Abstract
Macropinocytosis is a form of endocytosis which provides an effective way for non-selective uptakes of extracellular proteins, liquids, and particles. The endocytic process is initiated by the activation of the growth factors signaling pathways. After activation of the biochemical signal, the cell starts internalizing extracellular solutes and nutrients into the irregular endocytic vesicles, known as macropinosomes that deliver them into the lysosomes for degradation. Macropinocytosis plays an important role in the nutritional supply of cancer cells. Due to the rapid expansion of cancer cells and the abnormal vascular microenvironment, cancer cells are usually deprived of oxygen and nutrients. Therefore, they must transform their metabolism to survive and grow in this harsh microenvironment. To satisfy their energy needs, cancer cells enhance the activity of macropinocytosis. Therefore, this metabolic adaptation that is used by cancer cells can be exploited to develop new targeted cancer therapies. In this review, we discuss the molecular mechanism that actuates the process of macropinocytosis in a variety of cancers, and the novel anti-cancer therapeutics in targeting macropinocytosis.
Keywords: Macropinocytosis, growth factors, cancer mechanism, targeted cancer therapy
Introduction
Cancer macropinocytosis is an endocytic uptake process by which cancer cells internalize extracellular proteins or necrotic cell debris and deliver them to lysosomes for further degradation [1-4]. The decomposition of these macropinocytic cargos contributes to the supply of the desperately needed amino acids that support cancer cells’ survival and growth. As shown in Figure 1, the molecular mechanism of macropinocytosis in cancer cells is quite complicated. The Ras and PI3K signaling pathways are the most common in cancer macropinocytosis (Table 1). The macropinocytic induction is associated with the activation of oncogenes (e.g., RAS or EGFR) or deactivation of tumor suppressor genes (e.g., PTEN) in cancer cells [5-7]. Macropinocytosis is closely related to actin cytoskeleton remodeling and the macropinosomes’ generation process includes plasma membrane ruffling, macropinocytic cup formation and closure, and detachment from the plasma membrane. Several pivotal regulators of actin polymerization, such as small GTPases (e.g., Ras, Rac, Cdc42, Arf6, and Rab5), p21-activated kinase 1 (Pak1), and phosphoinositide 3-kinase (PI3K), have been linked to the formation of plasma membrane protrusions and macropinocytic activity [8]. Interestingly, RAS and PI3K activation is related to the stimulation of receptor tyrosine kinases (RTK), such as platelet-derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR) [1,9]. Certainly, there are also negative-regulatory factors that weaken macropinocytosis. For instance, the PTEN phosphatase blocks the PI3K signaling pathway by converting PI (3, 4, 5) P3 to PI (4, 5) P2. Importantly, PI (3, 4, 5) P3 is necessary for plasma membranes’ ruffling and macropinosomes’ closures [7]. In addition, one particular study demonstrated that mTORC1 could suppress the lysosomal catabolism of extracellular proteins [2]. Of course, there are also other specific pathways, such as the Wnt signaling pathway, that play an important role in micropinocytosis in colorectal and bladder cancers. Therefore, with the identification of the molecular mechanism of macropinocytosis, novel therapeutic approaches can be developed to treat cancers. At present, the major therapeutic methods in targeting macropinocytosis include small molecules and antibodies. Intriguingly, nucleic acids, as an emerging therapeutic drug, might become the third modality in cancer treatments using macropinocytosis [10]. Here, we aim to summarize the role of macropinocytosis in different type of cancers, including pancreatic, lung, colorectal, bladder, prostate, brain and nervous system cancers. Meanwhile, we discuss three major therapeutic modalities, including chemotherapy, immunotherapy, and nucleic acid therapy that are used for targeting macropinocytosis in cancers.
Figure 1.
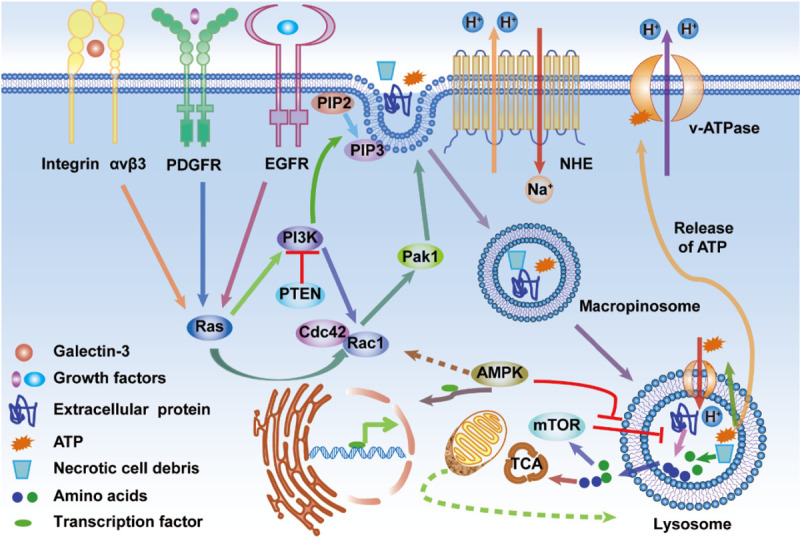
Schematic diagram of cancer cells ingesting extracellular small particles, such as protein, necrotic debris, and ATP, through macropinocytosis. The activation of Ras and PI3K pathways, by oncogenic mutations, integrin αvβ3, EGFR or PDGFR, activates upstream effectors (e.g., Ras and PI3K) which then activate downstream effectors, such as Rac1, Cdc42, and Pak1, that are significant regulators of macropinocytosis. PTEN loss can activate PI3K, which is closely linked to membrane phospholipid conversion. AMPK activation can activate Rac1, which can trigger macropinocytosis, and prompt the transport of transcription factors into the nucleus, resulting in elevated expression of lysosomal genes in the nucleus. The activation of Rac1 and Cdc42 and the lysosomal degradation of macropinocytic cargos are sensitive to pH changes, which are regulated by the Na+/H+ exchanger (NHE) and the vacuolar H+-ATPase (v-ATPase). In the lysosome, extracellular proteins, or necrotic debris, can be degraded into amino acids, which can fuel the TCA cycle, leading to increased cell growth and survival. The lysosomal degradation process of the macropinocytosed protein into amino acids can be inhibited by mTORC1. Interestingly, AMPK can antagonize the mTORC1 pathway and improve the degradation efficiency of internalized proteins in the lysosome.
Table 1.
Example of micropinocytosis in different cancer types
| Type of cancer | Molecular driver | Macropinocytic cargo | Signaling Pathway | Reference |
|---|---|---|---|---|
| pancreatic cancer | KRAS | proteins | RAS, PI3K | [1,9] |
| lung cancer | KRAS | proteins, ATP | RAS, PI3K, Rac | [26,28-31] |
| galectin-3 | ||||
| integrin αvβ3 | ||||
| AMPK | ||||
| colorectal cancer | KRAS | proteins | RAS, PI3K, Wnt | [39,42,43] |
| Fz | ||||
| Lrp6 | ||||
| APC | ||||
| PRMT1 | ||||
| bladder cancer | HRAS | proteins | RAS, PI3K, Wnt | [46,48] |
| KRAS | ||||
| PTEN | ||||
| brain and nervous system cancers | ||||
| GBM | HRAS | proteins | RAS, PI3K | [49] |
| KRAS | ||||
| neuroblastoma | IGF-1 | proteins | PI3K | [57] |
| PMA | proteins | ? | [57] | |
| medulloblastoma | TrkA | proteins | ? | [58] |
| prostate cancer | PTEN | necrotic cell debris | PI3K | [7,27,61-63] |
| AMPK |
Macropinocytosis in different types of cancer
Pancreatic cancer
Approximately 95% of pancreatic ductal adenocarcinomas (PDAC) have poor prognosis and are associated with KRAS mutation [11]. Using mouse models [12,13] and human specimens [14] of KRAS-driven PDAC, previous studies have shown that macropinocytosis acts as a nutrient supply pathway. It is interesting to note that macropinocytic inductions can be detected in PDAC autochthonous models. KrasLSL-G12D/+; p53loxP/loxP (KP) mice models could progress to PDAC via restricting the expression of the pancreatic cre-recombinase. In vitro, the levels of macropinocytic induction in KP mice were significantly higher than those in the wild-type group [12]. Similarly, the stimulation of macropinocytosis was also clearly increased in all human PDAC specimens [14]. Therefore, macropinocytosis is a PDAC metabolic adaptation that allows PDAC cells to uptake extracellular proteins and other small molecules [15].
As one of the extracellular proteins, serum albumin is the most abundant. It is a legitimate inference that PDAC cells afford to take up extracellular serum albumin by macropinocytosis [16]. Using immunofluorescence microscopy that tracked labeled amino acids and albumin, an in vitro study demonstrated that the presence of macropinosomes is involved in the degradation of proteins in lysosomes [14]. The free amino acids, such as glutamine, are produced by breakdown of proteins. As a consequence, PDAC cells have the capacity to conduct tricarboxylic acid (TCA) cycle due to the presence of these free amino acids [17]. Taken together, PDAC cells adapt to promote the catabolism of serum albumin when essential amino acids are lacking.
The initiation of RAS-driven macropinocytosis is closely associated with several signaling pathways, such as the RAS and PI3K pathways (Table 1). Firstly, growth factors that induce macropinocytosis, specifically bind to RTKs (e.g., EGFR, PDGFR), which stimulate membrane ruffling through the activation of Ras. Rac1 and Cdc42 activations by Ras are crucial for membrane ruffling and macropinocytic cups formation and the stimulation of Pak1 leads to induce actin polymerization. In addition, PI (3, 4, 5) P3 that can coordinate actin remodeling, is generated with the increase of PI3K. Therefore, both Pak1 and PI (3, 4, 5) P3 are important for the macropinocytic cups closure [1,9]. However, there are other factors that affect the process of macropinocytosis. For instance, Rac1 and Cdc42 require activation under appropriate submembranous alkaline pH that is maintained by Na+/H+ exchanger (NHE) and vacuolar H+-ATPase (v-ATPase) (Figure 1) [1,5,18]. Furthermore, Rac1 activity was markedly restrained in KRAS-driven PDAC cells with low syndecan 1 (SDC1, a protein mediator of macropinocytosis) expression [19]. The mechanistic targeting of rapamycin (mTOR) is associated with the presence of two distinct multiprotein complexes: mTORC1 and mTORC2 [20]. As a critical regulator of macropinocytosis, mTORC1 is activated by amino acids that are produced by lysosomal degradation of internalized extracellular proteins and in response to growth factors, signaling. Surprisingly, the activity mTORC1 can not only stimulate biosynthetic pathways but also inhibit lysosomal proteins’ degradation [21,22]. Importantly, one particular study has shown that mTORC1 inhibition could significantly increase lysosomal degradation of internalized proteins from the extracellular environment [2]. Moreover, mTORC2 plays a significant role in macropinocytosis and lysosomal degradation of extracellular proteins. One particular study demonstrated that mTORC2 disruption can deprive the ability of lysosomes to scavenge proteins, leading to the inhibition of proliferation and induction of apoptosis [23].
Lung cancer
Similarly, KRAS oncogene mutations are also the most common in non-small cell lung cancer (NSCLC) [24,25]. It is known that NSCLC cells, driven by KRAS mutations, stimulate macropinocytosis to survive and proliferate in nutrient-deprived environments (Table 1) [26]. Interestingly, KRAS addiction refers to NSCLC cell lines that carry KRAS mutations and that are dependent on the expression of the KRAS oncogene for their viability [27]. A recent report showed that KRAS-addicted NSCLC cells trigger macropinocytosis following direct binding between galectin-3 and integrin αvβ3 receptor on their cell surfaces (Table 1) [28]. Integrin αvβ3-positive cells induce the formation of macropinosomes that promote the internalization of extracellular proteins. This process depends on galectin-3 and integrin αvβ3, as the consumption of either molecule reduces the activity of macropinocytosis (Figure 1). In addition, AMP-activated protein kinase (AMPK) also plays a significant role in lysosomal induction in KRAS-mutant NSCLC. For example, a study has elaborated that energy depletion could activate AMPK that prompts the dephosphorylation and nuclear transport of transcription factors, such as Tfeb and Tfe3. Importantly, activated Tfe3 can promote the expression of lysosomal genes in the nucleus (Table 1; Figure 1) [29]. Hence, AMPK is required for the growth of KRAS-driven NSCLC by indirectly regulating the expression of lysosomal genes. Remarkably, a recent report has also demonstrated that NSCLC cell lines could survive in glucose starvation conditions through Rac-driven macropinocytosis which facilitated the internalization of extracellular proteins. Noteworthy, PI3K was shown to be the crucial upstream activator of macropinocytosis that was mediated by the Rac-Pak signaling (Table 1; Figure 1) [30].
In addition to the internalization of extracellular proteins, lung cancer cells could also assimilate extracellular ATP through macropinocytosis to maintain viability under low energy environments (Table 1) [31]. Therefore, the levels of extracellular ATP in lung cancer are much higher than those in normal tissues. Furthermore, several studies indicated that NSCLC cells could internalize extracellular ATP by macropinocytosis to enhance their intracellular ATP levels, which lead to cancer cell growth and drug resistance [32-34]. In summary, ATP internalization through macropinocytosis is a major example of macropinocytic cargo changes in cancer cells. According to different circumstances, the identification of new macropinocytic cargoes that play important roles in cancer cells’ proliferation or drug resistance can result in the development of novel cancer targeted therapies.
Colorectal cancer
Similar to lung and pancreatic cancers, mutations in KRAS oncogene are also the predominant oncogenic alterations in colorectal cancer [35]. It is well known that macropinocytosis plays a critical role in the proliferation and growth of cancer cells driven by oncogenic KRAS. Several studies demonstrated that macropinocytosis was conducive to cancer cells growth by improving the activity of mTORC1 [36-38]. Interestingly, the activation of the PI3K/Akt signaling pathway is closely linked to mTORC1 activity [20]. Furthermore, study showed that the activation of the protein kinase CK2 in colorectal cancer plays an important role in the PI3K/Akt signaling pathway that could activate mTORC1. By activating Akt through phosphorylation, CK2 could enhance the PI3K/Akt signaling pathway (Table 1) [39]. Subsequently, Akt inactivates tuberous sclerosis complex 2 (TSC2) through phosphorylation, leading to TSC1/2 lysosomal separation and the activation of Rheb that activates mTORC1 on the lysosomal membrane [40]. In addition, another study demonstrated that the Rag activation can recruit mTORC1 to the surface of the lysosome (Figure 2) [41]. Therefore, small GTPases Rag or Rheb plays a vital role in regulating mTORC1 activity, which stimulates protein synthesis and cell growth [20].
Figure 2.
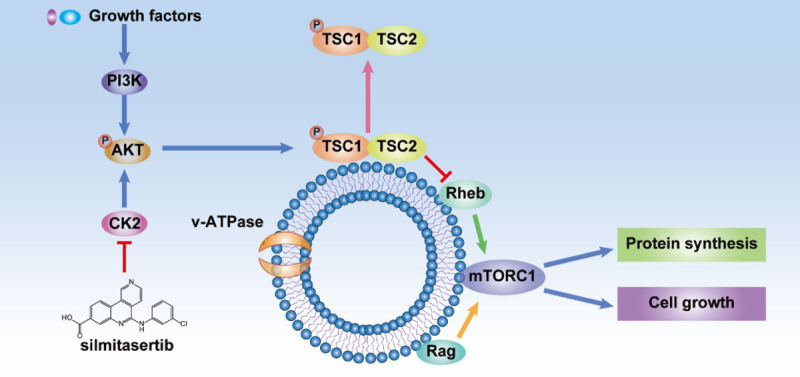
Growth factors stimulation induces the PI3K/Akt/mTORC1 pathway. CK2 can enhance the PI3K/Akt/mTORC1 signaling pathway by phosphorylating and activating Akt. Therefore, the CK2 inhibitor, silmitasertib, can result in mTORC1 inhibition. Akt inactivates tuberous sclerosis complex 2 (TSC2) through phosphorylation, leading to TSC1/2 separation from the lysosomal membrane. Rheb and Rag activation can activate and recruit mTORC1 on the lysosomal membrane, and thereby stimulates protein synthesis and cell growth.
Moreover, the Wnt signaling pathway is also an important player in colorectal cancer through its role in switching metabolic pathways according to nutrient stress requirements. For instance, a recent report indicated that the activation of the Wnt signaling pathway and the tumor suppressors APC, or Axin deletions, markedly increase macropinocytosis in colorectal cancer cells (Table 1) [42]. Interestingly, Wnt growth factors induce the initiation of macropinocytosis by binding to co-receptors Frizzled (Fz) and LDL receptor-related protein 6 (Lrp6) on the cell surface. The canonical Wnt pathway signals target the activation of WNT genes by stabilizing the transcriptional activator β-catenin, which can translocate into the nucleus and bind to the TCF transcriptional factors (Table 1) [43]. In colorectal cancer cells, the study demonstrated that β-catenin stabilization benefits from the functional incapacitation of the complex components, APC, Axin1, GSK3β, and CK1, that could destroy β-catenin. Importantly, it was also found that the ATPase vacuolar protein sorting 4 (Vps4), contributes to the accumulation of β-catenin in colorectal cancer cells [42]. The increased expression of Wnt3a, a WNT gene expression product, resulted in an increased uptake of extracellular proteins by stimulating actin rearrangements, which could be blocked by the inhibitor of NHE [42]. Fortunately, previous studies have shown that the protein arginine methyltransferase 1 (PRMT1) is important for Wnt signaling [44,45]. A study provided an intriguing evidence showing that Wnt-driven macropinocytosis requires PRMT1 in colorectal cancer cells (Table 1) [42]. Therefore, under nutrient-limited conditions, the Wnt signaling pathway plays an important role in macropinocytosis in colorectal cancer cells (Figure 3).
Figure 3.
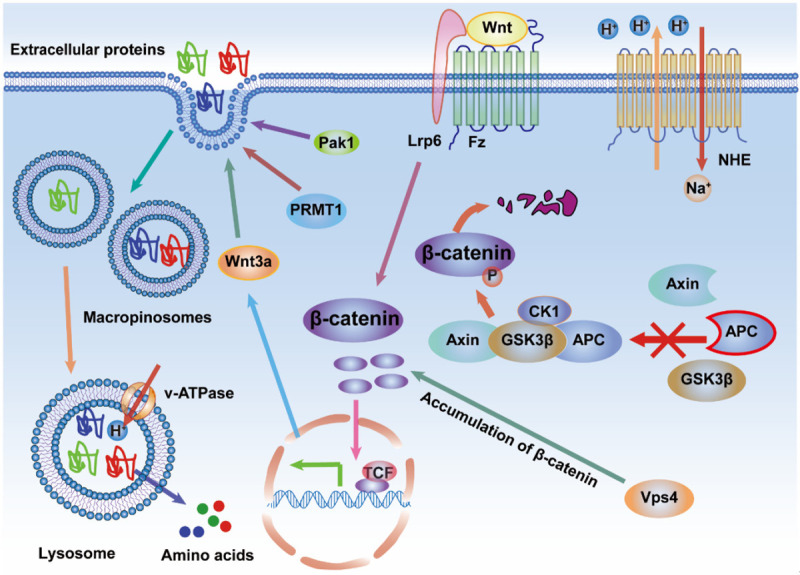
The Wnt growth factor triggers macropinocytosis by binding to co-receptors Frizzled (Fz) and LDL receptor-related protein 6 (Lrp6) on the cell surface. The stabilized upstream effector, β-catenin, can translocate into the nucleus and bind with TCF transcriptional factors to promote the expression of the downstream effector, Wnt3a, which can increase the uptake of extracellular proteins. The stabilization of β-catenin benefits from the functional incapacitation of the complex components, APC, Axin1, GSK3β, and CK1, that could destruct β-catenin. The vacuolar protein sorting 4 (Vps4) contributes to β-catenin accumulation in cancer cells. In addition, Wnt-driven macropinocytosis may require the protein arginine methyltransferase 1 (PRMT1) and Pak1 in cancer cells. However, the upstream effectors of PRMT1 and Pak1 in Wnt signaling pathway, are still unclear.
Bladder cancer
Interestingly, bladder cancer cells that are most commonly driven by HRAS mutations, also exhibit enhanced macropinocytosis for extracellular proteins uptake [13]. Bacille Calmette-Guerin (BCG), an attenuated strain of Mycobacterium bovis, is absorbed into bladder cancer cells by macropinocytosis. It is also well known that BCG can be effectively used in the treatment of superficial bladder cancer [46]. However, BCG mechanism of action in the treatment of bladder cancer is still unclear [47]. Interestingly, these HRAS-driven or KRAS-driven bladder cancer cells allowed the assimilation of BCG via macropinocytosis, which was dependent on small GTPases (e.g., Cdc42 and Rac1) and their downstream effector, Pak1 (Table 1). In addition, it was also identified to be connected to the deletion of PTEN, which could hinder the PI3K signaling pathway (Table 1) [46]. Therefore, these significant findings indicate that macropinocytosis not only supports the metabolism of bladder cancer cells but may also represents a mode of BCG transport into bladder cancer cells, which can optimize the therapeutic effect.
In addition to Ras signaling pathway, the activation of the Wnt signaling pathway has also been demonstrated to induce macropinocytic uptake in bladder cancer cells. Interestingly, several studies demonstrated that five negative regulators (DKK2, KREMEN1, NKD1, SMAD4, and MAPK91) of the Wnt signaling pathway were identified by performing a whole-genome shRNA screen in RAS-wild-type bladder cancer cells [48]. The knockdown of these genes caused activation of the canonical Wnt signaling pathway, which stimulated macropinocytic uptake through β-catenin accumulation and translocation. The use of a recombinant Wnt3a protein or the expression of a constitutively active form of β-catenin resulted in robust macropinocytosis in bladder cancer cells. Similar to the Ras pathway, it was also found that the Wnt pathway stimulates macropinocytosis in a Pak1-dependent mode that was demonstrated using the Pak1 inhibitor IPA-3 (Table 2; Figure 3). Therefore, the activation of the Wnt pathway is necessary to oncogenic Ras-driven macropinocytosis in bladder cancer cells (Table 1) [48]. In short, the Wnt signaling pathway may be closely related to Ras and PI3K signaling pathways through pak1, suggesting that these signaling pathways work together in cancer macropinocytosis.
Table 2.
Example of therapeutic modalities by exploiting micropinocytosis in cancers
| Therapeutic modalities | drugs | Mechanism | Type of cancer | Reference |
|---|---|---|---|---|
| chemotherapy | ||||
| NHE inhibitor | EIPA | impact on submembranous alkaline pH | RAS-driven cancer | [1,42,73] |
| v-ATPase inhibitor | bafilomycin A1 | impact on lysosomal acidic pH | RAS-driven cancer | [74] |
| EGFR inhibitor | gefitinib | inhibit the macropinocytosis pathway | NSCLC | [75] |
| Galectin-3 inhibitor | GCS-100 | inhibit the macropinocytosis pathway | Lung cancer or pancreatic cancer | [28] |
| DOCK1 inhibitor | TBOPP | repress DOCK1-mediated macropinocytosis | RAS-driven cancer | [76] |
| actin inhibitors | blebbstatin | inhibit actin polymerization | RAS-driven cancer | [77] |
| cytochalasin D | inhibit actin polymerization | RAS-driven cancer | [78] | |
| PI3K inhibitors | Wortmannin, LY294002 | inhibit PI3K signaling pathway | RAS-driven cancer | [79] |
| Pak1 inhibitor | IPA-3 | impact on actin polymerization | RAS-driven and WNT-driven cancer | [48] |
| lysosomal inhibitor | PPT1 | suppress lysosomal activity | pancreatic cancer or colorectal cancer | [80,81] |
| mTOR inhibitors | torin1 and AZD2014 | suppress proteins scavenging | RAS-driven cancer | [2,23] |
| AMPK activator | sepantronium bromide | block mTORC1 | prostate cancer | [64] |
| CK2 inhibitor | silmitasertib | massive macropinocytosis | colorectal cancer | [39] |
| inducers | MOMIPP | massive macropinocytosis | GBM | [55] |
| bacoside A | massive macropinocytosis | GBM | [56] | |
| NGF | massive macropinocytosis | medulloblastoma | [58] | |
| METH | massive macropinocytosis | neuroblastoma | [59] | |
| anti-cancer agents conjugation | albumin-conjugated DOX | increase anti-cancer drugs to cancer cells through micropinocytosis | PDAC | [82] |
| nab-paclitaxel with gemcitabine | increase anti-cancer drugs to cancer cells through micropinocytosis | PDAC | [83] | |
| T-UPSM | pH-triggered rapid drug release in lysosomes | PDAC | [84] | |
| TBM1 with 5-FU | induce macropinocytosis and increase 5-FU transport into cancer cells | colorectal cancer | [85] | |
| MOMIPP with temozolomide | massive micropinocytosis and increase uptake of temozolomide | GBM | [49] | |
| Immunotherapy | ||||
| mAbs | bevacizumab | target intracellular VEGF | NSCLC | [89] |
| ScFv | target EGFR | pancreatic cancer | [93] | |
| vaccines | BCG | using macropinocytosis | bladder cancer | [46] |
| MTBVAC | using macropinocytosis | bladder cancer | [94] | |
| ApoE3-incorporated biomimetic nanoparticle | target macropinocytosis pathway | metastatic cancer | [95] | |
| Nucleic acid therapy | ||||
| nucleic acid drugs | TCTP ASOs | decreased expression of TCTP | prostate cancer | [97] |
| TFEB siRNA | suppress TFEB | KRAS-mutant cancer | [101] | |
| ATF5 siRNA | inhibit cancer cell growth | GBM | [53] | |
| KRASG12D siRNA | decreased expression of KRASG12D | pancreatic cancer | [106] |
Brain and nervous system cancers
The activation of the Ras and PI3K signaling pathways are also significantly involved in the induction of macropinocytosis in glioblastoma (GBM) cells [49]. EGFR and PDGFR are activators of macropinocytosis in GBM cells [49]. Interestingly, previous studies have demonstrated that EGFR and PDGFR were upregulated in gliomas [50-52]. In gliomas, K-Ras and H-Ras are activated after stimulation by EGFR and PDGFR. Subsequently, Rac1 and Cdc42 activate both Arf6 and Pak1 that are initiators of actin polymerization. In addition, the transformation of PI (3, 4, 5) P3 by PI (3, 4) P2 under the control of PI3K, plays an important role in the formation of macropinosomes (Table 1) [49]. Therefore, the activated Ras in GBM cells can enhance the levels of intracellular macropinosomes and extracellular small molecules’ internalizations in nutrient-poor environments (Figure 1). Fortunately, this property has been used to study the absorption of potential therapeutic nano-drugs that could cross the blood-brain barrier [53]. However, the hyperstimulation of macropinocytosis in GBM cells that was induced by over-expressed Ras or by small molecules, could lead to methuosis, a form of cell death [54]. For example, one particular study has demonstrated that the 3-(5-methoxy-2-methyl-1Hindol-3-yl)-1-(4-pyridinyl)-2-propen-1-one (MOMIPP) can induce massive macropinocytosis, leading to methuosis in GBM cells (Table 2) [55]. Similarly, uncontrolled macropinocytotic effects were observed in GBM cells that were treated with Bacoside A, and that resulted in tumor cell death (Table 2) [56]. This event may be dependent on high intracellular calcium mediated by the phosphorylated calcium/calmodulin-dependent protein kinase IIA (CAMK2A).
Furthermore, macropinocytosis is also easy to find in medulloblastoma and neuroblastoma. For example, two different types of macropinocytosis were found in neuroblastoma cells, which were induced by insulin-like growth factor 1 (IGF-1) and phorbol 12-myristate 13-acetate (PMA) (Table 1) [57]. Interestingly, IGF-1 induced macropinocytosis predominantly occurred in the cell bodies and required the PI3K signaling for macropinosomes formation, while PMA induced macropinocytosis in the neurites did not require PI3K signaling. Other than GBM, the hyperstimulation of macropinocytosis, in medulloblastoma and neuroblastoma, have also been observed to result in cell death. In fact, uncontrolled macropinocytosis has been detected when medulloblastoma cells, with TrkA overexpression, were treated with nerve growth factor (NGF), leading to tumor cell death (Tables 1, 2) [58]. Moreover, in neuroblastoma cells, methamphetamine (METH) induced hyperstimulation of macropinocytosis that was mediated by activated Ras/Rac1, in response to PI3K signaling pathways, eventually resulting in tumor cell death (Table 2) [59].
Prostate cancer
Loss of the tumor suppressor gene PTEN is most common in prostate cancer [60]. PTEN is as a lipid phosphatase that plays an important role in opposing the activity of the PI3K pathway that signals through transforming PI (3, 4, 5) P3 into PI (4, 5) P2 (Table 1) [27,61]. Therefore, PTEN loss in prostate cancer results in increased PI (3, 4, 5) P3 [62]. Because PI (3, 4, 5) P3 is necessary for the plasma membrane ruffling and macropinosomes closure, PTEN loss in prostate cancer cells may be conducive in exploiting macropinocytosis to maintain survival and proliferation of cancer cells in nutrient-deleted conditions. However, the loss of PTEN alone is not enough to trigger macropinocytosis. Cells can effectively respond to energy shortages by activating AMPK (Table 1) [63]. The induction of macropinocytosis is also mediated by AMPK, which functions to activate Rac1 that promotes macropinosomes’ formation (Table 1; Figure 1) [7]. Strikingly, AMPK can also improve the effective lysosomal degradation of internalized proteins by antagonizing the mTORC1 pathway (Figure 1) [9]. One particular study has provided that sepantronium bromide could block mTORC1 through AMPK activation (Table 2) [64].
To proliferate and survive in nutrient poor environments, PTEN-deficient prostate cancer cells internalize and catabolize necrotic debris and extracellular proteins by macropinocytosis [65]. It is surprising that serum albumin uptake in PTEN-deficient prostate cancer cells is independent of macropinocytosis, and potentially depends on macropinocytosis-independent pathways for its internalization [7]. Interestingly, several studies indicated that prostate cancer associated fibroblasts could afford to ingest serum albumin to support cancer cells growth via macropinocytosis [66,67]. In fact, PTEN-deficient prostate cancer cells assimilate necrotic cell debris in nutrient-limiting conditions, solely by macropinocytosis. In prostate cancer cells, necrotic cell debris, as macropinocytic cargoes, are degraded to produce amino acids and lipids (Table 1; Figure 1) [7]. Both amino acids and lipids that are generated by catabolism of necrotic cell debris are beneficial to biosynthetic metabolism and material storage. Therefore, further studies are needed to explore the extent AMPK effects on cellular macropinocytosis in other pathological contexts and whether necrotic cell debris could be exploited as a nutrient source to support growth in other cancer types [68].
Therapeutic modalities for exploiting cancers macropinocytosis
Chemotherapy
Since cancer cells can absorb extracellular small molecules, such as serum albumin, necrotic debris and ATP, through macropinocytosis, small molecule drugs can therefore be used to block the macropinocytosis pathway and treat cancers by disrupting the metabolic activity of cancer cells [69-72]. For example, numerous studies have shown that 5-(N-ethyl-N-propyl) amiloride (EIPA) can inhibit both macropinocytosis and actin polymerization by blocking NHE (Table 2) [1,42,73]. Similarly, v-ATPase inhibitors, such as bafilomycin A1, that have an impact on lysosomal acidic pH, can also result in the inhibition of macropinocytosis and reduction of intracellular amino acid levels (Table 2) [74]. In addition, inhibitors of the signaling pathway networks that mediate the activity of macropinocytosis are also effectively used in anti-cancer treatment. For instance, a recent study indicated that the EGFR inhibitor, gefitinib, can suppress the macropinocytosis pathway in NSCLC cells (Table 2) [75]. Analogously, one particular study has demonstrated that the Galectin-3 inhibitor, GCS-100, could disrupt the interaction between Galectin-3 and integrin αvβ3, thereby inhibiting the macropinocytosis pathway in KRAS-addicted lung and pancreatic cancer cells (Table 2) [28]. In fact, DOCK1 is a Rac-specific guanine nucleotide exchange factor (GEF) and the selective inhibitor of DOCK1,1-(2-(3’-(trifluoromethyl)-[1,1’-biphenyl]-4-yl)-2-oxoethyl)-5-pyrrolidinylsulfonyl-2(1H)-pyridone (TBOPP), can repress DOCK1-mediated macropinocytosis in RAS-transformed cancer cells (Table 2) [76]. There are other frequently used small molecule inhibitors of macropinocytosis, such as actin inhibitors (blebbstatin [77], cytochalasin D [78]) and PI3K inhibitors (Wortmannin, LY294002 [79]) (Table 2). Moreover, recent studies reported that a novel lysosomal inhibitor, palmitoyl-protein thioesterase 1 (PPT1), can suppress lysosomal activity that play a critical role in degrading proteins during macropinocytosis (Table 2) [80,81]. Interestingly, it was shown that better effects could be obtained when mTOR inhibitors are delivered in combination with inhibitors that could suppress the uptake or the process of lysosomal extracellular proteins scavenging [2,23].
In addition to blocking cancer cell macropinocytosis, another anti-cancer method is to cause a significant increase in cancer cell catastrophic macropinocytosis by using related inhibitors or inducers. For example, one particular study has demonstrated that treatment with the CK2 inhibitor, silmitasertib, and gives rise to large number of vacuoles that derived from massive macropinocytosis, resulting in colorectal cancer cells methuosis-like death (Table 2; Figure 2) [39]. Similarly, several studies have shown that inducers can also cause massive macropinocytosis that lead to methuosis in brain and nervous system cancers cells (Table 2) [55,56,58,59].
Lastly, researchers also made great efforts to exploit macropinocytosis for delivering anti-cancer agents into cancer cells [71]. For example, a study demonstrated that albumin-conjugated doxorubicin (DOX) could be internalized into KRAS-driven PDAC cells by macropinocytosis, thereby releasing DOX in the PDAC cells to exert its toxic effect (Table 2) [82]. Similarly, it has been shown that macropinocytosis can be exploited for internalization of drug that combined albumin nanoparticles (nab-paclitaxel) with gemcitabine for PDAC therapy (Table 2) [83]. Interestingly, a recent research indicated that triptolide prodrug-loaded UPSM (T-UPSM) could be absorbed into KRAS-mutant PDAC cells through macropinocytosis, which pH triggered the rapid release of drug in lysosomes (Table 2) [84]. Furthermore, Tubeimoside-1 (TBM1) can induce macropinocytosis and increase 5-FU transport into colorectal cancer cells where it promotes synergistic anti-cancer effects (Table 2) [85]. Analogously, a recent study has shown that MOMIPP could also induce massive macropinocytosis, leading to increased uptake of temozolomide by GBM cells and resulting in additional anti-cancer effects (Table 2) [49]. Therefore, understanding the mechanism of cancer cells’ macropinocytosis is very helpful for cancer treatment.
Immunotherapy
Currently, therapeutic methods, such as monoclonal antibodies (mAbs) and vaccines, are widely used in cancer immunotherapy [86]. Interestingly, recent studies have elaborated that mAbs could be internalized into cancer cells through macropinocytosis [87,88]. In addition, macropinocytosis can be selectively upregulated in different types of cancer. Therefore, mAbs that can be effectively internalized via macropinocytosis are meaningful for the development of cancer therapies. For instance, bevacizumab nanoparticles, which can be internalized by macropinocytosis, were applied to target intracellular VEGF in NSCLC (Table 2) [89]. In recent years, more studies have reported on the application of antibody-drug conjugates (ADCs) in cancer therapy [90-92]. Intriguingly, one particular study has indicated that the ScFv antibody that was based on albumin domain and its cytotoxic conjugate, exhibits characteristics of targeted-EGFR, intensive-macropinocytosis and cytotoxicity, resulting in apparent growth inhibition of KRAS-mutant pancreatic cancer (Table 2) [93].
In addition to mAbs, vaccines that are associated with macropinocytosis also play an important role in cancer immunotherapy. For example, one particular study has shown that oncogenic activation of macropinocytosis resulted in BCG internalization into bladder cancer cells (Table 2) [46]. Additionally, mycobacterium tuberculosis vaccine (MTBVAC) could also be internalized into bladder cancer cells through macropinocytosis, resulting in the inhibition of cancer cells’ growth (Table 2) [94]. Thus, MTBVAC can be used as a new immunotherapy drug for bladder cancer. Similarly, ApoE3-incorporated biomimetic nanoparticle is also very likely to be used as a safe and effective nanovaccine for cancer immunotherapy by exploiting the macropinocytosis pathway (Table 2) [95]. Briefly, both mAbs and vaccines can be effectively applied to cancer immunotherapy through exploiting macropinocytosis.
Nucleic acid therapy
Nucleic acid therapy may become another emerging therapeutic modality that could use the role of macropinocytosis in cancer cells [10]. Currently, the fastest clinically progressing nucleic acid drugs for cancer therapy are antisense oligonucleotides (ASOs), DNA, short interfering RNA (siRNA), microRNA (miRNA), and messenger RNA (mRNA) (Figure 4) [96]. However, due to the cell membrane barrier, these nucleic acids require carriers to enter the cell. Fortunately, there are several delivery systems, such as lipids [97,98], polymers [99], and peptides [100], that can be used to carry nucleic acids into cancer cells and via macropinocytosis. For example, translationally controlled tumor protein (TCTP) ASOs that carried by lipids can be internalized into castration resistant prostate cancer cells through macropinocytosis, resulting in decreased expression of TCTP, which is tightly linked to cell growth (Table 2) [97]. Interestingly, siRNA, which is carried by lipofectamine, can drive the suppression of the transcription factor EB (TFEB) in KRAS-mutant cancer cells, leading to a substantial reduction in the lysosomal ability to degrade extracellular proteins (Table 2) [101]. Similarly, AGMA1 polyamidoamine that effectively carry siRNAs can be absorbed into prostate cancer cells by macropinocytosis and cause gene silencing without inducing cytotoxicity [102]. Furthermore, one particular study demonstrated that gold nanoparticles modified by cell-penetrating peptides (CPPs) can significantly increase cellular uptake and the absorption rate of nucleic acid drugs [103].
Figure 4.
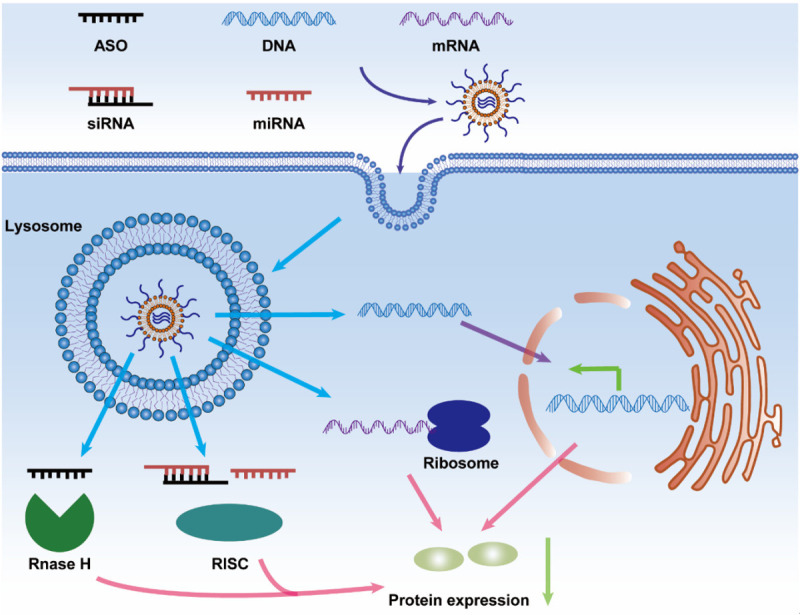
Nucleic acid drugs, including antisense oligonucleotide (ASO), DNA, short interfering RNA (siRNA), microRNA (miRNA), and messenger RNA (mRNA), can be applied to clinical cancer therapy. Carriers that contain nucleic acid drugs enter into cancer cells through macropinocytosis and are released from lysosomes. ASO is associated with the activity of RNase H endonuclease. MiRNAs and siRNAs are recognized by the RNA-induced silencing complex (RISC). Ribosomes and mRNAs participate in protein translation and expression. DNAs may be transported into the nucleus and affect the transcription of gene. Eventually, the expression of macropinocytosis-associated proteins will be reduced.
Interestingly, in addition to these carriers (e.g., lipids, polymers, and peptides), inorganic nanoparticles [53] and extracellular vesicles (EVs) [104-106] can also carry nucleic acids into cancer cells through macropinocytosis. For example, the nanostructure, lipoprotein, which carries the activating transcription factor-5 (ATF5) siRNA, can cross the blood-brain barrier, and be internalized by Ras-driven GBM cells via macropinocytosis. Subsequently, the release of ATF5 siRNA in the GBM cells causes the inhibition of cancer cell growth (Table 2) [53]. In addition, exosomes can carry siRNA that targets oncogenic KRASG12D, into pancreatic cancer cells through macropinocytosis, which inhibits cancer cell growth and increases overall survival (Table 2) [106]. Therefore, research on the use of macropinocytosis for intracellular delivery of nucleic acids drugs to cancer cells, is needed in the future.
Conclusions and perspectives
Under nutritional stress, cancer cells can initiate macropinocytosis through the activation of oncogenes and related complex signaling pathways, or the deactivation of tumor suppressor genes. The macropinocytic cargos may be extracellular proteins, ATP, necrotic cell debris or other macromolecules. Fortunately, an enhanced macropinocytic activity has been observed in various types of cancer. Not only does macropinocytosis provide a survival possibility under nutritional deficiencies, but it also provides the potential for tumors to limitlessly grow in harsh tumor microenvironments. In fact, the molecular mechanism that drive macropinocytosis in different cancers is quite complex. For example, the Ras, PI3K, and Wnt signaling pathways play significant roles in triggering macropinocytosis in different cancers. Therefore, it is extremely useful to better understand the molecular mechanism of macropinocytosis to enable the development of cancer targeted therapies.
At present, there are three main therapeutic modalities that exploit cancer macropinocytosis, including chemotherapy, immunotherapy, and nucleic acid therapy. For this reason, developing novel, specific and effective drugs (e.g., small molecules, mAbs, vaccines, and nucleic acids) for targeting cancer macropinocytosis will be the focus of future research. For instance, in addition to the molecular targets in Table 2, these molecules (e.g., Rac1, Cdc42, SDC1, Rag, Rheb, Fz, Lrp6, Vps4, β-catenin, Wnt3a, and PRMT1) could be used as cancer therapeutic targets by exploiting macropinocytosis.
However, macropinocytosis is not the only cancerous metabolic pathway in nutrient-poor conditions. For example, there are other metabolic pathways, such as clathrin-mediated endocytosis (CME) [107,108] and caveolae-mediated endocytosis (CVE) [108,109], that can also effectively internalize small particles into cancer cells. Therefore, studies that focused on combining macropinocytosis inhibitors with other metabolic pathway inhibitors may improve cancers therapeutic outcomes.
Acknowledgements
The work was supported by The National Natural Sciences Foundations of China (No. 81960456 and 81960457) and Jiangxi Provincial Natural Science Foundation (No. 20202ACB216004). Thank you very much for the language editing service provided by editsprings.
Disclosure of conflict of interest
None.
Abbreviations
- Pak1
p21-activated kinase 1
- PI3K
phosphoinositide 3-kinase
- RTK
receptor tyrosine kinases
- PDGFR
platelet-derived growth factor receptor
- EGFR
epidermal growth factor receptor
- PDAC
pancreatic ductal adenocarcinomas
- KP
KrasLSL-G12D/+; p53loxP /loxP
- TCA
tricarboxylic acid
- NHE
Na+/H+ exchanger
- v-ATPase
vacuolar H+-ATPase
- SDC1
syndecan 1
- mTOR
mechanistic targeting of rapamycin
- NSCLC
non-small cell lung cancer
- AMPK
AMP-activated protein kinase
- TSC
tuberous sclerosis complex
- Fz
Frizzled
- Lrp6
LDL receptor-related protein 6
- Vps4
vacuolar protein sorting 4
- PRMT1
protein arginine methyltransferase 1
- BCG
Bacille Calmette-Guerin
- GBM
glioblastoma
- MOMIPP
3-(5-methoxy-2-methyl-1Hindol-3-yl)-1-(4-pyridinyl)-2-propen-1-one
- CAMK2A
calcium/calmodulin-dependent protein kinase IIA
- IGF-1
insulin-like growth factor 1
- PMA
phorbol 12-myristate 13-acetate
- NGF
nerve growth factor
- METH
methamphetamine
- EIPA
5-(N-ethyl-N-propyl) amiloride
- GEF
guanine nucleotide exchange factor
- TBOPP
1-(2-(3’-(trifluoromethyl)-[1,1’-biphenyl]-4-yl)-2-oxoethyl)-5-pyrrolidinylsulfonyl-2(1H)-pyridone
- PPT1
palmitoyl-protein thioesterase 1
- DOX
doxorubicin
- T-UPSM
triptolide prodrug-loaded UPSM
- TBM1
Tubeimoside-1
- mAbs
monoclonal antibodies
- ADCs
antibody-drug conjugates
- MTBVAC
mycobacterium tuberculosis vaccine
- siRNA
short interfering RNA
- miRNA
microRNA
- mRNA
messenger RNA
- TCTP
translationally controlled tumor protein
- TFEB
transcription factor EB
- CPPs
cell-penetrating peptides
- EVs
extracellular vesicles
- ATF5
activating transcription factor-5
- CME
clathrin-mediated endocytosis
- CVE
caveolae-mediated endocytosis
- RISC
RNA-induced silencing complex
References
- 1.Recouvreux MV, Commisso C. Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front Endocrinol (Lausanne) 2017;8:261. doi: 10.3389/fendo.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayashankar V, Edinger AL. Macropinocytosis confers resistance to therapies targeting cancer anabolism. Nat Commun. 2020;11:1121. doi: 10.1038/s41467-020-14928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm W, Thompson CB. Nutrient acquisition strategies of mammalian cells. Nature. 2017;546:234–242. doi: 10.1038/nature22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez C, Hauser AD, Vucic EA, Bar-Sagi D. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature. 2019;576:477–481. doi: 10.1038/s41586-019-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unni AM, Lockwood WW, Zejnullahu K, Lee-Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. Elife. 2015;4:e06907. doi: 10.7554/eLife.06907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SM, Nguyen TT, Ravi A, Kubiniok P, Finicle BT, Jayashankar V, Malacrida L, Hou J, Robertson J, Gao D, Chernoff J, Digman MA, Potma EO, Tromberg BJ, Thibault P, Edinger AL. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 2018;8:866–883. doi: 10.1158/2159-8290.CD-17-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egami Y, Taguchi T, Maekawa M, Arai H, Araki N. Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front Physiol. 2014;5:374. doi: 10.3389/fphys.2014.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palm W. Metabolic functions of macropinocytosis. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180285. doi: 10.1098/rstb.2018.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai AS, Hunter MR, Kapustin AN. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180156. doi: 10.1098/rstb.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 12.Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, Wyckoff J, Del Rosario AM, Whitman M, Chin CR, Condon KJ, Lammers A, Kellersberger KA, Stall BK, Stephanopoulos G, Bar-Sagi D, Han J, Rabinowitz JD, Cima MJ, Langer R, Vander Heiden MG. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23:235–241. doi: 10.1038/nm.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perera RM, Bardeesy N. Pancreatic cancer metabolism: breaking it down to build it back up. Cancer Discov. 2015;5:1247–1261. doi: 10.1158/2159-8290.CD-15-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commisso C. The pervasiveness of macropinocytosis in oncological malignancies. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180153. doi: 10.1098/rstb.2018.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaziri-Gohar A, Zarei M, Brody JR, Winter JM. Metabolic dependencies in pancreatic cancer. Front Oncol. 2018;8:617. doi: 10.3389/fonc.2018.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao W, Rose JL, Wang W, Seth S, Jiang H, Taguchi A, Liu J, Yan L, Kapoor A, Hou P, Chen Z, Wang Q, Nezi L, Xu Z, Yao J, Hu B, Pettazzoni PF, Ho IL, Feng N, Ramamoorthy V, Jiang S, Deng P, Ma GJ, Den P, Tan Z, Zhang SX, Wang H, Wang YA, Deem AK, Fleming JB, Carugo A, Heffernan TP, Maitra A, Viale A, Ying H, Hanash S, DePinho RA, Draetta GF. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature. 2019;568:410–414. doi: 10.1038/s41586-019-1062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida S, Pacitto R, Inoki K, Swanson J. Macropinocytosis, mTORC1 and cellular growth control. Cell Mol Life Sci. 2018;75:1227–1239. doi: 10.1007/s00018-017-2710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez A, Hall MN. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36:397–408. doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalopoulou E, Auciello FR, Bulusu V, Strachan D, Campbell AD, Tait-Mulder J, Karim SA, Morton JP, Sansom OJ, Kamphorst JJ. Macropinocytosis renders a subset of pancreatic tumor cells resistant to mTOR inhibition. Cell Rep. 2020;30:2729–2742. e2724. doi: 10.1016/j.celrep.2020.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adderley H, Blackhall FH, Lindsay CR. KRAS-mutant non-small cell lung cancer: converging small molecules and immune checkpoint inhibition. EBioMedicine. 2019;41:711–716. doi: 10.1016/j.ebiom.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roman M, Baraibar I, Lopez I, Nadal E, Rolfo C, Vicent S, Gil-Bazo I. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17:33. doi: 10.1186/s12943-018-0789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pupo E, Avanzato D, Middonti E, Bussolino F, Lanzetti L. KRAS-driven metabolic rewiring reveals novel actionable targets in cancer. Front Oncol. 2019;9:848. doi: 10.3389/fonc.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Commisso C. Macropinocytosis in cancer: a complex signaling network. Trends Cancer. 2019;5:332–334. doi: 10.1016/j.trecan.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seguin L, Camargo MF, Wettersten HI, Kato S, Desgrosellier JS, von Schalscha T, Elliott KC, Cosset E, Lesperance J, Weis SM, Cheresh DA. Galectin-3, a druggable vulnerability for KRAS-addicted cancers. Cancer Discov. 2017;7:1464–1479. doi: 10.1158/2159-8290.CD-17-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, Shokhirev MN, Leblanc M, Vera LI, Hutchins A, Ross DS, Shaw RJ, Svensson RU. Genetic analysis reveals AMPK is required to support tumor growth in murine kras-dependent lung cancer models. Cell Metab. 2019;29:285–302. e287. doi: 10.1016/j.cmet.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodakoski C, Hopkins BD, Zhang G, Su T, Cheng Z, Morris R, Rhee KY, Goncalves MD, Cantley LC. Rac-mediated macropinocytosis of extracellular protein promotes glucose independence in non-small cell lung cancer. Cancers (Basel) 2019;11:37. doi: 10.3390/cancers11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Y, Wang X, Liu Y, Li Y, Colvin RA, Tong L, Wu S, Chen X. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 2014;351:242–251. doi: 10.1016/j.canlet.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Wang X, Li Y, Evers M, Zhang H, Chen X. Extracellular and macropinocytosis internalized ATP work together to induce epithelial-mesenchymal transition and other early metastatic activities in lung cancer. Cancer Cell Int. 2019;19:254. doi: 10.1186/s12935-019-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Li Y, Qian Y, Cao Y, Shriwas P, Zhang H, Chen X. Extracellular ATP, as an energy and phosphorylating molecule, induces different types of drug resistances in cancer cells through ATP internalization and intracellular ATP level increase. Oncotarget. 2017;8:87860–87877. doi: 10.18632/oncotarget.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y, Wang X, Li Y, Cao Y, Chen X. Extracellular ATP a new player in cancer metabolism: NSCLC cells internalize ATP in vitro and in vivo using multiple endocytic mechanisms. Mol Cancer Res. 2016;14:1087–1096. doi: 10.1158/1541-7786.MCR-16-0118. [DOI] [PubMed] [Google Scholar]
- 35.Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloomfield G, Kay RR. Uses and abuses of macropinocytosis. J Cell Sci. 2016;129:2697–2705. doi: 10.1242/jcs.176149. [DOI] [PubMed] [Google Scholar]
- 37.Sung S, Choi J, Cheong H. Catabolic pathways regulated by mTORC1 are pivotal for survival and growth of cancer cells expressing mutant Ras. Oncotarget. 2015;6:40405–40417. doi: 10.18632/oncotarget.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheong H. mTORC1 regulates nutrient access in Ras-mediated tumors. Aging (Albany NY) 2016;8:1165–1166. doi: 10.18632/aging.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva-Pavez E, Villar P, Trigo C, Caamano E, Niechi I, Perez P, Munoz JP, Aguayo F, Burzio VA, Varas-Godoy M, Castro AF, Colombo MI, Tapia JC. CK2 inhibition with silmitasertib promotes methuosis-like cell death associated to catastrophic massive vacuolization of colorectal cancer cells. Cell Death Dis. 2019;10:73. doi: 10.1038/s41419-019-1306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tejeda-Munoz N, Albrecht LV, Bui MH, De Robertis EM. Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc Natl Acad Sci U S A. 2019;116:10402–10411. doi: 10.1073/pnas.1903506116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht LV, Ploper D, Tejeda-Munoz N, De Robertis EM. Arginine methylation is required for canonical Wnt signaling and endolysosomal trafficking. Proc Natl Acad Sci U S A. 2018;115:E5317–E5325. doi: 10.1073/pnas.1804091115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albrecht LV, Bui MH, De Robertis EM. Canonical Wnt is inhibited by targeting one-carbon metabolism through methotrexate or methionine deprivation. Proc Natl Acad Sci U S A. 2019;116:2987–2995. doi: 10.1073/pnas.1820161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redelman-Sidi G, Iyer G, Solit DB, Glickman MS. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. 2013;73:1156–1167. doi: 10.1158/0008-5472.CAN-12-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15:615–625. doi: 10.1038/s41585-018-0055-4. [DOI] [PubMed] [Google Scholar]
- 48.Redelman-Sidi G, Binyamin A, Gaeta I, Palm W, Thompson CB, Romesser PB, Lowe SW, Bagul M, Doench JG, Root DE, Glickman MS. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 2018;78:4658–4670. doi: 10.1158/0008-5472.CAN-17-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colin M, Delporte C, Janky R, Lechon AS, Renard G, Van Antwerpen P, Maltese WA, Mathieu V. Dysregulation of macropinocytosis processes in glioblastomas may be exploited to increase intracellular anti-cancer drug levels: the example of temozolomide. Cancers (Basel) 2019;11:411. doi: 10.3390/cancers11030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westphal M, Maire CL, Lamszus K. EGFR as a target for glioblastoma treatment: an unfulfilled promise. CNS Drugs. 2017;31:723–735. doi: 10.1007/s40263-017-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Gines C, Navarro L, Munoz-Hidalgo L, Buso E, Morales JM, Gil-Benso R, Gregori-Romero M, Megias J, Roldan P, Segura-Sabater R, Almerich-Silla JM, Monleon D, Cerda-Nicolas M. Association between epidermal growth factor receptor amplification and ADP-ribosylation factor 1 methylation in human glioblastoma. Cell Oncol (Dordr) 2017;40:389–399. doi: 10.1007/s13402-017-0329-5. [DOI] [PubMed] [Google Scholar]
- 52.Muller S, Liu SJ, Di Lullo E, Malatesta M, Pollen AA, Nowakowski TJ, Kohanbash G, Aghi M, Kriegstein AR, Lim DA, Diaz A. Single-cell sequencing maps gene expression to mutational phylogenies in PDGF- and EGF-driven gliomas. Mol Syst Biol. 2016;12:889. doi: 10.15252/msb.20166969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang JL, Jiang G, Song QX, Gu X, Hu M, Wang XL, Song HH, Chen LP, Lin YY, Jiang D, Chen J, Feng JF, Qiu YM, Jiang JY, Jiang XG, Chen HZ, Gao XL. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat Commun. 2017;8:15144. doi: 10.1038/ncomms15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maltese WA, Overmeyer JH. Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am J Pathol. 2014;184:1630–1642. doi: 10.1016/j.ajpath.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trabbic CJ, Dietsch HM, Alexander EM, Nagy PI, Robinson MW, Overmeyer JH, Maltese WA, Erhardt PW. Differential induction of cytoplasmic vacuolization and methuosis by novel 2-indolyl-substituted pyridinylpropenones. ACS Med Chem Lett. 2014;5:73–77. doi: 10.1021/ml4003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.John S, Sivakumar KC, Mishra R. Bacoside A induces tumor cell death in human glioblastoma cell lines through catastrophic macropinocytosis. Front Mol Neurosci. 2017;10:171. doi: 10.3389/fnmol.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang J, Kolpak AL, Bao ZZ. Myosin IIB isoform plays an essential role in the formation of two distinct types of macropinosomes. Cytoskeleton (Hoboken) 2010;67:32–42. doi: 10.1002/cm.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, MacDonald JI, Talebian A, Leuenberger J, Seah C, Pasternak SH, Michnick SW, Meakin SO. Unravelling the mechanism of TrkA-induced cell death by macropinocytosis in medulloblastoma Daoy cells. Mol Cell Biol. 2016;36:2596–2611. doi: 10.1128/MCB.00255-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nara A, Aki T, Funakoshi T, Unuma K, Uemura K. Hyperstimulation of macropinocytosis leads to lysosomal dysfunction during exposure to methamphetamine in SH-SY5Y cells. Brain Res. 2012;1466:1–14. doi: 10.1016/j.brainres.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, Lotan TL. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15:222–234. doi: 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wise HM, Hermida MA, Leslie NR. Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond) 2017;131:197–210. doi: 10.1042/CS20160026. [DOI] [PubMed] [Google Scholar]
- 62.Koizumi A, Narita S, Nakanishi H, Ishikawa M, Eguchi S, Kimura H, Takasuga S, Huang M, Inoue T, Sasaki J, Yoshioka T, Habuchi T, Sasaki T. Increased fatty acyl saturation of phosphatidylinositol phosphates in prostate cancer progression. Sci Rep. 2019;9:13257. doi: 10.1038/s41598-019-49744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danielpour D, Gao Z, Zmina PM, Shankar E, Shultes BC, Jobava R, Welford SM, Hatzoglou M. Early cellular responses of prostate carcinoma cells to sepantronium bromide (YM155) involve suppression of mTORC1 by AMPK. Sci Rep. 2019;9:11541. doi: 10.1038/s41598-019-47573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelsey R. Macropinocytosis for proliferation. Nat Rev Urol. 2018;15:336–337. doi: 10.1038/s41585-018-0012-2. [DOI] [PubMed] [Google Scholar]
- 66.Mishra R, Bhowmick NA. Visualization of macropinocytosis in prostate fibroblasts. Bio Protoc. 2019;9:e3235. doi: 10.21769/BioProtoc.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra R, Haldar S, Suchanti S, Bhowmick NA. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocr Relat Cancer. 2019;26:R673–R688. doi: 10.1530/ERC-19-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Commisso C, Debnath J. Macropinocytosis fuels prostate cancer. Cancer Discov. 2018;8:800–802. doi: 10.1158/2159-8290.CD-18-0513. [DOI] [PubMed] [Google Scholar]
- 69.Jiao Z, Cai H, Long Y, Sirka OK, Padmanaban V, Ewald AJ, Devreotes PN. Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc Natl Acad Sci U S A. 2020;117:4158–4168. doi: 10.1073/pnas.1917938117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeitouni D, Pylayeva-Gupta Y, Der CJ, Bryant KL. KRAS mutant pancreatic cancer: no lone path to an effective treatment. Cancers (Basel) 2016;8:45. doi: 10.3390/cancers8040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ha KD, Bidlingmaier SM, Liu B. Macropinocytosis exploitation by cancers and cancer therapeutics. Front Physiol. 2016;7:381. doi: 10.3389/fphys.2016.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thu PM, Zheng ZG, Zhou YP, Wang YY, Zhang X, Jing D, Cheng HM, Li J, Li P, Xu X. Phellodendrine chloride suppresses proliferation of KRAS mutated pancreatic cancer cells through inhibition of nutrients uptake via macropinocytosis. Eur J Pharmacol. 2019;850:23–34. doi: 10.1016/j.ejphar.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 73.Delpeut S, Sisson G, Black KM, Richardson CD. Measles virus enters breast and colon cancer cell lines through a PVRL4-mediated macropinocytosis pathway. J Virol. 2017;91:e02191-16. doi: 10.1128/JVI.02191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitazawa S, Nishizawa S, Nakagawa H, Funata M, Nishimura K, Soga T, Hara T. Cancer with low cathepsin D levels is susceptible to vacuolar (H(+) )-ATPase inhibition. Cancer Sci. 2017;108:1185–1193. doi: 10.1111/cas.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takenaka T, Nakai S, Katayama M, Hirano M, Ueno N, Noguchi K, Takatani-Nakase T, Fujii I, Kobayashi SS, Nakase I. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int J Pharm. 2019;572:118762. doi: 10.1016/j.ijpharm.2019.118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tajiri H, Uruno T, Shirai T, Takaya D, Matsunaga S, Setoyama D, Watanabe M, Kukimoto-Niino M, Oisaki K, Ushijima M, Sanematsu F, Honma T, Terada T, Oki E, Shirasawa S, Maehara Y, Kang D, Cote JF, Yokoyama S, Kanai M, Fukui Y. Targeting Ras-driven cancer cell survival and invasion through selective inhibition of DOCK1. Cell Rep. 2017;19:969–980. doi: 10.1016/j.celrep.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 78.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Araki N, Egami Y, Watanabe Y, Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, Nofal M, Lim CY, Witze E, Chude CI, Zhang G, Alicea GM, Piao S, Murugan S, Ojha R, Levi SM, Wei Z, Barber-Rotenberg JS, Murphy ME, Mills GB, Lu Y, Rabinowitz J, Marmorstein R, Liu Q, Liu S, Xu X, Herlyn M, Zoncu R, Brady DC, Speicher DW, Winkler JD, Amaravadi RK. A unified approach to targeting the lysosome’s degradative and growth signaling roles. Cancer Discov. 2017;7:1266–1283. doi: 10.1158/2159-8290.CD-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Towers CG, Thorburn A. Targeting the lysosome for cancer therapy. Cancer Discov. 2017;7:1218–1220. doi: 10.1158/2159-8290.CD-17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H, Sun M, Liu Z, Kong C, Kong W, Ye J, Gong J, Huang DCS, Qian F. KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs. J Control Release. 2019;296:40–53. doi: 10.1016/j.jconrel.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Kota J, Hancock J, Kwon J, Korc M. Pancreatic cancer: stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. doi: 10.1016/j.canlet.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 84.Kong C, Li Y, Liu Z, Ye J, Wang Z, Zhang L, Kong W, Liu H, Liu C, Pang H, Hu Z, Gao J, Qian F. Targeting the oncogene KRAS mutant pancreatic cancer by synergistic blocking of lysosomal acidification and rapid drug release. ACS Nano. 2019;13:4049–4063. doi: 10.1021/acsnano.8b08246. [DOI] [PubMed] [Google Scholar]
- 85.Gong X, Sun R, Gao Z, Han W, Liu Y, Zhao L, Jing L, Yao X, Sun X. Tubeimoside 1 acts as a chemotherapeutic synergist via stimulating macropinocytosis. Front Pharmacol. 2018;9:1044. doi: 10.3389/fphar.2018.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimiz-Gebologlu I, Gulce-Iz S, Biray-Avci C. Monoclonal antibodies in cancer immunotherapy. Mol Biol Rep. 2018;45:2935–2940. doi: 10.1007/s11033-018-4427-x. [DOI] [PubMed] [Google Scholar]
- 87.Ha KD, Bidlingmaier SM, Su Y, Lee NK, Liu B. Identification of novel macropinocytosing human antibodies by phage display and high-content analysis. Methods Enzymol. 2017;585:91–110. doi: 10.1016/bs.mie.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller-Greven G, Carlin CR, Burgett ME, Ahluwalia MS, Lauko A, Nowacki AS, Herting CJ, Qadan MA, Bredel M, Toms SA, Lathia JD, Hambardzumyan D, Sarkaria JN, Hamerlik P, Gladson CL. Macropinocytosis of bevacizumab by glioblastoma cells in the perivascular niche affects their survival. Clin Cancer Res. 2017;23:7059–7071. doi: 10.1158/1078-0432.CCR-17-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srinivasan AR, Lakshmikuttyamma A, Shoyele SA. Investigation of the stability and cellular uptake of self-associated monoclonal antibody (MAb) nanoparticles by non-small lung cancer cells. Mol Pharm. 2013;10:3275–3284. doi: 10.1021/mp3005935. [DOI] [PubMed] [Google Scholar]
- 90.Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111:538–549. doi: 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- 91.Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394:793–804. doi: 10.1016/S0140-6736(19)31774-X. [DOI] [PubMed] [Google Scholar]
- 92.Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, Sheng W, Wang Y, Li L, Li Y, Zhang S, Liu X, Chen S, Zhen Y. A macropinocytosis-intensifying albumin domain-based scFv antibody and its conjugate directed against K-Ras mutant pancreatic cancer. Mol Pharm. 2018;15:2403–2412. doi: 10.1021/acs.molpharmaceut.8b00234. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez-Arguedas S, Uranga S, Martin M, Elizalde J, Gomez AB, Julian E, Nardelli-Haefliger D, Martin C, Aguilo N. Therapeutic efficacy of the live-attenuated Mycobacterium tuberculosis vaccine, MTBVAC, in a preclinical model of bladder cancer. Transl Res. 2018;197:32–42. doi: 10.1016/j.trsl.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Zhou S, Huang Y, Chen Y, Liu S, Xu M, Jiang T, Song Q, Jiang G, Gu X, Gao X, Chen J. Engineering ApoE3-incorporated biomimetic nanoparticle for efficient vaccine delivery to dendritic cells via macropinocytosis to enhance cancer immunotherapy. Biomaterials. 2020;235:119795. doi: 10.1016/j.biomaterials.2020.119795. [DOI] [PubMed] [Google Scholar]
- 96.Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karaki S, Benizri S, Mejias R, Baylot V, Branger N, Nguyen T, Vialet B, Oumzil K, Barthelemy P, Rocchi P. Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J Control Release. 2017;258:1–9. doi: 10.1016/j.jconrel.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 98.Lu M, Zhao X, Xing H, Xun Z, Zhu S, Lang L, Yang T, Cai C, Wang D, Ding P. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm. 2018;550:100–113. doi: 10.1016/j.ijpharm.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W, Kang X, Yuan B, Wang H, Zhang T, Shi M, Zheng Z, Zhang Y, Peng C, Fan X, Yang H, Shen Y, Huang Y. Nano-structural effects on gene transfection: large, botryoid-shaped nanoparticles enhance DNA delivery via macropinocytosis and effective dissociation. Theranostics. 2019;9:1580–1598. doi: 10.7150/thno.30302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niu X, Gao Z, Qi S, Su L, Yang N, Luan X, Li J, Zhang Q, An Y, Zhang S. Macropinocytosis activated by oncogenic Dbl enables specific targeted delivery of Tat/pDNA nano-complexes into ovarian cancer cells. Int J Nanomedicine. 2018;13:4895–4911. doi: 10.2147/IJN.S171361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeong S, Byun JK, Cho SJ, Chin J, Lee IK, Choi YK, Park KG. Transcription factor Eb is required for macropinocytosis-mediated growth recovery of nutrient-deprived Kras-mutant cells. Nutrients. 2018;10:1638. doi: 10.3390/nu10111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cavalli R, Primo L, Sessa R, Chiaverina G, di Blasio L, Alongi J, Manfredi A, Ranucci E, Ferruti P. The AGMA1 polyamidoamine mediates the efficient delivery of siRNA. J Drug Target. 2017;25:891–898. doi: 10.1080/1061186X.2017.1363215. [DOI] [PubMed] [Google Scholar]
- 103.He B, Yang D, Qin M, Zhang Y, He B, Dai W, Wang X, Zhang Q, Zhang H, Yin C. Increased cellular uptake of peptide-modified PEGylated gold nanoparticles. Biochem Biophys Res Commun. 2017;494:339–345. doi: 10.1016/j.bbrc.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 104.Nakase I, Noguchi K, Aoki A, Takatani-Nakase T, Fujii I, Futaki S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci Rep. 2017;7:1991. doi: 10.1038/s41598-017-02014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 106.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao GY, Mohanakrishnan A, Schmid SL. Role for ERK1/2-dependent activation of FCHSD2 in cancer cell-selective regulation of clathrin-mediated endocytosis. Proc Natl Acad Sci U S A. 2018;115:E9570–E9579. doi: 10.1073/pnas.1810209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei X, Wei R, Jiang G, Jia Y, Lou H, Yang Z, Luo D, Huang Q, Xu S, Yang X, Zhou Y, Li X, Ji T, Hu J, Xi L, Ma D, Ye F, Gao Q. Mechanical cues modulate cellular uptake of nanoparticles in cancer via clathrin-mediated and caveolae-mediated endocytosis pathways. Nanomedicine (Lond) 2019;14:613–626. doi: 10.2217/nnm-2018-0334. [DOI] [PubMed] [Google Scholar]
- 109.Chatterjee M, Ben-Josef E, Robb R, Vedaie M, Seum S, Thirumoorthy K, Palanichamy K, Harbrecht M, Chakravarti A, Williams TM. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017;77:5925–5937. doi: 10.1158/0008-5472.CAN-17-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]


