Abstract
A malignant serous effusion is one of the most common complications of advanced tumors, indicating a poor prognosis and having a profound impact on diagnosis, treatment, and prognosis. It is of great significance to identify benign and malignant effusions quickly and accurately. Both cellular and non-cellular components in the effusion can be employed for detection, diagnostic methods are necessary to obtain a definite diagnosis and more relevant information such as tumor classification. In this review, we focus on the comparison of several widespread cytological preparation methods, enrichment technology of exfoliated cells, and present tests for serous effusions, mainly including routine and special stains, immunocytochemistry, electron microscopy, enzyme-linked immunosorbent assay, flow cytometry, and molecular analysis.
Keywords: Serous effusions, cytological materials, preparation methods, cell enrichment procedures, molecular analysis
Introduction
Serous effusions include pleural, peritoneal, and pericardial effusions, which are the pathological accumulation of fluids in the body cavity caused by various benign or malignant diseases. A malignant serous effusion is one of the common complications of advanced tumors, indicating a poor prognosis and having a profound impact on systemic anti-tumor treatment and quality of life [1]. Therefore, it is critical to make a distinction quickly and accurately between benign and malignant effusions [2].
In clinical practice, effusion is a repeatable sample of tumor cells, sometimes even the only source of specimens available. The effusion is often removed for therapeutic purposes by minimally invasive operation. Many components can be detected including floating viable exfoliated cells, free proteins, nucleic acids and other molecular components in the serous fluids of patients with cancer [3]. The interference of numerous benign cells, such as reactive mesothelial cells or inflammatory cells, is troublesome. Metastatic adenocarcinoma, malignant mesothelioma, and reactive mesothelial cells show morphological overlaps, and a single morphological test has difficulty distinguishing among them [4-6]. Thus, it is necessary to apply ancillary methods for definite diagnosis and more relevant information such as tumor classification. Some markers are relatively organ-specific, giving clues to the origination of the tumor. Notably, the medical history, clinical features, and radiological findings should also be considered for a comprehensive analysis.
This review introduces the preparations of cytological materials, the enrichment process of interest exfoliated cells, and contemporary diagnostic methods. The diagnostic procedures of effusion specimens are showed in Figure 1. We comprehensively describe the basic steps, key details, latest developments, and pros and cons of each method, which helps researchers make an optimal choice.
Figure 1.
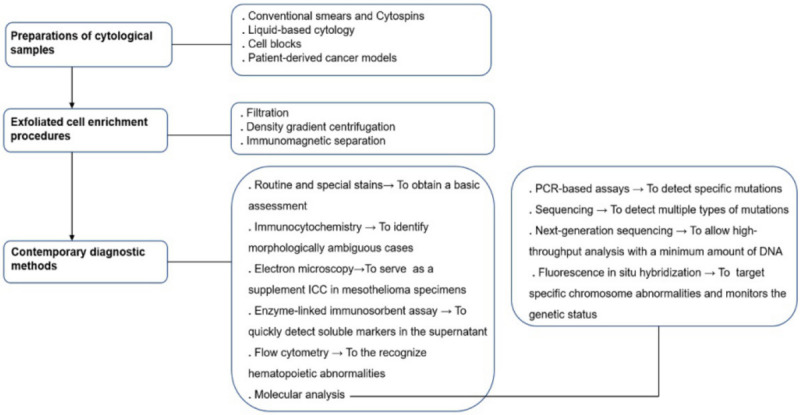
The diagnostic procedure of effusion specimen.
Preparations of cytological samples
Many factors account for the diagnostic efficiency of serous effusions, such as study size (large or small-scale studies), sample volume and types, pretreatment procedures, ancillary tests, and experience of analysts [7-10]. Here, we show the most frequent preparation methods of serous effusions, including direct smears, cytospins, liquid-based preparations, cell blocks (CBs), and patient-derived cancer models [11-13]. Because of the limited number of malignant cells, the diagnosis of effusion is difficult. Some methods can be applied to enrich the interest cells to improve the diagnostic performance. We mainly introduce filtration, density gradient centrifugation, and immunomagnetic cell separation [14-19].
The volume of the specimens is related to the adequacy and validity of the cytological diagnosis. To improve diagnostic performance, the optimal minimum cutoff volume of peritoneal, pleural, and pericardial effusions is 200 mL, 65 mL, and 60 mL, respectively [20-22]. As the clotting of collected effusions may disturb diagnosis, heparin-pretreated needles or tubes are recommended for anticoagulation, especially in bloody samples, which do not affect the morphological characteristics of cells but reduce pH values [23,24]. A fresh effusion is best for cytological tests or further study [25]. When the serous samples cannot be processed in time, they can be stored in a refrigerator at 4°C. The morphology and immunostaining patterns of the fluid may change slightly after 14 days, but they have no effect on the final diagnosis, and the fluid still retains sufficient DNA for molecular analysis [26].
The effusion can also be pre-fixed with an equal volume of 50% alcohol or methanol-based PreservCyt preservation solution, but the former fixation affects the staining forms of Diff-Quik staining and some immunocytochemistry (ICC) markers [8]. Notably, air-dried preps cannot be prepared once pre-fixed [25]. For hemorrhagic fluids, the lysate can be added to remove red blood cells, such as 1% glacial acetic acid or 0.15 M ammonium chloride solution [27,28]. After collecting, the effusion is centrifuged, and the pellet is taken into the subsequent process. The remaining materials can be frozen in 10% Roswell Park Memorial Institute/dimethyl sulfoxide (RPMI/DMSO) medium at -80°C for further investigations [28]. The comparison between different cytological preparations is described in Table 1 [13,25,29-38].
Table 1.
Comparison of different cytological preparation methods
| Cytological preparations | Strength | Shortcoming | Ref |
|---|---|---|---|
| Conventional direct smear | The process is simple, fast and economical Cells remain intact, with high quality DNA and RNA | Lower sensitivity due to cells overlapping, cell loss and chaotic background Remaining discarded fluids may be informational The coverslip needs to be removed | [25,29,34] |
| Cytospins | The collected samples can be fully utilized Cells remain intact, with high quality DNA and RNA | The coverslip needs to be removed | [38,41] |
| Liquid-based preparations | The background is clear Unstained slides can be used for other tests Cells are well preserved, with high quality DNA and RNA | The coverslip needs to be removed | [30,31] |
| Cell blocks | Cellular details are well preserved Numerous sections facilitate multiple analysis and archival storage Serving as a bridge connect cytology and histology | Formalin fixation affects DNA quality | [13,29,34-37] |
| The process is labor-intensive and time-consuming | |||
| Limited application when there are few cells | |||
| Patient-derived cancer models | Biological and molecular characteristics of human tumors are accurately recapitulated | These procedures are time-consuming, expensive, and not available in every laboratory | [12,47-50] |
| The models can be applied to learn biological behaviors, choose treatment plans, observe drug responsiveness, and predict treatment effects | Success rate is limited, discrepant in different tumors |
Conventional smears (CSs) and cytospins
CSs are made by spreading the centrifuged sediment onto glass slides. It is recommended to use a cotton swab instead of pipette-“hematologic” two-slide technic for better cellular aggregation and morphology [39]. Direct CS is the easiest, fastest, and most economical method [25]. The defects of CSs are (1) lower sensitivity due to cells overlapping, cell loss, and different laboratory procedures [34]; (2) relatively few and random materials are necessary to make smears, and the remaining discarded samples may contain important diagnostic information [29]; and (3) a large number of reactive mesothelial cells and inflammatory cells may disturb the observation of the detailed morphology of atypical or malignant cells, which increases the difficulty of diagnosis. Therefore, CSs are often applied in combination with other techniques such as CBs, especially in a diagnostic dilemma [40].
Cytospins refer to the preparation of smears by using cytocentrifuges, also known as cytospin smears, which coat the resuspended pellets on a slide at a certain speed [41]. Compared to direct smear, cytospin, as an enrichment method, can reduce diagnostic traps associated with unskilled technique and make full use of the collected samples, which is a highly accurate, convenient alternative method [38]. Both the cytospins and CSs must be fixed before staining. The fixation method is related to the type of staining. Air-drying is required before Romanowsky techniques such as Giemsa stain, and 95% ethanol fixation is generally applied before Papanicolaou and Hematoxylin-Eosin (HE) stain [37,42]. For immunocytochemical stains, 100% cold methanol is recommended to maintain the immunoreactivity of different antigens, such as hormone receptors, nuclear or lymphoid antigens [32,33].
Liquid-based cytology (LBC)
ThinPrep is the preferred liquid-based preparation in many laboratories. The general steps for ThinPrep are as follows [43]: first, the collected sample is fixed with preservative solution CytoLyt, then centrifuged, and second, the precipitate is resuspended and put into the ThinPrep® Processor, following the manufacturer’s instructions for automatic slide production. The remaining solutions can be stored for further research. The prepared slides are generally fixed with alcohol and analyzed by Papanicolaou stains; unstained slides can be air-dried and stored at -70°C for up to 1 week for immunocytochemical stains [11]. The advantages of the ThinPrep method are described as follows: (1) the cells are well preserved; (2) the overall background is clear because of reduced air-drying artifacts, blood, or inflammatory cells, which facilitates the observation of morphological details and the diagnosis of samples; (3) the additional unstained slides can be used for other auxiliary tests, such as ICC or molecular analysis [30,31].
CBs
Although no standard method for the preparation of CBs is available, 3 major acceptable steps are cell concentration, formation of a sticky pellet, and histological treatment of the pellet [36]. Cell concentration means discarding the supernatant after centrifugation. Plasma thrombin clot and the HistoGel technique have been widely adopted to form a sticky pellet by binding cells together with some viscous media. The former is to add 2-3 drops of plasma and thrombin to the precipitate to form a clot; the latter is to mix HistoGel heated into liquid with the sediment and solidify at room temperature. The remaining materials of ThinPrep stored in CytoLyt solution can also be processed by the plasma thrombin method [44,45]. There are many methods for CBs fixation, different fixation methods should be selected for corresponding auxiliary analysis [36]. Formalin fixation is a relatively conventional method. Subsequently, immunohistochemical staining and molecular tests can be performed. These tests can also be applied after alcohol fixation. Because formalin may cause chemical cross-links of DNA fragments affecting analysis, alcohol or a mixture of both are chosen for sample fixation [46]. The formalin-fixed paraffin-embedded (FFPE)-CB is usually cut to 4-6 μm, and its routine staining is HE.
The merits of CBs are described as follows [13,29,34,37]: (1) the architectural patterns are well preserved, such as cell balls, papillae, three-dimensional clusters, connections between cells, cytoplasmic, and nuclear morphology, which makes it convenient to interpret the staining forms and improves the diagnostic sensitivity; (2) a single sample can produce numerous sections facilitating archival storage for further research, and these sections can be tested simultaneously for multiple forms of staining or molecular analysis; (3) sufficient cells can be obtained and gathered in a small area for easy microscopic observation, serving as a bridge connecting cytology and histology. The shortcomings of CBs are as follows: highly technical, labor-intensive, time-consuming, and limited application when there are few cells [35,36].
The selection of cytological material preparations is related to, for example, sample types, estimated diagnosis, and laboratory preference [36]. CB is recommended as an adjunctive preparation for smear or LBC, especially when the diagnoses of both are negative or difficult to define, and their remaining fluids are usually exploited to prepare the CBs [34,37,40].
Patient-derived cancer models
Pre-clinical models include cancer cell lines, patient-derived xenograft (PDX), spheroid culture and patient-derived organoid (PDO), etc [12,47]. Among them, patient-derived cancer models gain extensive attention due to their accurate recapitulation of the biological and molecular characteristics of human tumors in vivo. Scholars isolated tumor cells from malignant effusions, cultured them in vitro to form PDO models, or injected them into immunodeficient mice to obtain PDX models [48,49]. Molecular analysis of effusion is a valuable tool providing useful information for precision medicine. Pre-clinical tumor models and molecular analysis are combined to learn biological behaviors, choose treatment plans, observe drug responsiveness, and predict treatment effects [12,50]. The disadvantages of these models are: (1) these models are time-consuming, expensive, and not available in every laboratory; (2) the success rate is limited, discrepant in different tumors.
Sarah J Hill et al. built a PDO model using pleural effusions to learn DNA damage repair and test therapeutic sensitivities for a rapid targeted drug screening in patients with high-grade serous ovarian cancer (HGSOC) [49]. Giuseppe Roscilli et al. established a PDO model using malignant pleural effusions to study the heterogeneity and predict chemosensitivity in patients with advanced non-small cell lung cancer (NSCLC) [51]. Benjamin Izar et al. generated a PDX model with malignant ascites [48]. They exploited single-cell RNA sequencing to identify inter- and intra-patient heterogeneity and assess the anti-tumor activity of inhibiting the JAK/STAT pathway in HGSOC. Akihito Machinaga et al. established a PDX model to test the efficacy of gemcitabine and clarify the potential mechanism of chemotherapy resistance in patients with refractory pancreatic ductal adenocarcinoma [52].
Exfoliated cell enrichment procedures
Many other exfoliated cells in the serous effusions interfere with the detection of malignant cells. As shown by Bertrum Sheid et al., ascites in patients with ovarian cancer contained only <0.1% of adenocarcinoma cells, 37% of lymphocytes, 32% of macrophages, and 29% of mesothelial cells [53]. Fortunately, there are slight differences in the size, density, and expression of surface biomarkers between malignant and normal cell groups, which can be utilized to isolate and enrich a sufficient number of tumor cells for analysis. The main enrichment measures are filtration, density gradient centrifugation, and immunomagnetic separation [15,18,19,54-62] (Table 2).
Table 2.
Comparison of different cell enrichment procedures
| Enrichment procedures | Basic principles | Strength | Shortcoming | Ref |
|---|---|---|---|---|
| Filtration | Divergence of cell size | Simple, fast, and cost-effective | Low purity and cell yield | [15,18,57] |
| Density gradient centrifugation | Divergence of cell density | Simple, fast, more accurate than filtration | Low purity and cell yield | [15,19,54,58] |
| Immunomagnetic separation | Specific binding of antibodies to different cellular markers | Higher yield and purity of interest cells Especially suitable for sorting rare cells Commercially available chips for continuous high-throughput analysis | Time-consuming and expensive Mostly applied for laboratory analysis | [15,56,60,62,71,52-56] |
Filtration
Filtration is a simple, fast, and cost-effective separation method based on cell size but has limited applications because the separation result is not as good as that for the latter 2 procedures. More than half of the tumor cells exist in clusters, whereas most non-malignant cells exist as a single cell, and the tumor cells derived from epithelium are usually larger than leukocytes [18,55]. Therefore, filtration membranes can be utilized for filtration. The pore size of the filtration membrane is the key factor, and it should be selected according to the different target cells. H. W. HIRTE et al. used a 30 μm nylon mesh filter to isolate ovarian cancer cells from ascites [18]. Elin Andersson et al. applied an 8 μm commercial track-etched polycarbonate filter to isolate bladder cancer cells from urine [55]. This approach is relatively inaccurate with many disadvantages. The malignant cells defined in the filtration procedure are clumps of cells, which is controversial because not all tumor cells exist in the form of aggregation [57].
Density gradient centrifugation
Density gradient centrifugation is a physical separation method based on the density divergence between different cells. It is applied to separate specific cells in serous effusions or peripheral blood. Density gradient media are, for example, Percoll, BSA, Ficoll, and Renograffin [19]. Percoll is the comparatively favored medium among them because it is commercially available, with relatively stable physical and chemical properties, simple density gradient adjustment, easy elution, and gentle damage to cells [19,54].
One example of the operation flow is as follows: a discontinuous Percoll density gradient of 60%, 50%, and 40% was prepared and placed in a centrifuge tube layer by layer; samples were collected for centrifugation and the precipitate was resuspended; cells were adjusted to a proper concentration (e.g., 107) and then layered on top of the gradient in 1 ml of 30% Percoll to centrifuge for 30 min at 800 g; finally, the cells of each layer were collected, eluted, and prepared for smears and staining tests. Approximately 90% of recovered malignant cells were found in the lowest density fraction, that is, less than 1.056 g/ml (corresponding to 30% Percoll gradient); 82% macrophages at a density of 1.056-1.067 g/ml (30%-50%); and 98% lymphocytes at 1.067-1.077 g/ml (40%-60%) [19]. Jerzy Rabczynski et al. pointed out that typical malignant ovarian cancer cells are concentrated in the density layer of 1.035-1.070 g/ml [63].
The loss of many target cells in the density gradient is due to the obvious difference in the density of tumor cells. This method can be combined with magnetic cell separation because it can effectively remove cellular debris and red blood cells, so as not to block the magnetic beads [58].
Immunomagnetic separation
The 2 key factors to assess the effectiveness of cell purification methods are yield and purity. Although the 2 former methods can obtain a large yield of cells, the purity is poor and the volumes of required effusions are large, and the immunomagnetic separation provides both higher yield and purity [15]. The general steps of immunomagnetic separation are as follows: incubate antibody-conjugated magnetic beads with collected effusions, and antibodies with high affinity for the specific surface antigen of the target cells connect the magnetic beads to the interest cells [58]; next, the mixed solution passes through a magnetic column, and the labeled cells are left to be eluted if necessary, with the unlabeled cells out [61].
According to the different targeting of the selected antibody, it is divided into positive and negative selection; the former antibody targets the desired interest cells, and the latter antibody targets the unwanted cells [64-66]. For example, antibodies exploited for positive separation of ovarian cancer cells include the following [14,15,28,67-69]: Ber-EP4, folic acid, human epithelial antigen, the extracellular domain of the MUC16 cell surface protein, carbohydrate antigen 125 (CA125), a receptor tyrosine kinase, EphA2, and monoclonal antibody (mAb) CC49. Although CD45 is exclusively expressed on hematopoietic cells, it is routinely applied in negative separation to remove contaminating leukocytes [70].
The use of positive sorting is limited in the absence of phenotype information on the target cells. Some researchers hope that the enrichment step does not directly modify or connect the target cells, although the binding of the antibody with magnetic particles does not affect cell function [71]. The 2 methods should be selected according to the actual situation, and sometimes they can be exploited in combination [28,65]. The cells remain intact and viable after magnetic isolation and could be tested for further morphological or immunocytochemical assays [56].
Immunomagnetic separation is employed in sorting the rare cells, which can achieve approximately 1000 times the enrichment of the initial samples, but it is time-consuming and expensive [56]. Commercially available chips have been introduced by some laboratories to support continuous high-throughput analysis and they are convenient, cost-effective, and promising for future applications [60,62].
Routine and special stains
Routine and special stains are important diagnostic techniques for effusion cytology, especially cytochemistry and ICC. All cytological preparations can be employed for staining; among them, the CB is optimal with sufficient material [35,37,40]. Giemsa staining is a conventional way to air-dry smears; Papanicolaou or HE staining is a traditional method for alcohol-fixed smears; HE staining is generally performed in CBs [13,37,42]. Some special stains can be adopted to identify pathogens in morphologically suspicious infected samples, such as the Grocott or PAS stain to identify fungi, and the acid-fast or Fite stain to recognize mycobacterium [72].
When evaluating and interpreting the slides, researchers have focused on the cellularity, cell arrangement, and cytoplasmic and nuclear details, as comprehensively illustrated by Mair et al. [34,40,73]: (1) volume of blood/clot obscuring background (large: 0, moderate: 1, minimal: 2); (2) amount of diagnostic cellular material present (minimal: 0, moderate: 1, abundant: 2); (3) degree of cellular degeneration and cellular trauma (marked: 0, moderate: 1, minimal: 2); and (4) retained architecture/cellular arrangement (minimal: 0, moderate: 1, excellent: 2). According to the aforementioned criteria, the quality of the slides is classified into 3 categories [34,40,73]: (1) diagnostically unsuitable (0 score); (2) diagnostically adequate (1-4 score); and (3) diagnostically superior (4-8 score). After the assessment, the final pathological diagnoses are usually divided into the following 5 groups [20,74,75]: benign, malignant, suspicious (more likely to be malignant), atypical (not completely consistent with benign or malignant cells in general morphology), and non-diagnostic (insufficient cells number or contaminated background).
ICC
The distinction between benign and malignant effusions is essential for diagnosis, treatment, and prognosis [76]. Cytochemistry staining is based on cellular morphology, and it is difficult to distinguish between benign and malignant cells in many cases, especially in metastatic adenocarcinoma, reactive mesothelial cells, and malignant mesothelioma. Because reactive mesothelial cells are hyperplastic and hypertrophic, closely mimicking malignant cells, metastatic carcinoma and malignant mesothelial cells exhibit morphological overlap, and a single cytological examination is insufficient for accurate diagnosis [4-6]. ICC is a valuable tool for solving this problem [76-78]. The FFPE-CB is the preferred preparation because of its clear background, multiple archival sections, and comparable performance to those of surgical pathological materials [35,37].
Due to the heterogeneous expressions of tumor antigens, the diagnostic performance of a single antibody is limited, and a panel of markers is recommended for detection [76,79]. No consensus on the best antibody combination has been reached. It should contain at least 2 antibodies, an epithelial marker, and a mesothelial one [8,80]. Among them, MOC-31 is one of the most sensitive, specific adenocarcinoma markers that recognizes the epithelial cell adhesion molecule (Ep-CAM) on the surface of epithelial cells [78]. Calretinin is considered a reliable marker of mesothelioma [81]. Epithelial membrane antigen (EMA) is strongly positive in almost all mesothelioma and only weakly positive in reactive mesothelial cells, but desmin is the opposite [82]. The combination of BerEp4/Calretinin, desmin/EMA, or WT1/AE1-AE3 shows satisfactory performance, for example, EMA positivity and desmin negativity were found in 98% (49 of 52) of malignant mesothelioma, and EMA negativity and desmin positivity were revealed in 86% (55 of 64) of reactive mesothelial hyperplasia [82,83]. Some markers can indicate the primary origin of metastatic cancer, such as Thyroid transcription factor-1 (TTF-1) to label pulmonary adenocarcinoma, CDX2 to mark intestinal adenocarcinomas, and GATA3 to suggest metastatic breast carcinoma [84-86].
Unlike immunohistochemical staining of surgical specimens, there remains no accurate immunocytochemical grading and scoring system for effusion. The immunostaining of some effusion samples is evaluated by semi-quantitative scoring, as has been described by Vickie Y et al. and Tomohiro Oda et al. [87,88]. A staining index is recorded as the sum of the intensity score (IS) and percentage score (PS). IS refers to the staining intensity of the target cells in the corresponding expression pattern (core 0: no staining; 1: weak; 2: moderate; 3: strong), and PS refers to the proportion of positively stained interest cells (score 0: no staining; 1: <10%; 2: 10%-50%; 3: >50%). Some inspiration may be from the calculation of the M-score, an immunohistochemical scoring algorithm that also includes the proportion and staining intensity of positive cells [89,90].
Electron microscopy (EM)
EM is applied to evaluate cellular ultrastructure, based on the morphology of microvilli, to identify mesothelial or epithelial cells [91]. The basic procedure is to fix the precipitate with glutaraldehyde after centrifugation, and then slice and embed it for EM evaluation [91]. Traditionally, EM has been the gold standard for the diagnosis of mesothelioma, but it is gradually being replaced by IHC because of its long time, high cost, and technical complexity [72,92]. Now, the EM is only applied as an alternative method when the ICC diagnoses are ambiguous or difficult to interpret [93].
Enzyme-linked immunosorbent assay (ELISA)
The supernatant in routine effusion analysis is usually discarded, and some significant information is ignored, such as soluble markers, free DNA, and RNA. ELISA is an effective, simple method to detect soluble markers in the supernatant, which is valuable for diagnosis, treatment, and prognosis [94-97]. The basic process is to centrifuge the fluid, draw the supernatant, aliquot and freeze the supernatant at -80°C for later analysis, and then exploit commercially available ELISA kits for the test according to the manufacturer’s steps [94,95].
Singer G et al. detected a significant increase in the level of secretory HLA-G (sHLA-G) in malignant ascites by ELISA, indicating that sHLA-G can be applied to distinguish between benign and malignant effusions [96]. The combined detection of effusion and blood markers provides a new idea of diagnosis and treatment. Liu D et al. employed ELISA to test the levels of human epididymis protein 4 (HE4) in ascitic supernatants and corresponding CA125 in the serum of ovarian cancer patients with chemotherapy and non-chemotherapy; a positive correlation was shown between the 2 markers, and a high level of HE4 may predict chemotherapy resistance and ascites formation [97].
Flow cytometry
ICC is widely accepted in the diagnosis of effusion except for specimens with limited cells, and the flow cytometry serves as an important complementary technique. Although flow cytometry is mainly used for the diagnosis and treatment guidance of hematopoietic malignancies, it is also chosen for cell detection and DNA ploidy analysis of malignant effusions [98-101]. Effusion samples used for flow cytometry are fresh, unfixed, or stored in 10% DMSO/RPMI medium at -80°C, and the general steps are as follows [28,99,102]: after centrifugation, the supernatant is discarded; a filtration procedure can be added to remove large cells clumps or viscous aggregates; the pellet is washed and resuspended with RPMI or phosphate buffer saline to adjust the number of cells for analysis; and cells prepared for immunotyping should be placed on ice with a gentle operation to reduce debris or dead cells.
Examples of ordinary markers are Ber-EP4, CD45, CD14, N-cadherin, EMA, MUC4, progesterone (PR), and TTF-1 [99,101-103]. An advantage of flow cytometry is the rapid multiparametric analysis of both surface markers and DNA aneuploidy, with no observer error [99]. Flow cytometry is a valuable method because it can provide immunotyping information to distinguish between benign and malignant cells and develop tailored treatment protocols; moreover, the fluorescence-activated cell sorting can highly purify tumor cells for subsequent molecular analysis [102]. In addition, the flow cytometry can quantitatively analyze the number of receptors per cell and whether the receptor protein is functional, by using fluorescently labeled ligands [28].
Molecular analysis
In an era of personalized medicine, targeted therapy has become a research hotspot. Individualized treatment plans based on the results of molecular tests help patients significantly improve progression-free survival and overall survival, providing them with substantial clinical benefits [104]. Cytological materials play an important role in molecular analysis because of their great accessibility, minimal invasion, safety, low cost, and easy patient acceptance [105,106]. Both the supernatant and cellular components of the effusion can be tested for nucleic acids, proteins, and other molecules. The relevant information can be employed to diagnose, predict therapeutic responses, assess prognoses, and identify new therapeutic targets [3].
The FFPE-CB is the preferred preparation for molecular tests, but some laboratories adopt methanol fixation as an alternative because formalin fixation may cause the cross-linking of nucleic acids and proteins, and fragments and sequence artifacts, to interfere with detection [107,108]. Other preparations such as smears exhibit a prior quality of DNA, and provide excellent resources for molecular analysis [109,110].
Current molecular strategies include polymerase chain reaction (PCR)-based assays, sequencing, and fluorescence in situ hybridization (FISH) [72,111-134] (Table 3). The choice of these methods is determined by the target gene/genes, mutation spectrum of the interested gene, sample size for screening, and available equipment [115]. The basic steps of molecular testing are as follows [83,135,136]: select and mark the appropriate area on the slides, place them in xylene overnight or use a deep-freeze method (put the slides in the freezer at -20°C for 1-2 minutes) to remove the coverslip, and then transfer the material to a small tube for cells lysis and DNA extraction. When there are limited tumor cells in serous examples, macrodissection, manual microdissection, or laser-capture microdissection is recommended for cell enrichment [106].
Table 3.
Comparison of different molecular analysis
| Molecular tests | Basic principles | Strength | Shortcoming | Ref |
|---|---|---|---|---|
| PCR-based assays | Primers targeting specific genes for multiple copy analysis | Quick, simple, reproducible, sensitive, specific | Limited number of detected genes | [115,121,127,131] |
| Sanger sequencing | Sequencing by termination | Detecting multiple types of mutations | Limited number of genes detected every single time | [114,115,118,122] |
| Low sensitivity | ||||
| Requiring at least 20% of tumor cells | ||||
| Pyrosequencing | Sequencing by synthesis | Sensitive Requiring malignant cells as low as 5% | Limited number of genes detected every single time | [114,122] |
| Next-generation sequencing (NGS) | Sequencing by synthesis | High-throughput analysis Single or multiple gene analysis Requiring a low number of tumor cells | Expensive Complicated results analysis | [111,114,120,122,125,126,128] |
| FISH | Probes targeting specific chromosomal abnormalities | Direct visualization of cytological materials | Limited number of probes each test containing Not available in every laboratory | [72,112,113,117,132,137] |
PCR-based assays
PCR is adopted to detect specific mutations. The synthetically designed primers are linked to the target sequence, many copies of the original target sequence are generated through a multicycle amplification process [115]. Quantitative real-time polymerase chain reaction (qRT-PCR) quantifies the number of target sequences in a sample by detecting the amount of fluorescent signal released in each cycle [127]. Rocco Cappellesso et al. exploited qRT-PCR to assess the expression levels of several microRNAs in cytological specimens, and the result showed that the combination of miR-21 and miR-126 achieved 86% sensitivity and 87% specificity in distinguishing malignant mesothelioma from reactive mesothelial cells [131]. Reverse transcription polymerase chain reaction (RT-PCR) reverses mRNA to complementary DNA to detect the expression of interest genes [121].
Sequencing
Sanger Sequencing is “sequencing by termination”, which randomly inhibits the extension process; then, different lengths of newly formed DNA fragments can be detected by an automatic reader after electrophoresis separation [115,122]. This method, which has long been regarded as the gold standard for direct DNA sequencing, can detect multiple types of mutations [118]. The disadvantage of Sanger Sequencing is low sensitivity, limited ability to recognize gene copy number changes, and least 20% of genetically altered tumor cells is required [114,122]. Pyrosequencing, as an alternative to Sanger Sequencing, is “sequencing by synthesis”, which recognizes the additions of specific bases by chemiluminescence detection of pyrophosphate released in the DNA PCR [114,122]. This sensitive method can detect mutations of genetically altered malignant cells as low as 5% [114].
Next-generation sequencing (NGS)
NGS is “sequencing by synthesis”, using the target gene as the template to synthesize complementary chain, which is visualized by 4 specific fluorescently labeled nucleic acids to obtain information of the interest gene [116,120,122]. NGS is the hottest, flexible sequencing method that allows simultaneous analysis of multiple gene targets with a minimum amount of DNA [125]. Many commercially available narrow-spectrum combinations can replace PCR to detect specific genetic variation, and broad-spectrum combinations can be applied to analyze whole-exome sequencing, accounting for approximately 2% of the entire genome [124,126]. Because the analysis of the NGS results is complicated, more investment is required in informatics to develop a more practical algorithm to convert digital information into a simple quantitative score [128].
Current guidelines recommend routine detection of epidermal growth factor receptor (EGFR) and v-raf murine sarcoma viral oncogene homolog B (BRAF) mutations, as well as ALK and ROS1 rearrangements, in patients with advanced or metastatic NSCLC [111]. EGFR detection involves screening and targeted tests: screening tests detect all EGFR mutations and potentially new mutations, and targeted tests detect the exactly known mutations that are clinically available [114]. Screening methods include Sanger sequencing, Pyrosequencing, NGS, and High Resolution Melt Analysis, and the targeted assays are the Agena MassARRAY Oncocarta panel, the Cobas EGFR Mutation Test (Roche Molecular Systems), the Therascreen EGFR Kit (Qiagen), and SNaPShot (by Life Technologies/Applied Biosystem) [114]. Molecular tests for ALK, ROS1, and RET rearrangements include FISH and RT-PCR [114]. Two experiments have shown 85% consistency between FISH and RT-PCR when detecting ALK rearrangements [133,134].
FISH
When the cytological results are negative or ambiguous, FISH serves as a valuable supplementary tool, substantially improving the sensitivity without affecting the specificity [113,137]. FISH is an accurate method that targets specific chromosome abnormalities and monitors the genetic status of cells to directly visualize cytological materials [72,113]. Various cytological materials can be employed for FISH, including conventional or liquid-based preparations, Papanicolaou- or Giemsa-stained, or immunocytochemical slides [132]. Among them, the alcohol-fixed or air-dried smears are more suitable for FISH analysis than FFPE-CBs because the cross-linking of nucleic acids and proteins caused by formalin fixation affects DNA quality [107]. The basic steps of FISH are as follows: after selecting and marking the appropriate hybridization area, remove the coverslip, denature by protease, incubate with fluorescently labeled specific DNA probe overnight, and then wash and observe at an appropriate wavelength after counterstaining the cell nuclei with DAPI [132].
EMA, desmin, and other immunocytochemical markers have limited ability to distinguish between reactive mesothelial cells and malignant mesothelioma in some cases, and molecular tests can identify several frequent genetic abnormalities in mesothelioma [112]. Among them, homozygous deletion of 9p21 is a relatively general alteration [119,123,129,130], which can be detected by the commercially available UroVysion FISH kit. The 4 probes of the kit can hybridize to the centromere region on chromosomes 3, 7, and 17 and to the p16 INK4A gene locus at 9p21 [113]. The UroVysion FISH kit can be applied with cytology to obtain better diagnostic performance for effusion diagnosis, as R. DCB et al. showed [117]. They exploited this kit to 70 samples of pleural and peritoneal fluids; the final diagnostic sensitivity, specificity, and accuracy were 87.3%, 71.4%, and 85.7% respectively, and the results after combining with cytology were 88.0%, 83.3%, and 87.8%.
Moreover, FISH can be exploited to detect ALK, ROS1, RET gene rearrangement and MET amplification to screen lung cancer patients suitable for targeted chemotherapy, and it has been employed in urinary cytology for the diagnosis of urothelial tumors [132].
Conclusion
Serous effusions can be classified into benign and malignant. Cytological specimens are important materials because of their great accessibility, minimal invasion, and easy patient acceptance. Both the cellular components and supernatant of collected effusions are informative for precise and timely diagnosis, treatment, and prognosis.
Various methods are performed for the different diagnostic purposes of fluid samples. Morphological analysis is the basis. ICC assists in identifying morphologically ambiguous cases and provides possible organ origin and treatment-related information. EM is only applied as a supplement to ICC in the diagnostic dilemma of mesothelioma specimens. ELISA is employed to quickly detect soluble markers in the supernatant. Flow cytometry is mainly exploited in the diagnosis of hematopoietic abnormalities, allowing rapid multi-parameter analysis of cell surface markers and DNA aneuploidy, especially in samples of limited cellularity. In the era of precision medicine, molecular detection has gained increasing attention, which escorts the tailored targeted therapy. Among them, NGS is the hottest for high-throughput analysis with a minimum amount of DNA. Pre-clinical tumor models and molecular analysis are combined to provide useful information. New molecular detection and related technologies continue to emerge and make the diagnosis of effusion more accurate, efficient, convenient, and economical than ever before.
Acknowledgements
This work was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (XJTU1AF-2018-017, XJTU1AF-CRF-2019-002), the Natural Science Basic Research Program of Shaanxi (2018JM7073, 2017ZDJC-11), the Key Research and Development Program of Shaanxi (2017ZDXM-SF-068, 2019QYPY-138), the Innovation Capability Support Program of Shaanxi (2017XT-026, 2018XT-002), and the Medical Research Project of Xi’an Social Development Guidance Plan (2017117SF/YX011-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13:273–282. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang R, Xie HY, Lin Y, Li Q, Yuan CL, Liu ZH, Li YQ. Intraperitoneal perfusion therapy of endostar combined with platinum chemotherapy for malignant serous effusions: a meta-analysis. Asian Pac J Cancer Prev. 2015;16:8637–8644. doi: 10.7314/apjcp.2015.16.18.8637. [DOI] [PubMed] [Google Scholar]
- 3.Davidson B. Molecular testing on serous effusions. Diagn Cytopathol. 2020 doi: 10.1002/dc.24392. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Patarapadungkit N, Jangsiriwitayakorn P, Chaiwiriyakul S, Sirivech P, Thongbor R, Phanomsri EO, Nititarakul L. Modified liquid-based cytology technique for immunocytochemistry in effusion specimen. Asian Pac J Cancer Prev. 2019;20:2611–2617. doi: 10.31557/APJCP.2019.20.9.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedrossian CW. Diagnostic problems in serous effusions. Diagn Cytopathol. 1998;19:131–137. doi: 10.1002/(sici)1097-0339(199808)19:2<131::aid-dc14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Kho-Duffin J, Tao LC, Cramer H, Catellier MJ, Irons D, Ng P. Cytologic diagnosis of malignant mesothelioma, with particular emphasis on the epithelial noncohesive cell type. Diagn Cytopathol. 1999;20:57–62. doi: 10.1002/(sici)1097-0339(199902)20:2<57::aid-dc2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Woo CG, Son SM, Han HS, Lee KH, Choe KH, An JY, Man Lee K, Lim YH, Lee HC, Lee OJ. Diagnostic benefits of the combined use of liquid-based cytology, cell block, and carcinoembryonic antigen immunocytochemistry in malignant pleural effusion. J Thorac Dis. 2018;10:4931–4939. doi: 10.21037/jtd.2018.07.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetsch PA, Abati A. Immunocytochemistry in effusion cytology: a contemporary review. Cancer. 2001;93:293–308. doi: 10.1002/cncr.9044.abs. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez NG. The immunohistochemical diagnosis of mesothelioma. Differentiation of mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 1989;13:276–291. [PubMed] [Google Scholar]
- 10.Betta PG, Andrion A, Donna A, Mollo F, Scelsi M, Zai G, Terracini B, Magnani C. Malignant mesothelioma of the pleura. The reproducibility of the immunohistological diagnosis. Pathol Res Pract. 1997;193:759–765. doi: 10.1016/S0344-0338(97)80054-4. [DOI] [PubMed] [Google Scholar]
- 11.Gong Y, Sun X, Michael CW, Attal S, Williamson BA, Bedrossian CW. Immunocytochemistry of serous effusion specimens: a comparison of ThinPrep vs. cell block. Diagn Cytopathol. 2003;28:1–5. doi: 10.1002/dc.10219. [DOI] [PubMed] [Google Scholar]
- 12.Maru Y, Hippo Y. Current status of patient-derived ovarian cancer models. Cells. 2019;8:505. doi: 10.3390/cells8050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivakumarswamy U, Arakeri SU, Karigowdar MH, Yelikar B. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol. 2012;29:11–15. doi: 10.4103/0970-9371.93210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Nie L, Li F, Aguilar ZP, Xu H, Xiong Y, Fu F, Xu H. Folic acid conjugated magnetic iron oxide nanoparticles for nondestructive separation and detection of ovarian cancer cells from whole blood. Biomater Sci. 2016;4:159–166. doi: 10.1039/c5bm00207a. [DOI] [PubMed] [Google Scholar]
- 15.Barker SD, Casado E, Gomez-Navarro J, Xiang J, Arafat W, Mahasreshti P, Pustilnik TB, Hemminki A, Siegal GP, Alvarez RD, Curiel DT. An immunomagnetic-based method for the purification of ovarian cancer cells from patient-derived ascites. Gynecol Oncol. 2001;82:57–63. doi: 10.1006/gyno.2001.6226. [DOI] [PubMed] [Google Scholar]
- 16.Buick RN, MacKillop WJ. Measurement of self-renewal in culture of clonogenic cells from human ovarian carcinoma. Br J Cancer. 1981;44:349–355. doi: 10.1038/bjc.1981.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura Y, Harada Y, Yasuda N, Ishidao T, Yusa S, Matsusaki K, Yonemitsu Y. Effective recovery of highly purified CD326(+) tumor cells from lavage fluid of patients treated with a novel cell-free and concentrated ascites reinfusion therapy (KM-CART) Springerplus. 2015;4:780. doi: 10.1186/s40064-015-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirte HW, Clark DA, Mazurka J, O’Connell G, Rusthoven J. A rapid and simple method for the purification of tumor cells from ascitic fluid of ovarian carcinoma. Gynecol Oncol. 1992;44:223–226. doi: 10.1016/0090-8258(92)90046-l. [DOI] [PubMed] [Google Scholar]
- 19.Hamburger AW, Dunn FE, White CP. Percoll density gradient separation of cells from human malignant effusions. Br J Cancer. 1985;51:253–258. doi: 10.1038/bjc.1985.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooper LM, Ali SZ, Olson MT. A minimum fluid volume of 75 mL is needed to ensure adequacy in a pleural effusion: a retrospective analysis of 2540 cases. Cancer Cytopathol. 2014;122:657–665. doi: 10.1002/cncy.21452. [DOI] [PubMed] [Google Scholar]
- 21.Rooper LM, Ali SZ, Olson MT. A minimum volume of more than 60 mL is necessary for adequate cytologic diagnosis of malignant pericardial effusions. Am J Clin Pathol. 2016;145:101–106. doi: 10.1093/ajcp/aqv021. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Feng Z, Zhang Y, Liu Z, Sun X, Jin S. Determination of the optimal volume of ascitic fluid for the precise diagnosis of malignant ascites. Saudi J Gastroenterol. 2019;25:327–332. doi: 10.4103/sjg.SJG_547_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roffe BD, Wagner FH, Derewicz HJ, Gill GW. Heparinized bottles for the collection of body cavity fluids in cytopathology. Am J Hosp Pharm. 1979;36:211–214. [PubMed] [Google Scholar]
- 24.Bou-Khalil PK, Jamaleddine GW, Debek AH, El-Khatib MF. Use of heparinized versus non-heparinized syringes for measurements of the pleural fluid pH. Respiration. 2007;74:659–662. doi: 10.1159/000106844. [DOI] [PubMed] [Google Scholar]
- 25.Michael CW, Davidson B. Pre-analytical issues in effusion cytology. Pleura Peritoneum. 2016;1:45–56. doi: 10.1515/pp-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manosca F, Schinstine M, Fetsch PA, Sorbara L, Maria Wilder A, Brosky K, Erickson D, Raffeld M, Filie AC, Abati A. Diagnostic effects of prolonged storage on fresh effusion samples. Diagn Cytopathol. 2007;35:6–11. doi: 10.1002/dc.20587. [DOI] [PubMed] [Google Scholar]
- 27.Khurram N, Anis T, Yusuf NW. Diagnostic accuracy of a limited immuno-panel of calretinin and Ber-EP4 for diagnosis of malignant effusions. J Coll Physicians Surg Pak. 2019;29:33–36. doi: 10.29271/jcpsp.2019.01.33. [DOI] [PubMed] [Google Scholar]
- 28.Forster MD, Ormerod MG, Agarwal R, Kaye SB, Jackman AL. Flow cytometric method for determining folate receptor expression on ovarian carcinoma cells. Cytometry A. 2007;71:945–950. doi: 10.1002/cyto.a.20456. [DOI] [PubMed] [Google Scholar]
- 29.Dekker A, Bupp PA. Cytology of serous effusions. An investigation into the usefulness of cell blocks versus smears. Am J Clin Pathol. 1978;70:855–860. doi: 10.1093/ajcp/70.6.855. [DOI] [PubMed] [Google Scholar]
- 30.Leung CS, Chiu B, Bell V. Comparison of ThinPrep and conventional preparations: nongynecologic cytology evaluation. Diagn Cytopathol. 1997;16:368–371. doi: 10.1002/(sici)1097-0339(199704)16:4<368::aid-dc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Guiter GE, Gatscha RM, Zakowski MF. ThinPrep vs. conventional smears in fine-needle aspirations of sarcomas: a morphological and immunocytochemical study. Diagn Cytopathol. 1999;21:351–354. doi: 10.1002/(sici)1097-0339(199911)21:5<351::aid-dc11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Srebotnik Kirbis I, Us Krasovec M, Pogacnik A, Strojan Flezar M. Optimization and validation of immunocytochemical detection of oestrogen receptors on cytospins prepared from fine needle aspiration (FNA) samples of breast cancer. Cytopathology. 2015;26:88–98. doi: 10.1111/cyt.12143. [DOI] [PubMed] [Google Scholar]
- 33.Kirbis IS, Flezar MS, Krasovec MU. MIB-1 immunostaining on cytological samples: a protocol without antigen retrieval. Cytopathology. 2004;15:154–159. doi: 10.1111/j.1365-2303.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 34.Thapar M, Mishra RK, Sharma A, Goyal V, Goyal V. Critical analysis of cell block versus smear examination in effusions. J Cytol. 2009;26:60–64. doi: 10.4103/0970-9371.55223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fetsch PA, Simsir A, Brosky K, Abati A. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:61–66. doi: 10.1002/dc.10039. [DOI] [PubMed] [Google Scholar]
- 36.Saqi A. The state of cell blocks and ancillary testing: past, present, and future. Arch Pathol Lab Med. 2016;140:1318–1322. doi: 10.5858/arpa.2016-0125-RA. [DOI] [PubMed] [Google Scholar]
- 37.Bhanvadia VM, Santwani PM, Vachhani JH. Analysis of diagnostic value of cytological smear method versus cell block method in body fluid cytology: study of 150 cases. Ethiop J Health Sci. 2014;24:125–131. doi: 10.4314/ejhs.v24i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirkin W, Auger M, Donat E, Lipa M. Cytospins--an alternative method for fine-needle aspiration cytology of the breast: a study of 148 cases. Diagn Cytopathol. 1995;13:266–269. doi: 10.1002/dc.2840130316. [DOI] [PubMed] [Google Scholar]
- 39.Dekker A, Bupp PA. Cytology of serous effusions: a comparative study of two slightly different preparative methods. Acta Cytol. 1976;20:394–399. [PubMed] [Google Scholar]
- 40.Matreja SS, Malukani K, Nandedkar SS, Varma AV, Saxena A, Ajmera A. Comparison of efficacy of cell block versus conventional smear study in exudative fluids. Niger Postgrad Med J. 2017;24:245–249. doi: 10.4103/npmj.npmj_150_17. [DOI] [PubMed] [Google Scholar]
- 41.Marcos R, Santos M, Marrinhas C, Correia-Gomes C, Caniatti M. Cytocentrifuge preparation in veterinary cytology: a quick, simple, and affordable manual method to concentrate low cellularity fluids. Vet Clin Pathol. 2016;45:725–731. doi: 10.1111/vcp.12423. [DOI] [PubMed] [Google Scholar]
- 42.Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67:536–539. doi: 10.1378/chest.67.5.536. [DOI] [PubMed] [Google Scholar]
- 43.Rossi ED, Bizzarro T, Schmitt F, Longatto-Filho A. The role of liquid-based cytology and ancillary techniques in pleural and pericardic effusions: an institutional experience. Cancer Cytopathol. 2015;123:258–266. doi: 10.1002/cncy.21518. [DOI] [PubMed] [Google Scholar]
- 44.Jing X, Li QK, Bedrossian U, Michael CW. Morphologic and immunocytochemical performances of effusion cell blocks prepared using 3 different methods. Am J Clin Pathol. 2013;139:177–182. doi: 10.1309/AJCP83ADULCXMAIX. [DOI] [PubMed] [Google Scholar]
- 45.Carneiro FP, Muniz-Junqueira MI, De Vasconcelos Carneiro M, De Araújo Oliveira Í, Soares AC, De Vargas Haar N, Takano GHS, De Sousa Vianna LM, De Carvalho Caldas G, Vieira DLM, Frutuoso LL, Brito LMR, De Siqueira RVM, Parente AM, De Castro TMML, Peres I, Mendes LMS, Dos Santos Borges TK, Ferreira VM, Motoyama AB. Anti-EpCAM antibodies for detection of metastatic carcinoma in effusions and peritoneal wash. Oncol Lett. 2019;18:2019–2024. doi: 10.3892/ol.2019.10468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 47.Liu HD, Xia BR, Jin MZ, Lou G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin Transl Oncol. 2020;22:1240–1251. doi: 10.1007/s12094-019-02276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izar B, Tirosh I, Stover EH, Wakiro I, Cuoco MS, Alter I, Rodman C, Leeson R, Su MJ, Shah P, Iwanicki M, Walker SR, Kanodia A, Melms JC, Mei S, Lin JR, Porter CBM, Slyper M, Waldman J, Jerby-Arnon L, Ashenberg O, Brinker TJ, Mills C, Rogava M, Vigneau S, Sorger PK, Garraway LA, Konstantinopoulos PA, Liu JF, Matulonis U, Johnson BE, Rozenblatt-Rosen O, Rotem A, Regev A. A single-cell landscape of high-grade serous ovarian cancer. Nat Med. 2020;26:1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill SJ, Decker B, Roberts EA, Horowitz NS, Muto MG, Worley MJ Jr, Feltmate CM, Nucci MR, Swisher EM, Nguyen H, Yang C, Morizane R, Kochupurakkal BS, Do KT, Konstantinopoulos PA, Liu JF, Bonventre JV, Matulonis UA, Shapiro GI, Berkowitz RS, Crum CP, D’Andrea AD. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–1421. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins A, Miles GJ, Wood J, MacFarlane M, Pritchard C, Moss E. Patient-derived explants, xenografts and organoids: 3-dimensional patient-relevant pre-clinical models in endometrial cancer. Gynecol Oncol. 2020;156:251–259. doi: 10.1016/j.ygyno.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Roscilli G, De Vitis C, Ferrara FF, Noto A, Cherubini E, Ricci A, Mariotta S, Giarnieri E, Giovagnoli MR, Torrisi MR, Bergantino F, Costantini S, Fenizia F, Lambiase M, Aurisicchio L, Normanno N, Ciliberto G, Mancini R. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J Transl Med. 2016;14:61. doi: 10.1186/s12967-016-0816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machinaga A, Hori Y, Shimizu K, Okahara K, Yanagita E, Miyoshi M, Itoh T, Sasai K. Xenografts derived from patients’ ascites recapitulate the gemcitabine resistance observed in pancreatic cancer patients. Pancreas. 2019;48:1294–1302. doi: 10.1097/MPA.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 53.Sheid B. Angiogenic effects of macrophages isolated from ascitic fluid aspirated from women with advanced ovarian cancer. Cancer Lett. 1992;62:153–158. doi: 10.1016/0304-3835(92)90186-y. [DOI] [PubMed] [Google Scholar]
- 54.Pertoft H. Fractionation of cells and subcellular particles with Percoll. J Biochem Biophys Methods. 2000;44:1–30. doi: 10.1016/s0165-022x(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 55.Andersson E, Steven K, Guldberg P. Size-based enrichment of exfoliated tumor cells in urine increases the sensitivity for DNA-based detection of bladder cancer. PLoS One. 2014;9:e94023. doi: 10.1371/journal.pone.0094023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kielhorn E, Schofield K, Rimm DL. Use of magnetic enrichment for detection of carcinoma cells in fluid specimens. Cancer. 2002;94:205–211. doi: 10.1002/cncr.10193. [DOI] [PubMed] [Google Scholar]
- 57.Balconi G, Broggini M, Erba E, D’Incalci M, Colombo N, Mangioni C, Bertolero F. Human ovarian tumors in primary culture: growth, characterization and initial evaluation of the response to cis platinum treatment in vitro. Int J Cancer. 1988;41:809–818. doi: 10.1002/ijc.2910410606. [DOI] [PubMed] [Google Scholar]
- 58.Chan JK, Hamilton CA, Anderson EM, Cheung MK, Baker J, Husain A, Teng NN, Kong CS, Negrin RS. A novel technique for the enrichment of primary ovarian cancer cells. Am J Obstet Gynecol. 2007;197:507, e1–5. doi: 10.1016/j.ajog.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Cotter MJ, Norman KE, Hellewell PG, Ridger VC. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am J Pathol. 2001;159:473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pamme N, Wilhelm C. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip. 2006;6:974–980. doi: 10.1039/b604542a. [DOI] [PubMed] [Google Scholar]
- 61.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 62.Peterson VM, Castro CM, Chung J, Miller NC, Ullal AV, Castano MD, Penson RT, Lee H, Birrer MJ, Weissleder R. Ascites analysis by a microfluidic chip allows tumor-cell profiling. Proc Natl Acad Sci U S A. 2013;110:E4978–4986. doi: 10.1073/pnas.1315370110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabczynski J, Bar JK, Noworolska A, Cislo M, Richer R, Harlozinska A. Morphologic heterogeneity of cell populations isolated by density gradient centrifugation from serous fluids of ovarian tumors. Tumori. 1987;73:539–545. doi: 10.1177/030089168707300601. [DOI] [PubMed] [Google Scholar]
- 64.Munoz NM, Leff AR. Highly purified selective isolation of eosinophils from human peripheral blood by negative immunomagnetic selection. Nat Protoc. 2006;1:2613–2620. doi: 10.1038/nprot.2006.340. [DOI] [PubMed] [Google Scholar]
- 65.Di Ianni M, Del Papa B, Cecchini D, Bonifacio E, Moretti L, Zei T, Ostini RI, Falzetti F, Fontana L, Tagliapietra G, Maldini C, Martelli MF, Tabilio A. Immunomagnetic isolation of CD4+CD25+FoxP3+ natural T regulatory lymphocytes for clinical applications. Clin Exp Immunol. 2009;156:246–253. doi: 10.1111/j.1365-2249.2009.03901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Henao GA, Picon M, Amill B, Querol S, Ferra C, Granena A, Garcia J. Combined positive and negative cell selection from allogeneic peripheral blood progenitor cells (PBPC) by use of immunomagnetic methods. Bone Marrow Transplant. 2001;27:683–687. doi: 10.1038/sj.bmt.1702860. [DOI] [PubMed] [Google Scholar]
- 67.Canney PA, Moore M, Wilkinson PM, James RD. Ovarian cancer antigen CA125: a prospective clinical assessment of its role as a tumour marker. Br J Cancer. 1984;50:765–769. doi: 10.1038/bjc.1984.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Chen L, Liu Y, Xu Y, Zhang X, Shi Y, Wang C, Zhang PL, Liu Y. Improving the cytological diagnosis of high-grade serous carcinoma in ascites with a panel of complementary biomarkers in cell blocks. Cytopathology. 2018;29:247–253. doi: 10.1111/cyt.12514. [DOI] [PubMed] [Google Scholar]
- 70.Lu Y, Liang H, Yu T, Xie J, Chen S, Dong H, Sinko PJ, Lian S, Xu J, Wang J, Yu S, Shao J, Yuan B, Wang L, Jia L. Isolation and characterization of living circulating tumor cells in patients by immunomagnetic negative enrichment coupled with flow cytometry. Cancer. 2015;121:3036–3045. doi: 10.1002/cncr.29444. [DOI] [PubMed] [Google Scholar]
- 71.Lara O, Tong X, Zborowski M, Chalmers JJ. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp Hematol. 2004;32:891–904. doi: 10.1016/j.exphem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Yu GH, Glaser LJ, Gustafson KS. Role of ancillary techniques in fluid cytology. Acta Cytol. 2020;64:52–62. doi: 10.1159/000496568. [DOI] [PubMed] [Google Scholar]
- 73.Mair S, Dunbar F, Becker PJ, Du Plessis W. Fine needle cytology--is aspiration suction necessary? A study of 100 masses in various sites. Acta Cytol. 1989;33:809–813. [PubMed] [Google Scholar]
- 74.Chen LM, Lazcano O, Katzmann JA, Kimlinger TK, Li CY. The role of conventional cytology, immunocytochemistry, and flow cytometric DNA ploidy in the evaluation of body cavity fluids: a prospective study of 52 patients. Am J Clin Pathol. 1998;109:712–721. doi: 10.1093/ajcp/109.6.712. [DOI] [PubMed] [Google Scholar]
- 75.Collins GR, Thomas J, Joshi N, Zhang S. The diagnostic value of cell block as an adjunct to liquid-based cytology of bronchial washing specimens in the diagnosis and subclassification of pulmonary neoplasms. Cancer Cytopathol. 2012;120:134–141. doi: 10.1002/cncy.20181. [DOI] [PubMed] [Google Scholar]
- 76.He DN, Zhu HS, Zhang KH, Jin WJ, Zhu WM, Li N, Li JS. E-cadherin and calretinin as immunocytochemical markers to differentiate malignant from benign serous effusions. World J Gastroenterol. 2004;10:2406–2408. doi: 10.3748/wjg.v10.i16.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tickman RJ, Cohen C, Varma VA, Fekete PS, DeRose PB. Distinction between carcinoma cells and mesothelial cells in serous effusions. Usefulness of immunohistochemistry. Acta Cytol. 1990;34:491–496. [PubMed] [Google Scholar]
- 78.Malle D, Valeri RM, Photiou C, Kaplanis K, Andreadis C, Tsavdaridis D, Destouni C. Significance of immunocytochemical expression of E-cadherin, N-cadherin and CD44 in serous effusions using liquid-based cytology. Acta Cytol. 2005;49:11–16. doi: 10.1159/000326088. [DOI] [PubMed] [Google Scholar]
- 79.Ko EC, Jhala NC, Shultz JJ, Chhieng DC. Use of a panel of markers in the differential diagnosis of adenocarcinoma and reactive mesothelial cells in fluid cytology. Am J Clin Pathol. 2001;116:709–715. doi: 10.1309/PJ7H-A52V-M3XB-V94Y. [DOI] [PubMed] [Google Scholar]
- 80.Arora R, Agarwal S, Mathur SR, Verma K, Iyer VK, Aron M. Utility of a limited panel of calretinin and Ber-EP4 immunocytochemistry on cytospin preparation of serous effusions: a cost-effective measure in resource-limited settings. Cytojournal. 2011;8:14. doi: 10.4103/1742-6413.83233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ordonez NG. Immunohistochemical diagnosis of epithelioid mesothelioma: an update. Arch Pathol Lab Med. 2005;129:1407–1414. doi: 10.5858/2005-129-1407-IDOEMA. [DOI] [PubMed] [Google Scholar]
- 82.Hasteh F, Lin GY, Weidner N, Michael CW. The use of immunohistochemistry to distinguish reactive mesothelial cells from malignant mesothelioma in cytologic effusions. Cancer Cytopathol. 2010;118:90–96. doi: 10.1002/cncy.20071. [DOI] [PubMed] [Google Scholar]
- 83.Engels M, Michael C, Dobra K, Hjerpe A, Fassina A, Firat P. Management of cytological material, pre-analytical procedures and bio-banking in effusion cytopathology. Cytopathology. 2019;30:31–38. doi: 10.1111/cyt.12654. [DOI] [PubMed] [Google Scholar]
- 84.Ng WK, Chow JC, Ng PK. Thyroid transcription factor-1 is highly sensitive and specific in differentiating metastatic pulmonary from extrapulmonary adenocarcinoma in effusion fluid cytology specimens. Cancer. 2002;96:43–48. doi: 10.1002/cncr.10310. [DOI] [PubMed] [Google Scholar]
- 85.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Shield PW, Papadimos DJ, Walsh MD. GATA3: a promising marker for metastatic breast carcinoma in serous effusion specimens. Cancer Cytopathol. 2014;122:307–312. doi: 10.1002/cncy.21393. [DOI] [PubMed] [Google Scholar]
- 87.Jo VY, Cibas ES, Pinkus GS. Claudin-4 immunohistochemistry is highly effective in distinguishing adenocarcinoma from malignant mesothelioma in effusion cytology. Cancer Cytopathol. 2014;122:299–306. doi: 10.1002/cncy.21392. [DOI] [PubMed] [Google Scholar]
- 88.Oda T, Ogata S, Kawaguchi S, Minabe S, Dokyu M, Takahashi H, Kumazawa F, Shimazaki H, Takano M, Hase K, Ozeki Y, Kanoh S, Nakanishi K. Immunocytochemical utility of claudin-4 versus those of Ber-EP4 and MOC-31 in effusion cytology. Diagn Cytopathol. 2016;44:499–504. doi: 10.1002/dc.23476. [DOI] [PubMed] [Google Scholar]
- 89.O’Shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, Somers EB, Vachani A. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. doi: 10.18632/oncotarget.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Shannessy DJ, Somers EB, Smale R, Fu YS. Expression of folate receptor-alpha (FRA) in gynecologic malignancies and its relationship to the tumor type. Int J Gynecol Pathol. 2013;32:258–268. doi: 10.1097/PGP.0b013e3182774562. [DOI] [PubMed] [Google Scholar]
- 91.Dominguez-Malagon H, Cano-Valdez AM, Gonzalez-Carrillo C, Campos-Salgado YE, Lara-Garcia A, Lopez-Mejia M, Corona-Cruz JF, Arrieta O. Diagnostic efficacy of electron microscopy and pleural effusion cytology for the distinction of pleural mesothelioma and lung adenocarcinoma. Ultrastruct Pathol. 2016;40:254–260. doi: 10.1080/01913123.2016.1195469. [DOI] [PubMed] [Google Scholar]
- 92.Oczypok EA, Oury TD. Electron microscopy remains the gold standard for the diagnosis of epithelial malignant mesothelioma: a case study. Ultrastruct Pathol. 2015;39:153–158. doi: 10.3109/01913123.2014.960542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng WK, Yau BW, Ma L. Diagnostic utility and limitations of electron microscopy in effusion fluid cytology smears. Diagn Cytopathol. 2003;29:46–48. doi: 10.1002/dc.10299. [DOI] [PubMed] [Google Scholar]
- 94.Lane D, Matte I, Rancourt C, Piche A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao J, Song L, Li D, Peng L, Ding H. Clinical value of haptoglobin and soluble CD163 testing for the differential diagnosis of tuberculous and malignant pleural effusions. Medicine (Baltimore) 2019;98:e17416. doi: 10.1097/MD.0000000000017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, Grosse-Wilde H, Cheng CC, Kurman RJ, Shih Ie M. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4464. [PubMed] [Google Scholar]
- 97.Liu D, Kong D, Li J, Gao L, Wu D, Liu Y, Yang W, Zhang L, Zhu J, Jin X. HE4 level in ascites may assess the ovarian cancer chemotherapeutic effect. J Ovarian Res. 2018;11:47. doi: 10.1186/s13048-018-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 99.Krishan A, Ganjei-Azar P, Hamelik R, Sharma D, Reis I, Nadji M. Flow immunocytochemistry of marker expression in cells from body cavity fluids. Cytometry A. 2010;77:132–143. doi: 10.1002/cyto.a.20824. [DOI] [PubMed] [Google Scholar]
- 100.Kentrou NA, Tsagarakis NJ, Tzanetou K, Damala M, Papadimitriou KA, Skoumi D, Stratigaki A, Anagnostopoulos NI, Malamou-Lada E, Athanassiadou P, Paterakis G. An improved flow cytometric assay for detection and discrimination between malignant cells and atypical mesothelial cells, in serous cavity effusions. Cytometry B Clin Cytom. 2011;80:324–334. doi: 10.1002/cyto.b.20608. [DOI] [PubMed] [Google Scholar]
- 101.Davidson B, Dong HP, Berner A, Christensen J, Nielsen S, Johansen P, Bryne M, Asschenfeldt P, Risberg B. Detection of malignant epithelial cells in effusions using flow cytometric immunophenotyping: an analysis of 92 cases. Am J Clin Pathol. 2002;118:85–92. doi: 10.1309/M877-QABM-D9GB-FJAX. [DOI] [PubMed] [Google Scholar]
- 102.Pillai V, Cibas ES, Dorfman DM. A simplified flow cytometric immunophenotyping procedure for the diagnosis of effusions caused by epithelial malignancies. Am J Clin Pathol. 2013;139:672–681. doi: 10.1309/AJCP4HIFSHMO9WTK. [DOI] [PubMed] [Google Scholar]
- 103.Risberg B, Davidson B, Dong HP, Nesland JM, Berner A. Flow cytometric immunophenotyping of serous effusions and peritoneal washings: comparison with immunocytochemistry and morphological findings. J Clin Pathol. 2000;53:513–517. doi: 10.1136/jcp.53.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, Veillon R, Blons H, Audigier-Valette C, Bringuier PP, Lamy R, Beau-Faller M, Pujol JL, Sabourin JC, Penault-Llorca F, Denis MG, Lantuejoul S, Morin F, Tran Q, Missy P, Langlais A, Milleron B, Cadranel J, Soria JC, Zalcman G Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 105.Corcoran JP, Psallidas I, Wrightson JM, Hallifax RJ, Rahman NM. Pleural procedural complications: prevention and management. J Thorac Dis. 2015;7:1058–1067. doi: 10.3978/j.issn.2072-1439.2015.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roy-Chowdhuri S, Aisner DL, Allen TC, Beasley MB, Borczuk A, Cagle PT, Capelozzi V, Dacic S, da Cunha Santos G, Hariri LP, Kerr KM, Lantuejoul S, Mino-Kenudson M, Moreira A, Raparia K, Rekhtman N, Sholl L, Thunnissen E, Tsao MS, Vivero M, Yatabe Y. Biomarker testing in lung carcinoma cytology specimens: a perspective from members of the pulmonary pathology society. Arch Pathol Lab Med. 2016;140:1267–1272. doi: 10.5858/arpa.2016-0091-SA. [DOI] [PubMed] [Google Scholar]
- 107.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356–371. doi: 10.1111/cyt.12174. [DOI] [PubMed] [Google Scholar]
- 109.da Cunha Santos G. Standardizing preanalytical variables for molecular cytopathology. Cancer Cytopathol. 2013;121:341–343. doi: 10.1002/cncy.21290. [DOI] [PubMed] [Google Scholar]
- 110.Gailey MP, Stence AA, Jensen CS, Ma D. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin-fixed, paraffin-embedded tissue. Cancer Cytopathol. 2015;123:30–39. doi: 10.1002/cncy.21476. [DOI] [PubMed] [Google Scholar]
- 111.NCCN Guidelines: Nonsmall cell lung cancer, version 4.2019. https://www.nccn.org/professionals/physician_gls/. Accessed 29 April 2019.
- 112.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Mesothelioma. Cancer Genet Cytogenet. 2001;127:93–110. doi: 10.1007/3-540-30792-3_7. [DOI] [PubMed] [Google Scholar]
- 113.Flores-Staino C, Darai-Ramqvist E, Dobra K, Hjerpe A. Adaptation of a commercial fluorescent in situ hybridization test to the diagnosis of malignant cells in effusions. Lung Cancer. 2010;68:39–43. doi: 10.1016/j.lungcan.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Khoo C, Rogers TM, Fellowes A, Bell A, Fox S. Molecular methods for somatic mutation testing in lung adenocarcinoma: EGFR and beyond. Transl Lung Cancer Res. 2015;4:126–141. doi: 10.3978/j.issn.2218-6751.2015.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy-Chowdhuri S. Advances in molecular testing techniques in cytologic specimens. Surg Pathol Clin. 2018;11:669–677. doi: 10.1016/j.path.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosolen DCB, Faria DK, Faria CS, Antonangelo L. Performance of the UroVysion® FISH assay for the diagnosis of malignant effusions using two cutoff strategies. Cancer Med. 2018;7:1967–1977. doi: 10.1002/cam4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Onofre FB, Onofre AS, Pomjanski N, Buckstegge B, Grote HJ, Bocking A. 9p21 deletion in the diagnosis of malignant mesothelioma in serous effusions additional to immunocytochemistry, DNA-ICM, and AgNOR analysis. Cancer. 2008;114:204–215. doi: 10.1002/cncr.23413. [DOI] [PubMed] [Google Scholar]
- 120.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 121.Mocharla H, Mocharla R, Hodes ME. Coupled reverse transcription-polymerase chain reaction (RT-PCR) as a sensitive and rapid method for isozyme genotyping. Gene. 1990;93:271–275. doi: 10.1016/0378-1119(90)90235-j. [DOI] [PubMed] [Google Scholar]
- 122.Carter J, Miller JA, Feller-Kopman D, Ettinger D, Sidransky D, Maleki Z. Molecular profiling of malignant pleural effusion in metastatic non-small-cell lung carcinoma. The effect of preanalytical factors. Ann Am Thorac Soc. 2017;14:1169–1176. doi: 10.1513/AnnalsATS.201609-709OC. [DOI] [PubMed] [Google Scholar]
- 123.Cheng JQ, Jhanwar SC, Klein WM, Bell DW, Lee WC, Altomare DA, Nobori T, Olopade OI, Buckler AJ, Testa JR. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994;54:5547–5551. [PubMed] [Google Scholar]
- 124.Kumar KR, Cowley MJ, Davis RL. Next-generation sequencing and emerging technologies. Semin Thromb Hemost. 2019;45:661–673. doi: 10.1055/s-0039-1688446. [DOI] [PubMed] [Google Scholar]
- 125.Cronin M, Ross JS. Comprehensive next-generation cancer genome sequencing in the era of targeted therapy and personalized oncology. Biomark Med. 2011;5:293–305. doi: 10.2217/bmm.11.37. [DOI] [PubMed] [Google Scholar]
- 126.Porcel JM. Diagnosis and characterization of malignant effusions through pleural fluid cytological examination. Curr Opin Pulm Med. 2019;25:362–368. doi: 10.1097/MCP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 127.Green MR, Sambrook J. Analysis and normalization of real-time polymerase chain reaction (PCR) experimental data. Cold Spring Harb Protoc. 2018;2018 doi: 10.1101/pdb.top095000. [DOI] [PubMed] [Google Scholar]
- 128.Fassan M. Molecular diagnostics in pathology: time for a next-generation pathologist? Arch Pathol Lab Med. 2018;142:313–320. doi: 10.5858/arpa.2017-0269-RA. [DOI] [PubMed] [Google Scholar]
- 129.Illei PB, Ladanyi M, Rusch VW, Zakowski MF. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer. 2003;99:51–56. doi: 10.1002/cncr.10923. [DOI] [PubMed] [Google Scholar]
- 130.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–2113. [PubMed] [Google Scholar]
- 131.Cappellesso R, Nicole L, Caroccia B, Guzzardo V, Ventura L, Fassan M, Fassina A. Young investigator challenge: MicroRNA-21/MicroRNA-126 profiling as a novel tool for the diagnosis of malignant mesothelioma in pleural effusion cytology. Cancer Cytopathol. 2016;124:28–37. doi: 10.1002/cncy.21646. [DOI] [PubMed] [Google Scholar]
- 132.Savic S, Bubendorf L. Common fluorescence in situ hybridization applications in cytology. Arch Pathol Lab Med. 2016;140:1323–1330. doi: 10.5858/arpa.2016-0202-RA. [DOI] [PubMed] [Google Scholar]
- 133.Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH, Tsai MF, Yu CJ, Yang CH, Yang PC. EML4-ALK translocation predicts better outcome in lung adenocarcinoma patients with wild-type EGFR. J Thorac Oncol. 2012;7:98–104. doi: 10.1097/JTO.0b013e3182370e30. [DOI] [PubMed] [Google Scholar]
- 134.Chen YL, Lee CT, Lu CC, Yang SC, Chen WL, Lee YC, Yang CH, Peng SL, Su WC, Chow NH, Ho CL. Epidermal growth factor receptor mutation and anaplastic lymphoma kinase gene fusion: detection in malignant pleural effusion by RNA or PNA analysis. PLoS One. 2016;11:e0158125. doi: 10.1371/journal.pone.0158125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.da Cunha Santos G, Schroder M, Zhu JB, Saieg MA, Geddie WR, Boerner SL, McDonald S. Minimizing delays in DNA retrieval: the “freezer method” for glass coverslip removal. Letter to the editor regarding comparative study of epidermal growth factor receptor mutation analysis on cytology smears and surgical pathology specimens from primary and metastatic lung carcinomas. Cancer Cytopathol. 2013;121:533. doi: 10.1002/cncy.21306. [DOI] [PubMed] [Google Scholar]
- 136.Jain D, Ramachandrappa VS, Singh V, Malik PS, Madan K, Faruq M, Guleria R. Use of exfoliative specimens and fine-needle aspiration smears for mutation testing in lung adenocarcinoma. Acta Cytol. 2017;61:455–461. doi: 10.1159/000479217. [DOI] [PubMed] [Google Scholar]
- 137.Fiegl M, Massoner A, Haun M, Sturm W, Kaufmann H, Hack R, Krugmann J, Fritzer-Szekeres M, Grunewald K, Gastl G. Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH) Br J Cancer. 2004;91:558–563. doi: 10.1038/sj.bjc.6601942. [DOI] [PMC free article] [PubMed] [Google Scholar]


