expressed at high levels in immune cells (7,8). It was reported that TIPE2-deficient macrophages produced more IL-6 and IL-12 upon stimulation with lipopolysaccharide (LPS), and TIPE2-deficient mice were more susceptible to LPS-induced septic shock (9). In recent years, many studies have revealed that TIPE2 expression is associated with aggressive tumor growth, metastasis, and poor patient survival (10–13). A recent report demonstrated that TIPE2 was lowly expressed in human lung cancer cell lines, and TIPE2 overexpression significantly suppressed lung cancer cell proliferation, colony formation, and cell invasion, and reduced the expression of N-cadherin in vitro (14). However, to date, the role of TIPE2 in breast cancer is still unclear. Thus, the aim of this study is to explore the role of TIPE2 in breast cancer. Our results demonstrated that ectopic expression of TIPE2 inhibits the proliferation and tumorigenesis in breast cancer. These results suggest that TIPE2 functions as a tumor suppressor in the development of breast cancer.
MATERIALS AND METHODS
Breast Cancer Tissue Samples
All cases examined were of breast cancer patients who underwent surgical resection at the Department of Breast Surgery, the First Affiliated Hospital of Xi’an Jiaotong University (China) during the period from 2012 to 2014. Normal tissue adjacent to the tumor from the same breast was used as a control. All fresh samples were immediately frozen after resection and stored at −80°C until use. All experiments were performed after obtaining approval of the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University and were in accordance with the Declaration of Helsinki Principles.
Cell Culture
Human breast cancer cell lines (MDA-MB-231, MCF7, and HCC1937) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD, USA) and 100 U/ml streptomycin and penicillin (Gibco) in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. Human mammary epithelial cell line (HMEC) was used as control.
Construction of TIPE2-Overexpressing Cell Lines
Full-length human TIPE2 was generated from human peripheral blood mononuclear cell (PBMC) cDNA by PCR and cloned into pcDNA3.1 vector. Breast cancer cells were transfected with TIPE2 or vector using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. The transfection efficiency was confirmed using Western blot.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from breast cancer tissues and cells using the TRIzol reagent (Invitrogen) according to the supplier’s instructions. About 5 μg of total RNA for each sample was reverse transcribed into first-strand cDNA. qRT-PCR was performed using an ABI Prism 7500 System (Applied Biosystems) with the SYBR Green Supermix (Invitrogen). The specific primers for TIPE2 were 5′-ACTGA GTAAGATGGCGGGTCG-3′ (sense) and 5′-TTCTGGCGAAAGCGGGTAG-3′ (antisense), and for β-actin were 5′-AAATCGTGCGTGACATCAAAGA-3′ (sense) and 5′-GGCCATCTCCTGCTCGAA-3′ (antisense). Data were analyzed using the formula: R = 2−[ΔCt sample − ΔCt control].
Western Blot
Cells or tumor tissues were homogenized and lysed with RIPA lysis buffer [100 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1% Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate, and protease inhibitor]. Equal amounts of protein samples were separated by 10% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). The membranes were then blocked with 5% nonfat milk in Tris-buffered saline containing 0.2% Tween 20 (TBST; Invitrogen) and then incubated with specific primary antibodies at 4°C overnight. The antibodies were as follows: mouse anti-TIPE2, mouse anti-E-cadherin, mouse anti-N-cadherin, mouse anti-β-catenin, mouse cyclin D1, mouse c-myc, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) and visualized using the ECL chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA). The fluorescence was scanned using a Typhoon scanner (Amersham Biosciences).
Cell Proliferation Assay
Breast cancer cells transfected with TIPE2 or vector were seeded in 96-well plates (1 × 105 cells/well) and then cultured at 24-h intervals for 4 days. Cell proliferation was measured daily for 96 h using the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer’s instructions. Absorbance at 570 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Migration and Invasion Assays
For the migration assay, breast cancer cells (1 × 105 cells per well) transfected with TIPE2 or vector were suspended in serum-free medium and plated on the upper chamber of Boyden chambers (BD Biosciences, Eugene, OR, USA) that were not coated with Matrigel. For the invasion assay, infected breast cancer cells were seeded into the upper chamber that was precoated with Matrigel (BD Biosciences, San Jose, CA, USA). For both assays, the lower chamber was filled with DMEM containing 10% FBS. After incubating for 24 h at 37°C, noninvasive cells in the upper chamber were removed using a cotton swab, and cells adhering to the lower membrane were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min, and then counted in five random fields per well under a light microscope (magnification: 100×).
In Vivo Tumorigenicity
Female Balb/c nude mice (4–5 weeks old) were obtained from the Animal Breeding Center of the First Affiliated Hospital of Xi’an Jiaotong University. MDA-MB-231 cells (1 × 106 cells in 0.2 ml of PBS) stably transfected with TIPE2 or vector were injected subcutaneously into the dorsal flank of female Balb/c nude mice. The tumor size was measured every 7 days for 4 weeks beginning 7 days after implantation. The tumor volume was calculated according to the following formula: V = L × W 2/2, where V is the volume (mm3); L, the biggest diameter (mm); and W, the smallest diameter (mm). About 4 weeks after inoculation, mice were euthanized by subcutaneous injection with sodium pentobarbital (40 mg/kg), and the tumors were weighed. Animal experiment was carried out with the approval of the Animal Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Statistical Analysis
Data are presented as the mean ± SD. Statistical significance was analyzed with one-way ANOVA or Student’s two-tailed t-test. A value of p < 0.05 was considered to be significant.
RESULTS
The Expression of TIPE2 Is Downregulated in Breast Cancer Tissues and Cell Lines
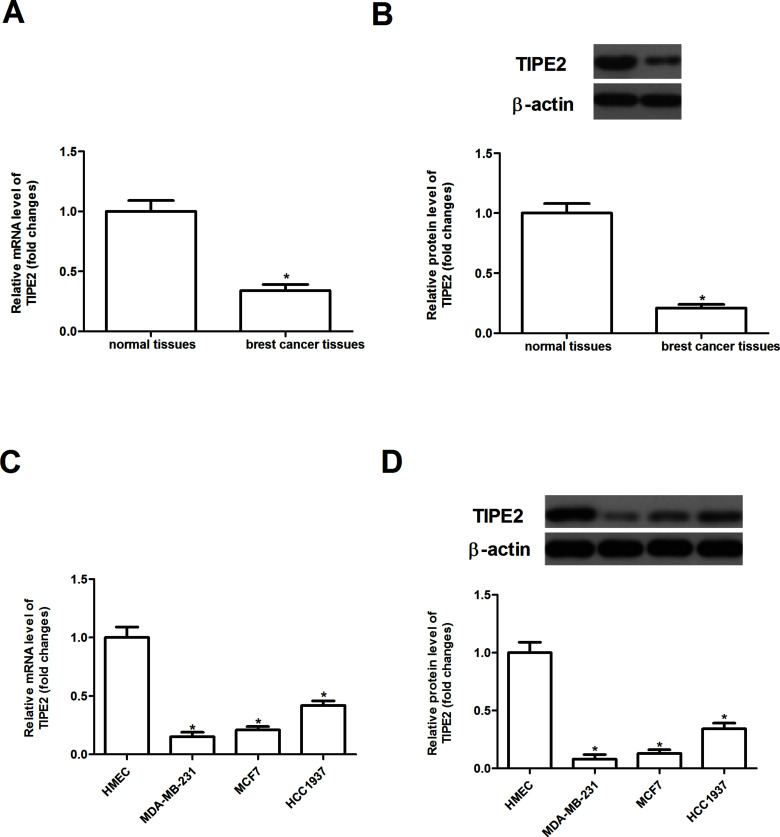
We first evaluated the expression of TIPE2 in primary breast cancer tissues and their corresponding adjacent nontumor tissues by RT-qPCR and Western blot. As shown in Figure 1A and B, the expression levels of TIPE2 at both mRNA and protein were significantly decreased in breast cancer tissues compared with that in the adjacent normal tissues. In addition, the expression of TIPE2 was also downregulated in human breast cancer cell lines, compared with HMEC (Fig. 1C and D).
Figure 1.
The expression of TIPE2 is downregulated in breast cancer tissues and cell lines. The mRNA expression levels of TIPE2 in human breast cancer tissues (A) and cell lines (C) were analyzed by qRT-PCR. The protein expression levels of TIPE2 in human breast cancer tissues (B) and cell lines (D) were detected by Western blot analysis. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
TIPE2 Inhibits the Proliferation of Breast Cancer Cells
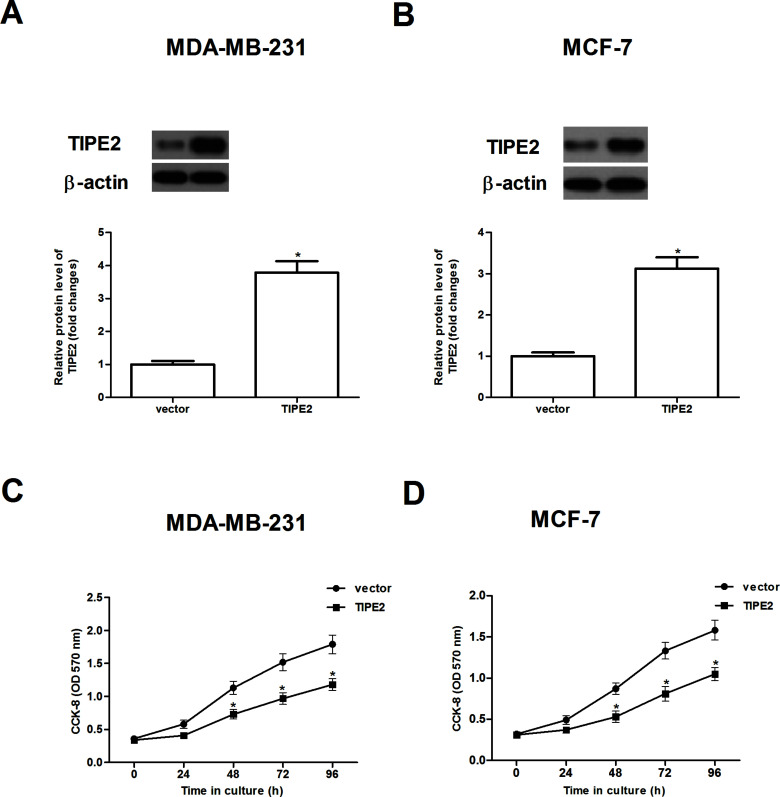
To investigate the effect of TIPE2 on cell proliferation, we treated MDA-MB-231 and MCF-7 cells with overexpression of TIPE2, respectively. As expected, after transfection, the protein expression level of TIPE2 was obviously increased in MDA-MB-231 and MCF-7 cells, respectively (Fig. 2A and B). Cell proliferation was then evaluated using the CCK-8 assay; overexpression of TIPE2 greatly inhibited the proliferation of both types of cells in a time-dependent manner (Fig. 2C and D).
Figure 2.
TIPE2 inhibits the proliferation of breast cancer cells. MDA-MB-231 and MCF-7 cells were transfected with TIPE2 or vector for 48 h. Western blot assay was performed to detect the expression of TIPE2 in MDA-MB-231 (A) and MCF-7 cells (B) after transfection. (C, D) In vitro cell proliferation was examined by the CCK-8 assay. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
TIPE2 Inhibits Breast Cancer Cell Migration and Invasion
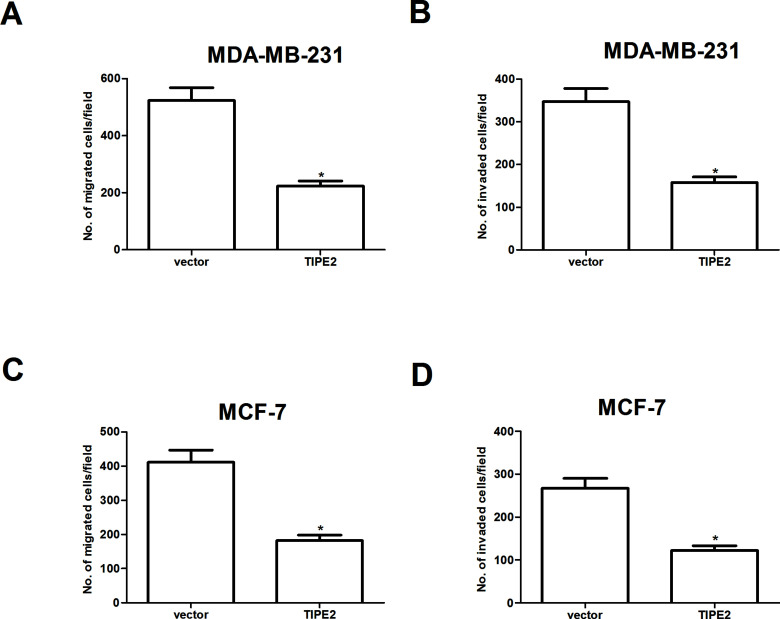
We investigated the effect of TIPE2 on breast cancer cell migration by Transwell migration assay. The results indicated that overexpression of TIPE2 significantly inhibited the migration of MDA-MB-231 cells (Fig. 3A). It also inhibited the invasion of MDA-MB-231 cells through Matrigel-coated polycarbonate filter in the Transwell chamber (Fig. 3B). Similar results were observed in MCF-7 cells (Fig. 3C and D).
Figure 3.
TIPE2 inhibits breast cancer cell migration and invasion. MDA-MB-231 and MCF-7 cells were transfected with TIPE2 or vector for 48 h. (A, B) Cell migration of MDA-MB-231 and MCF-7 cells was measured by Transwell migration assay. (C, D) Cell invasion of MDA-MB-231 and MCF-7 cells was assessed by the Matrigel invasion chamber. Then we investigated the effect of TIPE2 on breast cancer cell migration by Transwell migration assay. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
TIPE2 Inhibits the Epithelial-to-Mesenchymal Transition (EMT) Phenotype in Breast Cancer Cells
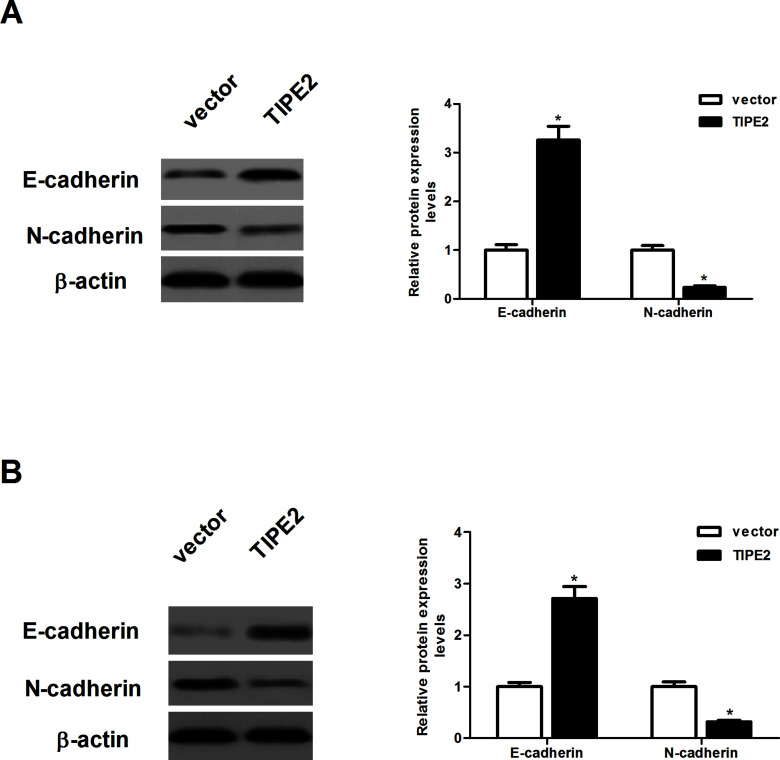
In order to examine the effect of TIPE2 on the EMT progression of breast cancer cells, we evaluated the expression of E-cadherin and N-cadherin by Western blot. As indicated in Figure 4A, overexpression of TIPE2 increased the protein expression of E-cadherin and decreased the protein expression of N-cadherin in MDA-MB-231 cells, compared with the vector group. Similarly, ectopic expression of TIPE2 led to an increase in E-cadherin expression and a decrease in N-cadherin expression in MCF-7 cells (Fig. 4B).
Figure 4.
TIPE2 inhibits the epithelial-to-mesenchymal transition (EMT) phenotype in breast cancer cells. MDA-MB-231 and MCF-7 cells were transfected with TIPE2 or vector for 48 h. (A) The protein expression levels of E-cadherin and N-cadherin were determined by Western blot. (B) The protein expression levels of E-cadherin and N-cadherin were determined by Western blot. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
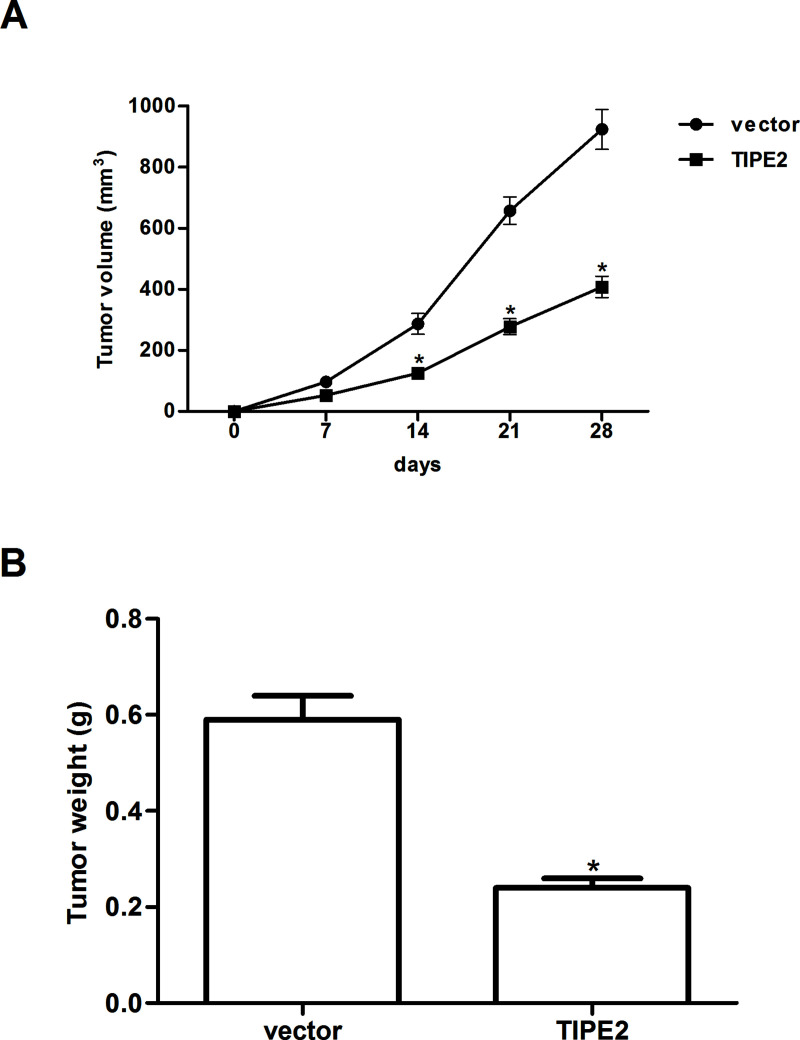
TIPE2 Inhibits Xenografted Tumor Growth In Vivo
To further examine the role of TIPE2 on breast cancer growth in vivo, the xenografted tumor in nude mouse was employed. Overexpression of TIPE2 remarkably reduced the size (Fig. 5A) and weight (Fig. 5B) of the xenografted tumor, compared with the vector group.
Figure 5.
TIPE2 inhibits xenografted tumor growth in vivo. MDA-MB-231 cells (1 × 106 cells in 0.2 ml of PBS) stably transfected with TIPE2 or vector were injected subcutaneously into the dorsal flank of female Balb/c nude mice. (A) The tumor volumes were calculated in each group every 7 days from day 0 to day 28; (B) the tumor weights were measured at day 29. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
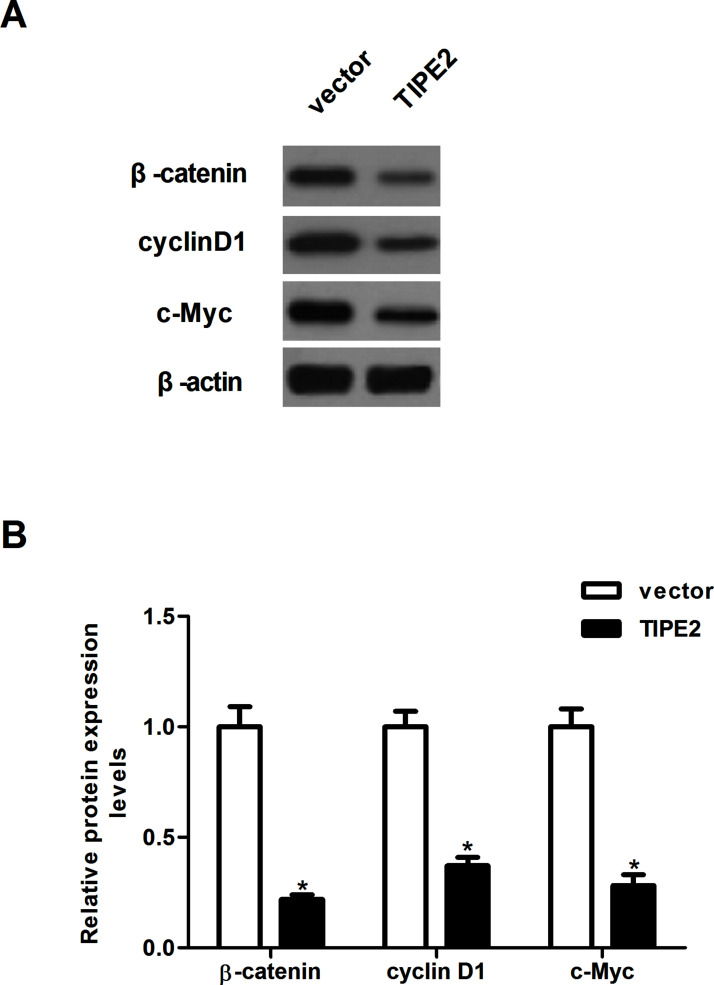
TIPE2 Inhibits the Activation of Wnt/β-Catenin Pathway in Breast Cancer Cells
The Wnt/β-catenin signaling pathway is involved in breast cancer cell proliferation and invasion. We thus explored whether TIPE2 level affected the expression of β-catenin, cyclin D1, and c-Myc in breast cancer cells. As shown in Figure 5, overexpression of TIPE2 significantly inhibited the expression of β-catenin, cyclin D1, and c-Myc in MDA-MB-231 cells, compared with the vector group (Fig. 6A), and the relative protein levels of β-catenin, cyclin D1, and c-Myc were quantified using Image-Pro Plus 6.0 software (Fig. 6B).
Figure 6.
TIPE2 inhibits the activation of Wnt/β-catenin pathway in breast cancer cells. MDA-MB-231 cells were infected with TIPE2 or vector for 48 h. (A) The protein expression levels of β-catenin, cyclin D1, and c-Myc were determined by Western blot. (B) The relative protein levels were quantified using Image-Pro Plus 6.0 software. Data are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
DISCUSSION
In this study, we showed that TIPE2 is involved in the pathogenesis of breast cancer. TIPE2 is downregulated in breast cancer tissues and cell lines. Overexpression of TIPE2 inhibited the proliferation in vitro and tumor xenograft growth in vivo. TIPE2 also inhibited the migration/invasion of breast cancer cell by preventing the EMT phenotype. Mechanically, TIPE2 inhibited the expression of β-catenin, cyclin D1, and c-Myc in breast cancer cells.
Recent studies have shown that TIPE2 plays an important role in tumorigenesis and tumor development. For example, Cao et al. confirmed that TIPE2 is downregulated in human primary hepatocellular carcinoma, and the forced expression of TIPE2 in hepatocellular carcinoma-derived cell lines markedly inhibits tumor cell growth, migration, and invasion in vitro and suppresses growth and metastasis of hepatocellular carcinoma in vivo (15). In addition, the expression of TIPE2 was found to be decreased in human gastric cancer tissues, and restoration of TIPE2 expression in gastric cells significantly suppressed cell proliferation (16). Consistent with the above-mentioned results, herein we detected the expression of TIPE2 in clinical tissue samples and cell lines and found that the expression of TIPE2 was significantly downregulated in human breast cancer tissues and cell lines. In addition, we observed that overexpression of TIPE2 inhibited the proliferation in vitro and tumor xenograft growth in vivo. These data suggest that TIPE2 may be a candidate tumor suppressor gene in the development of breast cancer.
The migration and invasion of cancer cells have been shown to be related to tumor progression (17). EMT is also a crucial step in tumor progression. During the EMT process, the actin cytoskeleton is reorganized, and cells acquire increased cell–matrix contacts, leading to dissociation from surrounding cells and enhanced migration and invasion (18). Reduction or a loss of E-cadherin expression is one of the well-established hallmarks of EMT and promotes breast cancer cell migration and invasion (19). In this study, we found that ectopic expression of TIPE2 greatly inhibited migration/invasion in breast cancer cells. Moreover, TIPE2 increased the expression level of E-cadherin and decreased the expression level of N-cadherin in breast cancer cells. These results suggest that TIPE2 inhibited the migration/invasion of breast cancer cells by preventing the EMT phenotype.
Increasing evidence has reported that the Wnt/β-catenin signaling pathway plays an important role in breast cancer progression (20–22). β-Catenin is a central factor in canonical Wnt signaling and induces the transcription of several target genes, which are implicated in cell survival, proliferation, and metastasis (23). A recent study showed that β-catenin knockdown breast cancer cells were significantly impaired in their ability to migrate in wound-filling assays and form anchorage-independent colonies in soft agar (24). Therefore, inhibition of the Wnt/β-catenin signaling pathway is an important therapeutic strategy for breast cancer (25–27). Moreover, it was reported that TIPE2 remarkably reduced the levels of β-catenin as well as the nuclear level of β-catenin in gastric cancer cells (28). In this study, we found that ectopic expression of TIPE2 inhibited the expression of β-catenin, cyclin D1, and c-Myc in breast cancer cells. All these results suggest that TIPE2 inhibits the proliferation and tumorigenesis in breast cancer, perhaps through suppressing the Wnt/β-catenin signaling pathway.
In conclusion, our findings show that TIPE2 may play an important role in breast cancer cell proliferation, invasion, and tumorigenesis in vivo. Therefore, TIPE2 may be a potential molecular target for the treatment of breast cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre L. A.; Bray F.; Siegel R. L.; Ferlay J.; Lortet-Tieulent J.; Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 65:87–108; 2015. [DOI] [PubMed] [Google Scholar]
- 2. Fan L.; Strasser-Weippl K.; Li J.-J.; Finkelstein D. M.; Yu K.-D.; Chen W.-Q.; Shao Z.-M.; Goss P. E. Breast cancer in China. Lancet Oncol. 15:e279–e289; 2014. [DOI] [PubMed] [Google Scholar]
- 3. DeSantis C. E.; Lin C. C.; Mariotto A. B.; Siegel R. L.; Stein K. D.; Kramer J. L.; Alteri R.; Robbins A. S.; Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 64:252–271; 2014. [DOI] [PubMed] [Google Scholar]
- 4. Chen L.; Linden H. M.; Anderson B. O.; Li C. I. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res. Treat. 147:609–616; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong G.; Wang D.; Liang X.; Gao H.; Wang L.; Yu X.; Liu J. Factors related to survival rates for breast cancer patients. Int. J. Clin Exp. Med. 7:3719–3724; 2014. [PMC free article] [PubMed] [Google Scholar]
- 6. Sun H.; Gong S.; Carmody R. J.; Hilliard A.; Li L.; Sun J.; Kong L.; Xu L.; Hilliard B.; Hu S. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133:415–426; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly B.; O’Neill L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25:771–784; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L.; Shi Y.; Wang Y.; Zhu F.; Wang Q.; Ma C.; Chen Y. H.; Zhang L. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol. Immunol. 48:1209–1215; 2011. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y.; Wei X.; Liu L.; Liu S.; Wang Z.; Zhang B.; Fan B.; Yang F.; Huang S.; Jiang F. TIPE2, a novel regulator of immunity, protects against experimental stroke. J. Biol. Chem. 287:32546–32555; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Q.-Q.; Zhang F.-F.; Wang F.; Qiu J.-H.; Luo C.-H.; Zhu G.-Y.; Liu Y.-F. TIPE2 inhibits lung cancer growth attributing to promotion of apoptosis by regulating some apoptotic molecules expression. PLoS One 10:e0126176; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y.; Tao M.; Wu J.; Meng Y.; Xu C.; Tian Y.; Zhou X.; Xiang J.; Zhang H.; Xie Y. Adenovirus-directed expression of TIPE2 suppresses gastric cancer growth via induction of apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther. 23:98–106; 2016. [DOI] [PubMed] [Google Scholar]
- 12. Deng B.; Feng Y. TIPE2 mediates the suppressive effects of shikonin on MMP13 in osteosarcoma cells. Cell. Physiol. Biochem. 37:2434–2443; 2015. [DOI] [PubMed] [Google Scholar]
- 13. Li X. TIPE2 regulates tumor-associated macrophages in skin squamous cell carcinoma. Tumor Biol. 37:5585–5590; 2016. [DOI] [PubMed] [Google Scholar]
- 14. Li Y.; Li X.; Liu G.; Sun R.; Wang L.; Wang J.; Wang H. Downregulated TIPE2 is associated with poor prognosis and promotes cell proliferation in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 457:43–49; 2015. [DOI] [PubMed] [Google Scholar]
- 15. Cao X.; Zhang L.; Shi Y.; Sun Y.; Dai S.; Guo C.; Zhu F.; Wang J.; Wang X.; Chen Y. H. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol. Cancer 12:149–158; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Q.; Zhao M.; Dong T.; Zhou C.; Peng Y.; Zhou X.; Fan B.; Ma W.; Han M.; Liu S. Tumor necrosis factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to decrease gastric cancer cell proliferation. J. Cell. Biochem. 116:1121–1129; 2015. [DOI] [PubMed] [Google Scholar]
- 17. Wan L.; Pantel K.; Kang Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 19:1450–1464; 2013. [DOI] [PubMed] [Google Scholar]
- 18. Ye X.; Weinberg R. A. Epithelial–mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 25:675–686; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dang T. T.; Esparza M. A.; Maine E. A.; Westcott J. M.; Pearson G. W. ΔNp63α promotes breast cancer cell motility through the selective activation of components of the epithelial-to-mesenchymal transition program. Cancer Res. 75:3925–3935; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howe L. R.; Brown A. M. Wnt signaling and breast cancer. Cancer Biol. Ther. 3:36–41; 2004. [DOI] [PubMed] [Google Scholar]
- 21. Prasad C. P.; Gupta S. D.; Rath G.; Ralhan R. Wnt signaling pathway in invasive ductal carcinoma of the breast: Relationship between β-catenin, disheveled and cyclin D1 expression. Oncology 73:112–117; 2008. [DOI] [PubMed] [Google Scholar]
- 22. Khramtsov A. I.; Khramtsova G. F.; Tretiakova M.; Huo D.; Olopade O. I.; Goss K. H. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176:2911–2920; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moon R. T.; Kohn A. D.; De Ferrari G. V.; Kaykas A. WNT and β-catenin signalling: Diseases and therapies. Nat. Rev. Genet. 5:691–701; 2004. [DOI] [PubMed] [Google Scholar]
- 24. Xu J.; Prosperi J. R.; Choudhury N.; Olopade O. I.; Goss K. H. β-Catenin is required for the tumorigenic behavior of triple-negative breast cancer cells. PLoS One 10:e0117097; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King T. D.; Suto M. J.; Li Y. The wnt/β-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J. Cell. Biochem. 113:13–18; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasad C. P.; Rath G.; Mathur S.; Bhatnagar D.; Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/β-catenin signaling. Chem. Biol. Interact. 181:263–271; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Cai J.; Guan H.; Fang L.; Yang Y.; Zhu X.; Yuan J.; Wu J.; Li M. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J. Clin. Invest. 123:566–579; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J.; Zhang H.; Xu C.; Xu H.; Zhou X.; Xie Y.; Tao M. TIPE2 functions as a metastasis suppressor via negatively regulating β-catenin through activating GSK3β in gastric cancer. Int. J. Oncol. 48:199–206; 2016. [DOI] [PubMed] [Google Scholar]