Abstract
Both cholangiocarcinoma (CCA) and gallbladder carcinoma (GBC) are belong to biliary tract carcinomas (BTCs) with a high degree of malignancy and a poor prognosis. Therefore, an in vitro model is urgently needed to increase our understanding of the pathogenesis of BTCs. Tumor organoids are a novel three-dimensional (3D) culture technology that utilizes samples from removed tumors. Therefore, it can maintain the histological features, expression profiles and marker expression of the parental tissues. Recently, with the widespread use of this technique, increasing research is beginning to use organoid to study the cellular metabolism, pathogenesis, chemotherapy resistance, and new therapy methods of BTCs. In this review, we will discuss the advantages and disadvantages of BTC organoids compared with other cell culture techniques. In addition, the construction methods, research directions and current limitations of BTC organoids will be described.
Keywords: Cholangiocarcinoma, gallbladder carcinoma, biliary tract carcinomas, organoids
Introduction
Origin of organoids
Organoids is a new type of 3D cell culture technology that can culture primary tissue grafts or single cells into self-organized tissues. Organoids can retain the histological characteristics, expression profiles, unique markers and many other characteristics of the original tissues [1]. Organoids originate from stem cells and are mainly derived from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and tissue resident adult stem cells (ASCs). Among them, ESC organoids and iPSC organoids are mainly used to study the growth and development of different organs, while ASC organoids can be used to study regenerative medicine in organs [2]. In 2009, Sato and his colleagues [3] made single mouse Lgr5+ intestinal stem cells grow into intestinal epithelial organoids with crypt-villus structures by combining Matrigel with different growth factors for the first time. Since then, organoids have been used in many different human and mouse organs, such as the gastrointestinal tract, lung, liver, gallbladder, pancreas, ovary, etc.
Tumor organoids
Tumor cell lines, tumor spheroids and animal models are important tools for studying tumors. They have many advantages in exploring the pathogenesis of tumors, but they still have their limitations (see Table 1). Tumor cell lines are traditional two-dimensional (2D) cell culture technology in which cells grow in conventional culture medium; thus, these cells are easy to culture, fast growing, and suitable for high-throughput drug screening and functional analyses of tumors. However, in the process of subculture, tumor cell lines are prone to accumulate mutations and thus cannot retain the characteristics of the original tumors and cannot simulate the interaction between tumor cells and other surrounding cells. Tumor spheroids are 3D microtissues that are typically obtained from single-cell suspensions that are self-assembling or forced to aggregate. Because of their unique layered structure, they are suitable for studying the phenotype, function and metabolic behavior of different layered tumor cells [4]. Compared with animal models, this model lacks the histomorphology of human tumors and cannot mimic the tumor microenvironment, so animal models are important for the systematic study of tumors. Unfortunately, animal models often take several months to establish, and the cost is higher; therefore, we need a model that not only considers the interaction between cells and cells, but can also reduce the cost and time. The successful construction of tumor organoids can solve the above problems; more importantly, these models can be directly derived from patients’ tissues and retain the specific tissue structure and genomic characteristics of the tumors. Therefore, organoids provide a new platform for precise tumor treatment [5].
Table 1.
Comparison of common tumor models
| Feature | 2D tumor cell line | Tumor spheroids | Animal models | Organoids |
|---|---|---|---|---|
| Time | Few days | Few days | Several months | Several weeks |
| Cost | Low | Low | High | Medium |
| Construction success rate | High | High | Low | Medium (depending on the specific tumor) |
| Long-term cultivation capacity | Yes | Yes | No | Yes |
| High-throughput drug screening | Suitable | Suitable | Inappropriate | Suitable |
| Other advantage | Low maintenance cost, high reproducibility | Easy to construct, high reproducibility | The ability to study tumors as a whole | Personal and precision treatment |
| Main disadvantage | Prone to accumulation of mutations, lack of interaction between tumor cells and other surrounding cells, unable to mimic tumor microenvironment | Lack of histological and morphological characteristics, unstable size of spheroids | Different from the tumor environment of the human body | Unable to mimic tumor microenvironment by itself, strong heterogeneity |
Construction methods of tumor organoids
Tumor organoids can be constructed by 3D culture of tumor cells in mice or patients, or through gene editing in 3D culture of nontumor cells in mice or patients (see Figure 1). To date, liver cancer [6], pancreatic cancer [7], ovarian cancer [8], breast cancer [9], lung cancer [10]and so on have been successfully constructed. Tumor organoids can be used to study the occurrence and development [11], personalized treatment [12], drug screening, discovery of prognostic markers [13] and other aspects of tumors.
Figure 1.
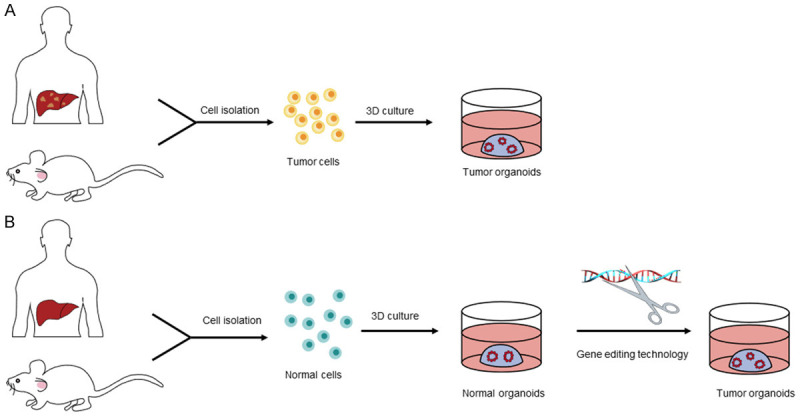
Construction methods of tumor organoids. A. Tumor organoids can be constructed by 3D culture of tumor cells in mice or patients. B. At the same time, after isolating normal cells from healthy humans or mice, constructing normal organoids through 3D culture technology. Then using gene editing technology to transform them into tumor organoids.
Cholangiocarcinoma organoids
Construction of cholangiocarcinoma organoids
Patient-derived cholangiocarcinoma organoids can be constructed directly from resected or biopsied tumor tissues. Saito’s team [14] successfully constructed six patient-derived organoid lines that have been stably cultured for more than one year: intrahepatic cholangiocarcinomas (ICCs), pancreatic ductal adenocarcinoma (PDA), gallbladder carcinomas (GBCs) and ampullary neuroendocrine carcinoma (NEC). The success rates were 50% (ICCs, 3/6), 20% (GBC, 1/5), 50% (PDA, 1/2) and 100% (NEC, 1/1). Subsequently, the organoids were identified by HE staining, PAS staining and immunohistochemistry. The results showed that the histopathological characteristics of the organoids were consistent with those of the primary tissues, and their tumorigenicity was verified by subcutaneous tumor formation experiments in severe combined immunodeficiency mice.
In addition, organoids can be combined with CRISPR/Cas9 gene editing technology to construct cholangiocarcinoma organoids. Artegiani and his group [15] introduced TP53, Smad4, NF1 and PTEN (four tumor suppressor genes co-mutated with BAP1) into human ductal organoid lines through CRISPR/Cas9. TP53/SMAD4/NF1/PTEN mutants grew in the form of vesicles, which showed a morphology consistent with that of wild-type (WT) organs. However, when additional BAP1 mutations were introduced, obvious loss of polarity, disruption of epithelial organization and increased motility were observed. They also verified the tumorigenicity of TP53/SMAD4/NF1/PTEN/BAP1WT and TP53/SMAD4/NF1/PTEN/BAP1-/- by in vivo experiments. When BAP1 was not mutated, the cysts presented adenoma morphology and were defined as biliary cyst adenoma, which is a preneoplastic lesion. When BAP1 was mutated, there were obvious pleomorphic nuclei, irregular cell morphology, impaired cell polarity, and high levels of mucin secretion and collagen fiber deposition, which are consistent with cholangiocarcinoma.
Application of patient derived cholangiocarcinoma organoids
Research on the occurrence and development of cholangiocarcinoma
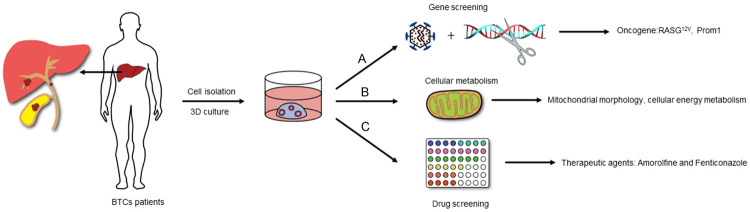
Organoids have been used to study many aspects of BTCs (see Figure 2). Now, it has been confirmed that in different culture environments, liver organoids can show both hepatocyte phenotype and bile duct phenotype [16]. Therefore, many studies have attempted to elucidate the relationship between hepatocytes and cholangiocarcinoma through organoids to determine the etiology of cholangiocarcinoma. Sun and his colleagues [17] used human induced hepatocytes to generate liver organoids and overexpressed ten ICC-enriched genes. These researchers found that overexpressed RASG12V resulted in loss of the circular morphology, separation from the original organoids and production of mucous vacuoles at the edges. Histopathological analysis revealed an increased nucleus-to-cytoplasm ratio, the occurrence of nuclear atypia, the formation of duct or cavity structures, the secretion of mucin, and the expression of cholangiocarcinoma-specific genes and NOTCH signaling genes. Subsequently, RASG12V-overexpressing organoids were orthotopically transplanted into mice. The tumor formation rate was 100%, and all morphological characteristics of the tumor tissue were consistent with those of ICCs. These tumors expressed specific markers of ICCs (CK19, SOX9); thus, the RASG12V gene was believed to be a key factor driving the transformation of liver cells to ICCs.
Figure 2.
Application of BTCs patients-derived organoids. A. Study the oncogenes of BTCs by lentiviral transfection and gene editing technology. B. Study the mitochondrial morphology and cellular energy metabolism of BTCs. C. Drug screening and drug sensitivity.
Moreover, some studies have confirmed that liver cancer organoids constructed from PPTR (overexpression of Prom1, deletion of tumor suppressor genes Pten and TP53) mice have strong stem cell characteristics [18]. This method can produce extensive invasion and metastasis after orthotopic transplantation. Because these organoids have the characteristics of hepatocellular carcinoma and cholangiocarcinoma, hepatocellular carcinoma can lead to the occurrence of cholangiocarcinoma in the process of developing highly invasive and metastatic tumors. Interestingly, Saito and his partners [19] found in their study that the cultivation of intrahepatic cholangiocarcinoma organoids in differentiation medium could show markers of liver cells, differentiating into mature liver cells. Both in vitro and in vivo experiments have confirmed that this differentiation can reduce the malignancy of intrahepatic cholangiocarcinoma by inhibiting the Wnt signaling pathway. Above studies showed that although cholangiocarcinoma can be generated by malignant transformation of hepatocytes, it also has the ability to differentiate into hepatocytes. Inhibiting the expression of some genes or signaling pathways may be a potential method to prevent cholangiocarcinoma.
Research on cellular metabolism of cholangiocarcinoma
According to the Warburg effect, glycolysis is considered the main process of energy production and metabolic growth of tumor cells, and mitochondria play a key role in the energy metabolism of tumors. Mitochondria also control the redox and calcium homeostasis of tumor cells and participate in transcriptional regulation and cell death; thus, they have been a key subcellular structure in the study of cancer metabolism [20]. Li’s group [21] found that the morphology of mitochondria increased and fusion was overactivated in cholangiocarcinoma organoids by comparing adjacent normal tissue organoids from the same patient. After the mitochondrial fusion regulatory genes (OPA1 and MFN1) were knocked out in cholangiocarcinoma organoids by lentivirus, the mitochondria was shortened and the mitochondrial metabolism was slowed (reducing oxygen consumption and ATP production). Importantly, the growth of cholangiocarcinoma organoids was inhibited by promoting apoptosis.
Another study cultured cholangiocarcinoma organoids under glucose-free conditions from two different patients and detected stem cell markers, LGR5+ cell number, gemcitabine drug sensitivity and AKT phosphorylation [22]. The researchers found that the proliferative activity of cholangiocarcinoma organoids and the morphology were decreased and the morphology was altered. Stem cell markers and the number of LGR5+ cells were significantly increased. In addition, gemcitabine drug sensitivity decreased and AKT phosphorylation increased. Subsequently, after inhibition of AKT phosphorylation, the stem cell characteristics and drug resistance to gemcitabine were decreased. The researcher believed that when cholangiocarcinoma cells proliferate rapidly, even when they are in a state of glucose depletion, tumor cells can survive by enhancing the characteristics of stem cells through AKT phosphorylation.
An interesting study constructed isocitrate dehydrogenase 1 (IDH1, mutated in 10-30% of ICC patients) mutants in intrahepatic biliary organoids of mice through lentiviral vectors [23]. The upregulated expression of the platelet isoform of the phosphofructokinase-1 (PFKP-1) gene in the mutants resulted in enhanced glycolysis, and its formation efficiency was significantly higher than that of the wild type. In addition, this study observed consistent results in surgically resected ICCs; that is, the expression of PFKP was significantly upregulated when IDH1 was mutated. Since many tumors can obtain growth advantages through PFKP, IDH1 mutations can be used as metabolic targets for ICCs.
In summary, many studies have studied the mitochondrial function and energy metabolism of cholangiocarcinoma through organoids, and tried to provide a new direction for the treatment of cholangiocarcinoma from the perspective of energy metabolism.
Drug screening and drug sensitivity
As cholangiocarcinoma is characterized by a high degree of malignancy, poor prognosis and chemotherapy resistance, it is important to find new drugs that can effectively treat cholangiocarcinoma through drug screening and drug sensitivity experiments. Saito and his cooperators [14] successfully screened 29 of 339 drugs that have been clinically used to significantly inhibit the growth of intrahepatic cholangiocarcinoma organoids. In addition to anticancer drugs, two antifungal agents (amorolfine and fenticonazole), cerivastatin (hyperlipidemia drug) and talipexole (drug for Parkinson disease) were included. The application of two antifungal agents to normal human intrahepatic biliary epithelial cells proved that they were not toxic to normal biliary cells and could be promising therapeutic drugs for the treatment of cholangiocarcinoma.
Broutier’s group [13] found that the sensitivity of five chemotherapeutic drugs (taselisib, gemcitabine, AZD8931, SCH772984 and dasatanib) was different in 6 primary liver cancer-derived organoids (2 cases of hepatocellular carcinoma, 2 cases of mixed liver cancer and 2 cases of cholangiocarcinoma). This parameter was correlated with tumor subtypes and the mutation of different subtypes. Among them, dasatanib inhibited one of the cholangiocarcinoma organoids. Then, the researchers confirmed that SCH772984 can selectively inhibit ERK phosphorylation and induce tumor cell apoptosis by in vivo experiments; thus, it may be a potential drug for the treatment of cholangiocarcinoma. Heat shock protein 90 (HSP90) inhibitors were screened from 486 targeted small molecular compounds to inhibit the growth of different subtypes of cholangiocarcinoma cells [24], and then, the activity of AUY922 (HSP90 inhibitor) was tested in chemotherapy-resistant intrahepatic cholangiocarcinoma patients-derived organoids. Inhibiting the expression of miRNA-21 was shown to enhance the sensitivity to AUY922; therefore, the combination of HSP90 inhibitor and a miRNA-21 inhibitor can be used as a new strategy to treat cholangiocarcinoma.
Gallbladder cancer organoids
Construction and application of gallbladder organoids
At present, there are few studies on gallbladder organoids, so in this section, we will introduce the construction and application of gallbladder organoids. Lugli and his cooperators [25] isolated gallbladders from 2-month-old mice and incubated them with PBS/EDTA for two hours after collection. The small cell clusters separated by the above operation were implanted into Matrigel and cultured in serum-free medium containing nicotinamide and a cocktail of growth factors (including epidermal growth factor, fibroblast growth factor 10, hepatocyte growth factor, R-spondin-1 and Noggin). In this way, the gallbladder organoids can be propagated stably for more than a year; thus, the researchers hypothesized that there are stem cells in gallbladder organoids. In addition, they found that gallbladder organoids can express the stem cell marker Prom1 and the transcription factor Sox17. Two weeks after removing R-spondin-1, Noggin and nicotinamide from the culture medium, gallbladder organoids could be induced to differentiate and express liver-specific genes (Cyp2c40 and Cyp2c68). Therefore, the researchers believed that gallbladder organoids have the ability to differentiate into hepatocytes, and gallbladder organoid transplantation is expected to become a new treatment for liver diseases. Surprisingly, subsequent studies have also confirmed that extrahepatic cholangiocyte organoids (ECOs) isolated from the gallbladder can regenerate in vivo. Sampaziotis’s team [26] implanted polyglycolic acid scaffolds containing ECOs into NSG mice to successfully reconstruct the surgical defect in the gallbladder wall.
In addition, some studies have studied the pathogenesis of rare gallbladder diseases through organoids. Zarei and his partners [27] compared gallbladder epithelial organoids of pigs with cystic fibrosis (CF) to normal gallbladder epithelial organoids and found that there is a difference in the expression of CF transmembrane conductance regulator (CTFR). The loss of CTFR will damage Cl-/HCO3 - anion transportation and fluid secretion and promote mucin accumulation in gallbladder epithelial cells, resulting in tiny gallbladders in newborn pigs. This research explained the pathogenesis of CF-related gallbladder disease.
Application of gallbladder cancer organoids
Analysis of the occurrence and development of gallbladder cancer
Gallbladder cancer, as one of the digestive tract tumors with the worst prognosis, has extensive regional differences, and the specific pathogenesis is still unclear. This disease is related to many factors, such as environmental factors, microbial factors, metabolic factors and molecular factors [28]. Therefore, elucidating the mechanism of the occurrence and development of gallbladder cancer through organoids can lay a theoretical foundation for treatment. Erlangga and his colleagues [29] used the CRISPR-Cas9 gene editing technique after isolating organoids from the mouse gallbladder and confirmed that Kras activation, PTEN and p53 deletion were involved in the process of gallbladder carcinogenesis. Then two ErbB2-overexpressing mutants with p53 deletion were constructed by retroviral transduction and gene editing, which confirmed that both of them could lead to gallbladder cancer. Gallbladder cancer induced by Kras activation is tubular adenocarcinoma, while most gallbladder cancers induced by ErbB2 mutation show papillary/tubulo-papillary differentiation.
Another study obtained gallbladder organoids from mice with TP53 gene inactivation and cultured them after infection with Salmonella [30]. It was found that organoids with a history of infection lost cohesion and polarity, the nucleus was enlarged and irregular and prominent nucleoli showed neoplastic transformation. Organoids without a history of infection had normal epithelial tissue. Thus, Salmonella bacteria can promote cell tumorigenesis with a predisposing genetic background.
Drug screening and drug sensitivity
At present, the latest methods for the treatment of gallbladder cancer include chemotherapy, radiotherapy, targeted therapy and local regional therapy [31]. However, the first-line chemotherapy regimen for clinical treatment is still gemcitabine combined with cisplatin, and the median survival time is less than one year [32]. Therefore, there is an urgent need for a new treatment to improve the survival rate of patients. García’s group [33] found that Hippo-Yes-associated protein 1 (YAP1) is highly expressed in 60% of patients with chronic cholecystitis and advanced gallbladder cancer. The high expression of nuclear YAP1 is related to the low overall survival rate of patients with subserosal gallbladder cancers. The researchers also confirmed that the inhibition of YAP1 can reduce the migration and invasion of gallbladder cancer cell lines by using verteporfin (VP). The researchers further verified the therapeutic effect of VP on patients-derived organoids and found that VP could inhibit the activity of gemcitabine-resistant GBC organoids. These results suggest that VP sensitizes cancer cells to gemcitabine and enhances cytotoxicity. VP plus gemcitabine may be a promising new regimen for the treatment of GBC. Erlangga’s study transplanted GBC organoids into mice and found that Nal-IR (topoisomerase inhibitor) could effectively pass through the stroma of gallbladder cancer cells and was mainly concentrated in tumor cells; its therapeutic effect was better than that of conventional irinotecan [29].
Deficiency of organoids for biliary tract carcinomas
With the successful construction of different organoids, the application of organoids in tumors has become a research hotspot in recent years. Although tumor organoids have been successfully used to study the occurrence and development, energy metabolism, drug screening, precision medicine treatment and many other aspects of various types of tumors, the success rate of their construction is still the primary limitation to expanding their applications. For cholangiocarcinoma organoids, the success rates of surgical resection and fine needle biopsy are 50% and 60%, respectively [14]. Compared with 76% for liver cancer [34], 80% for ovarian cancer [35] and 63% for pancreatic cancer [36], there is still much for improvement. Therefore, it is very important to analyze the factors that affect the construction of cholangiocarcinoma organoids.
First, cholangiocarcinoma organoids are derived from patient tissues, which tend to contain some noncancerous tissues, thus affecting the construction of cholangiocarcinoma organoids. Saito’s study compared the growth trends of patients’ noncancerous cells and cholangiocarcinoma cells in the construction of organoids and found that noncancerous organoids with a limited proliferation cycle (ceased proliferation at approximately passage 15) had more robust proliferation than cholangiocarcinoma organoids at the early stage [14]. This proliferation difference was also demonstrated in colorectal cancer [37]. Therefore, when organoids are constructed directly from surgically resected tissues, if the source of cells is not carefully analyzed, the noncancerous cells may predominate and the proliferation will overcome the tumor cells, leading to the failure of the construction of tumor organoids. Second, cholangiocarcinomas are often small in size, and the number of cholangiocarcinoma cells in surgically resected tissues is less than that in liver cancer and ovarian cancer. Therefore, the number of tumor cells may also be one of the factors affecting the construction of cholangiocarcinoma organoids. In previous studies, it was also reported that the construction of gastrointestinal cancer organoids was closely related to the number of tumor cells in parental biopsies [38].
In addition, although cholangiocarcinoma organoids can effectively simulate the interaction between tumor cells and the extracellular matrix, the factors that affect the pathogenesis and treatment of cholangiocarcinoma include blood vessels, CAFs (cancer-associated fibroblasts), immune cells (T cells, macrophages, NK cells), etc. Methods to link cholangiocarcinoma organoids with the tumor microenvironment will be a future research direction. At present, studies have applied co-culture technology and air-liquid interface (ALI) system technology to tumor organoids to better simulate the actual situation of tumors. Exposito’s team [39] cultivated organoids from multidrug-resistant metastatic colorectal cancer patients and successfully cocultured them with allogeneic CD8+ T cells. These researchers found that cibisatamab can effectively inhibit the growth rate of multidrug-resistant colorectal cancer cells and that its sensitivity is related to the expression of CEA. Inhibiting the WNT/β-catenin pathway can enhance the expression of CEA, which indicates a new direction to improve the immunotherapeutic effect of cibisatamab in the clinic. Interestingly, another study found that adipocytes can provide energy for the growth of colorectal cancer and regulate the metabolism of colorectal cancer cells by coculturing adipocytes with colorectal cancer organoids. This research effectively explains why obesity is a high risk factor for colorectal cancers [40]. Obesity has also been proven to be closely related to the occurrence of cholangiocarcinoma [41]. Therefore, in the future coculturing adipocytes or other immune cells with cholangiocarcinoma organoids will be an important research strategy to explain the pathogenesis of cholangiocarcinoma.
In ALI system technology, the bottom surface of the organoid is implanted into the culture medium, and the top surface is directly in contact with air. Usui and his cooperators [42] successfully established colorectal cancer organoids by using ALI system technology. Normal colorectal organoids have a cystic structure, containing epithelial cells, goblet cells and fibroblasts, while tumor organoids contain epithelial cells, goblet cells, myofibroblasts and tumor stem cells. Therefore, organoids cultured by ALI system technology can better mimic the tumor microenvironment. In addition, tumor organoids were found to be more resistant to 5-FU and irinotecan compared with 2D cell cultured cells. Neal’s group [43] confirmed that patient derived organoids could retain the T cell receptor spectrum of the original tumor and found that blocking immune checkpoint blockade by anti-PD-1/anti-PD-L1 could lead to tumor cell death by activating tumor antigen-specific tumor invasive lymphocytes. Similarly, anti-PD-1 has also shown progress in the treatment of cholangiocarcinomas in recent years [44], but it has not been applied it to cholangiocarcinoma organoids. In the future, combining tumor organoids with ALI system technology to better mimic the tumor microenvironment and detect the effect of immunotherapy may be a new strategy.
The existing research about the construction and application of gallbladder cancer organoids is still in its infancy, which may be related to the low incidence of gallbladder cancer and obvious regional differences. Methods to address the low success rate of gallbladder cancer organoid construction (20%) will help overcoming the primary limitation to applying organoids to gallbladder cancer.
Summary and outlook
Although biliary tract carcinomas still face many problems, such as a high degree of malignancy, single treatment methods and a low five-year survival rate, these problems will be gradually solved with an in-depth understanding of the pathogenesis, clinical diagnosis and treatment of biliary tract carcinomas in the future. Organoids have become the focus of current research because they can mimic the cell-to-cell interactions and the cell-to-ECM interplay. More importantly, tumor organoids can be directly derived from patients and express specific gene phenotypes of patients, which is important for clinical diagnosis and personalized medical treatment of tumors. In particular, the newly developed coculture technology and ALI system technology can simulate the tumor microenvironment to better recapitulate the complexity of tumors.
In addition, a new type of immunotherapy has aroused our interest. Löffler reported a successful case of using multi-peptide vaccine to treat a cholangiocarcinoma patient with a lung metastasis [45]. This vaccine induced T cell responses and increased T cell infiltration. The patient is currently tumor-free, 41 months after initiation of vaccination, suggesting therapeutic effectiveness. In order to make this promising new therapy better pass ethical review and be used in clinical trials, it could be a good choice to apply it to patient-derive organoids. With the rise of single-cell sequencing technology, finding the pathogenesis of cholangiocarcinoma by comparing the heterogeneity between different cells has become a current research hotspot. Single-cell sequencing of cholangiocarcinoma with different interventions will also become an important guide for clinical diagnosis and treatment in the future. However, for some treatments that have not been approved clinically, it is often difficult to obtain post-treatment samples of patients. Through the culturing patient-derived organoids, it is possible to obtain the single-cell sequencing results of directly interfered organoid, thereby providing a basis for clinical diagnosis and treatment.
At present, research on the organoids of biliary system carcinomas is still in its infancy. The construction success rate, applications and prospects still require more studies. However, this does not affect the research value and the bright future of organoids for biliary tract carcinomas.
Acknowledgements
This work was supported by National Natural Science Foundation of China, No. 81970569 and No. 81773293; and Natural Science Foundation of Hunan Province, No. 2017SK50121.
Disclosure of conflict of interest
None.
References
- 1.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 2.Murrow LM, Weber RJ, Gartner ZJ. Dissecting the stem cell niche with organoid models: an engineering-based approach. Development. 2017;144:998–1007. doi: 10.1242/dev.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 4.Zanoni M, Cortesi M, Zamagni A, Arienti C, Pignatta S, Tesei A. Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol. 2020;13:97. doi: 10.1186/s13045-020-00931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol. 2019;12:142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuciforo S, Fofana I, Matter MS, Blumer T, Calabrese D, Boldanova T, Piscuoglio S, Wieland S, Ringnalda F, Schwank G, Terracciano LM, Ng CKY, Heim MH. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24:1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiriac H, Belleau P, Engle DD, Plenker D, Deschenes A, Somerville TDD, Froeling FEM, Burkhart RA, Denroche RE, Jang GH, Miyabayashi K, Young CM, Patel H, Ma M, LaComb JF, Palmaira RLD, Javed AA, Huynh JC, Johnson M, Arora K, Robine N, Shah M, Sanghvi R, Goetz AB, Lowder CY, Martello L, Driehuis E, LeComte N, Askan G, Iacobuzio-Donahue CA, Clevers H, Wood LD, Hruban RH, Thompson E, Aguirre AJ, Wolpin BM, Sasson A, Kim J, Wu M, Bucobo JC, Allen P, Sejpal DV, Nealon W, Sullivan JD, Winter JM, Gimotty PA, Grem JL, DiMaio DJ, Buscaglia JM, Grandgenett PM, Brody JR, Hollingsworth MA, O’Kane GM, Notta F, Kim E, Crawford JM, Devoe C, Ocean A, Wolfgang CL, Yu KH, Li E, Vakoc CR, Hubert B, Fischer SE, Wilson JM, Moffitt R, Knox J, Krasnitz A, Gallinger S, Tuveson DA. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopper O, de Witte CJ, Lohmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, van Wijk LM, Revilla SA, Theeuwsen R, van de Ven M, van Roosmalen MJ, Ponsioen B, Ho VWH, Neel BG, Bosse T, Gaarenstroom KN, Vrieling H, Vreeswijk MPG, van Diest PJ, Witteveen PO, Jonges T, Bos JL, van Oudenaarden A, Zweemer RP, Snippert HJG, Kloosterman WP, Clevers H. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 9.Pan B, Zhao D, Liu Y, Li N, Song C, Li N, Li X, Li M, Zhao Z. Establishment and characterization of breast cancer organoids from a patient with mammary Paget’s disease. Cancer Cell Int. 2020;20:365. doi: 10.1186/s12935-020-01459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, Choi EK, Jeong SY, Taylor AM, Jain S, Meyerson M, Jang SJ. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, de Ligt J, Behjati S, Grolleman JE, van Wezel T, Nik-Zainal S, Kuiper RP, Cuppen E, Clevers H. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234–238. doi: 10.1126/science.aao3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Witte CJ, Espejo Valle-Inclan J, Hami N, Lohmussaar K, Kopper O, Vreuls CPH, Jonges GN, van Diest P, Nguyen L, Clevers H, Kloosterman WP, Cuppen E, Snippert HJG, Zweemer RP, Witteveen PO, Stelloo E. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 2020;31:107762. doi: 10.1016/j.celrep.2020.107762. [DOI] [PubMed] [Google Scholar]
- 13.Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo BK, Dietmann S, Davies SE, Praseedom RK, Lieshout R, IJzermans JNM, Wigmore SJ, Saeb-Parsy K, Garnett MJ, van der Laan LJ, Huch M. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito Y, Muramatsu T, Kanai Y, Ojima H, Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, Yoshikawa N, Furukawa R, Saito H. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27:1265–1276. e1264. doi: 10.1016/j.celrep.2019.03.088. [DOI] [PubMed] [Google Scholar]
- 15.Artegiani B, van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I, Tapia P, Lopez-Iglesias C, Postrach D, Dayton T, Oka R, Hu H, van Boxtel R, van Es JH, Offerhaus J, Peters PJ, van Rheenen J, Vermeulen M, Clevers H. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell. 2019;24:927–943. e926. doi: 10.1016/j.stem.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Wang Y, Cen J, Ma X, Cui L, Qiu Z, Zhang Z, Li H, Yang RZ, Wang C, Chen X, Wang L, Ye Y, Zhang H, Pan G, Kang JS, Ji Y, Zheng YW, Zheng S, Hui L. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 2019;21:1015–1026. doi: 10.1038/s41556-019-0359-5. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Qian M, Chen IH, Finkelstein D, Onar-Thomas A, Johnson M, Calabrese C, Bahrami A, Lopez-Terrada DH, Yang JJ, Tao WA, Zhu L. Acquisition of cholangiocarcinoma traits during advanced hepatocellular carcinoma development in mice. Am J Pathol. 2018;188:656–671. doi: 10.1016/j.ajpath.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito Y, Nakaoka T, Muramatsu T, Ojima H, Sukeda A, Sugiyama Y, Uchida R, Furukawa R, Kitahara A, Sato T, Kanai Y, Saito H. Induction of differentiation of intrahepatic cholangiocarcinoma cells to functional hepatocytes using an organoid culture system. Sci Rep. 2018;8:2821. doi: 10.1038/s41598-018-21121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2017;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Wang L, Wang Y, Zhang S, Zhou G, Lieshout R, Ma B, Liu J, Qu C, Verstegen MMA, Sprengers D, Kwekkeboom J, van der Laan LJW, Cao W, Peppelenbosch MP, Pan Q. Mitochondrial fusion via OPA1 and MFN1 supports liver tumor cell metabolism and growth. Cells. 2020;9:121. doi: 10.3390/cells9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshikawa N, Saito Y, Manabe H, Nakaoka T, Uchida R, Furukawa R, Muramatsu T, Sugiyama Y, Kimura M, Saito H. Glucose depletion enhances the stem cell phenotype and gemcitabine resistance of cholangiocarcinoma organoids through AKT phosphorylation and reactive oxygen species. Cancers (Basel) 2019;11:1933. doi: 10.3390/cancers11121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara H, Tateishi K, Misumi K, Hayashi A, Igarashi K, Kato H, Nakatsuka T, Suzuki N, Yamamoto K, Kudo Y, Hayakawa Y, Nakagawa H, Tanaka Y, Ijichi H, Kogure H, Nakai Y, Isayama H, Hasegawa K, Fukayama M, Soga T, Koike K. Mutant IDH1 confers resistance to energy stress in normal biliary cells through PFKP-induced aerobic glycolysis and AMPK activation. Sci Rep. 2019;9:18859. doi: 10.1038/s41598-019-55211-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampis A, Carotenuto P, Vlachogiannis G, Cascione L, Hedayat S, Burke R, Clarke P, Bosma E, Simbolo M, Scarpa A, Yu S, Cole R, Smyth E, Mateos JF, Begum R, Hezelova B, Eltahir Z, Wotherspoon A, Fotiadis N, Bali MA, Nepal C, Khan K, Stubbs M, Hahne JC, Gasparini P, Guzzardo V, Croce CM, Eccles S, Fassan M, Cunningham D, Andersen JB, Workman P, Valeri N, Braconi C. MIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinoma. Gastroenterology. 2018;154:1066–1079. e1065. doi: 10.1053/j.gastro.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugli N, Kamileri I, Keogh A, Malinka T, Sarris ME, Talianidis I, Schaad O, Candinas D, Stroka D, Halazonetis TD. R-spondin 1 and noggin facilitate expansion of resident stem cells from non-damaged gallbladders. EMBO Rep. 2016;17:769–779. doi: 10.15252/embr.201642169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, Gieseck RL 3rd, de Brito MC, Berntsen NL, Gomez-Vazquez MJ, Ortmann D, Yiangou L, Ross A, Bargehr J, Bertero A, Zonneveld MCF, Pedersen MT, Pawlowski M, Valestrand L, Madrigal P, Georgakopoulos N, Pirmadjid N, Skeldon GM, Casey J, Shu W, Materek PM, Snijders KE, Brown SE, Rimland CA, Simonic I, Davies SE, Jensen KB, Zilbauer M, Gelson WTH, Alexander GJ, Sinha S, Hannan NRF, Wynn TA, Karlsen TH, Melum E, Markaki AE, Saeb-Parsy K, Vallier L. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954–963. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]
- 27.Zarei K, Stroik MR, Gansemer ND, Thurman AL, Ostedgaard LS, Ernst SE, Thornell IM, Powers LS, Pezzulo AA, Meyerholz DK, Stoltz DA. Early pathogenesis of cystic fibrosis gallbladder disease in a porcine model. Lab Invest. 2020;100:1388–1399. doi: 10.1038/s41374-020-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra SK, Kumari N, Krishnani N. Molecular pathogenesis of gallbladder cancer: an update. Mutat Res. 2019;816-818:111674. doi: 10.1016/j.mrfmmm.2019.111674. [DOI] [PubMed] [Google Scholar]
- 29.Erlangga Z, Wolff K, Poth T, Peltzer A, Nahnsen S, Spielberg S, Timrott K, Woller N, Kuhnel F, Manns MP, Saborowski A, Vogel A, Saborowski M. Potent antitumor activity of liposomal irinotecan in an organoid- and CRISPR-Cas9-based murine model of gallbladder cancer. Cancers (Basel) 2019;11:1904. doi: 10.3390/cancers11121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanu T, Spaapen RM, Bakker JM, Pratap CB, Wu LE, Hofland I, Broeks A, Shukla VK, Kumar M, Janssen H, Song JY, Neefjes-Borst EA, te Riele H, Holden DW, Nath G, Neefjes J. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe. 2015;17:763–774. doi: 10.1016/j.chom.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Oneda E, Abu Hilal M, Zaniboni A. Biliary tract cancer: current medical treatment strategies. Cancers (Basel) 2020;12:1237. doi: 10.3390/cancers12051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigt J, Malfertheiner P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2010;4:395–397. doi: 10.1586/egh.10.45. [DOI] [PubMed] [Google Scholar]
- 33.Garcia P, Rosa L, Vargas S, Weber H, Espinoza JA, Suarez F, Romero-Calvo I, Elgueta N, Rivera V, Nervi B, Obreque J, Leal P, Vinuela E, Aguayo G, Muniz S, Sagredo A, Roa JC, Bizama C. Hippo-YAP1 is a prognosis marker and potentially targetable pathway in advanced gallbladder cancer. Cancers (Basel) 2020;12:778. doi: 10.3390/cancers12040778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruun J, Kryeziu K, Eide PW, Moosavi SH, Eilertsen IA, Langerud J, Røsok B, Totland MZ, Brunsell TH, Pellinen T, Saarela J, Bergsland CH, Palmer HG, Brudvik KW, Guren T, Dienstmann R, Guren MG, Nesbakken A, Bjørnbeth BA, Sveen A, Lothe RA. Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Clin Cancer Res. 2020;26:4107–4119. doi: 10.1158/1078-0432.CCR-19-3637. [DOI] [PubMed] [Google Scholar]
- 35.Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F, Aoki D. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10:12581. doi: 10.1038/s41598-020-69488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, Stigter ECA, Burgering B, Geurts V, Gracanin A, Bounova G, Morsink FH, Vries R, Boj S, van Es J, Offerhaus GJA, Kranenburg O, Garnett MJ, Wessels L, Cuppen E, Brosens LAA, Clevers H. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci U S A. 2019;116:26580–90. doi: 10.1073/pnas.1911273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Exposito R, Semiannikova M, Griffiths B, Khan K, Barber LJ, Woolston A, Spain G, von Loga K, Challoner B, Patel R, Ranes M, Swain A, Thomas J, Bryant A, Saffery C, Fotiadis N, Guettler S, Mansfield D, Melcher A, Powles T, Rao S, Watkins D, Chau I, Matthews N, Wallberg F, Starling N, Cunningham D, Gerlinger M. CEA expression heterogeneity and plasticity confer resistance to the CEA-targeting bispecific immunotherapy antibody cibisatamab (CEA-TCB) in patient-derived colorectal cancer organoids. J Immunother Cancer. 2019;7:101. doi: 10.1186/s40425-019-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, Weiss HL, Mark Evers B, Gao T. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrick JL, Thistle JE, Zeleniuch-Jacquotte A, Zhang X, Wactawski-Wende J, Van Dyke AL, Stampfer MJ, Sinha R, Sesso HD, Schairer C, Rosenberg L, Rohan TE, Robien K, Purdue MP, Poynter JN, Palmer JR, Newton CC, Linet MS, Liao LM, Lee IM, Koshiol J, Kitahara CM, Hofmann JN, Graubard BI, Giovannucci E, Gaziano MJ, Gapstur SM, Freedman ND, Chong DQ, Chan AT, Buring JE, Freeman LBE, Campbell PT, McGlynn KA. Body mass index, diabetes and intrahepatic cholangiocarcinoma risk: the liver cancer pooling project and meta-analysis. Am J Gastroenterol. 2018;113:1494–1505. doi: 10.1038/s41395-018-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usui T, Sakurai M, Enjoji S, Kawasaki H, Umata K, Ohama T, Fujiwara N, Yabe R, Tsuji S, Yamawaki H, Hazama S, Takenouchi H, Nakajima M, Tsunedomi R, Suzuki N, Nagano H, Sato K. Establishment of a novel model for anticancer drug resistance in three-dimensional primary culture of tumor microenvironment. Stem Cells Int. 2016;2016:7053872. doi: 10.1155/2016/7053872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng YY, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988. e1916. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sui M, Li Y, Wang H, Luo Y, Wan T, Wang X, Hu B, Cheng Y, Lv X, Xin X, Xu Q, Wang G, Lu S. Two cases of intrahepatic cholangiocellular carcinoma with high insertion-deletion ratios that achieved a complete response following chemotherapy combined with PD-1 blockade. J Immunother Cancer. 2019;7:125. doi: 10.1186/s40425-019-0596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Löffler M, Chandran P, Laske K, Schroeder C, Bonzheim I, Walzer M, Hilke F, Trautwein N, Kowalewski D, Schuster H, Günder M, Carcamo Yañez V, Mohr C, Sturm M, Nguyen H, Riess O, Bauer P, Nahnsen S, Nadalin S, Zieker D, Glatzle J, Thiel K, Schneiderhan-Marra N, Clasen S, Bösmüller H, Fend F, Kohlbacher O, Gouttefangeas C, Stevanović S, Königsrainer A, Rammensee HG. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016;65:849–855. doi: 10.1016/j.jhep.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]