Abstract
Collagen triple helix repeat containing 1 (CTHRC1), an extracellular matrix-related protein, has been found to be upregulated in many solid tumors and contributes to tumorigenesis. We found that CTHRC1 is overexpressed in glioblastoma tissues and cells. By using the technique of RNA interference, the expression of CTHRC1 in the human glioblastoma U-87MG cell line was downregulated, and the proliferation and migration of U-87MG cells were examined. The results showed that the knockdown of CTHRC1 exerts inhibitory effects on the proliferation and migration ability of U-87MG cells. Knockdown of CTHRC1 expression in U-87MG cells resulted in upregulation in the expression of E-cadherin and downregulation in the expression of N-cadherin, SNAIL, and Slug, suggesting that CTHRC1 inhibits glioblastoma cell migration by suppressing epithelial–mesenchymal transition (EMT). Knockdown of CTHRC1 led to remarkably decreased β-catenin protein levels in the nucleus. These results indicate that CTHRC1 might play an important role in the development of glioblastoma and offer a candidate molecular target for glioblastoma prevention and therapy.
Key words: Collagen triple helix repeat containing 1 (CTHRC1), Glioblastoma, RNA interference, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Glioblastoma, with a median survival of approximately 1 year, remains one of the most lethal tumors because of its refractory features against conventional therapies (1). Glioma tumor cells grow infiltratively via diffusion; therefore, there are no clear boundaries, leading to unlimited proliferation and high invasiveness (2). Unfortunately, glioblastoma is the most common malignancy of all brain tumors, and currently effective treatment options are still lacking (3). Despite advances in surgical techniques, radiotherapy, and chemotherapy, the prognosis for glioblastoma patients has not significantly improved in recent decades (4). Thus, it is crucial to find an approach in understanding the pathogenesis of glioblastoma and the development of more effective therapeutic targets for this disease.
Collagen triple helix repeat containing 1 (CTHRC1), an extracellular matrix-related protein, is a secreted protein involved in vascular remodeling, bone formation, and developmental morphogenesis. CTHRC1 is aberrantly overexpressed in multiple malignant tumors, including gastric cancer, non-small cell lung cancer, breast cancer, hepatocellular cancer, colorectal cancer, and pancreatic cancer (5–7). CTHRC1 was initially found in a screen for differentially expressed genes in balloon-injured versus normal rat arteries (8). It may contribute to tissue repair by limiting collagen matrix deposition and promoting cell migration (9). A previous study showed that the knockdown of CTHRC1 resulted in a decrease in migration ability of melanoma cancer cells in vitro (10). Currently, there is little information about the role and mechanism of CTHRC1 in glioblastoma cell.
In the present study, we intended to examine CTHRC1 expression in both glioblastoma tissue and glioblastoma cells by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot analysis and to observe the biological function of U-87MG cells following CTHRC1 knockdown by the RNA interference technique. These data might provide scientific information for prognosis prediction and targeted therapy for glioblastoma.
MATERIALS AND METHODS
Reagents
Antibodies against CTHRC1, E-cadherin, N-cadherin, SNAIL, Slug, and β-catenin were purchased from Abcam (Cambridge, UK). The antibodies against β-actin and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Specimens
The pathological diagnosis and grading for each glioblastoma were assessed by neuropathologists according to the 2007 World Health Organization (WHO) Classification of Nervous System Tumors (11). Primary tissue samples were obtained from 46 patients in the Department of Pathology of the First Hospital of Jilin University (Changchun, Jilin, P.R. China) between January and November 2015. No patients underwent radiation or chemotherapy prior to surgical therapy. In addition, 16 tissue specimens obtained from the normal cortex of patients without glioblastoma were used as the negative control. The patients included 36 (58.06%) males and 26 (41.94%) females, aged 19–60 years, with a median age of 40 years. Informed consent was obtained from all patients before entering this study, and the study was approved by the Clinical Research Ethics Committee of the First Hospital of Jilin University (12).
Cell Culture
Human malignant glioma cell lines U-118MG, U-373MG, and U-87MG and the normal human astrocyte cell line 1800 were obtained from the Cell Library of the Chinese Academy of Sciences (Shanghai, P.R. China) and cultured as described. Cells were cultured in DMEM supplemented with 10% (v/v) heat-inactivated FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37°C under a humidified atmosphere of 5% CO2 and 95% air.
CTHRC1 Knockdown by siRNA
U-87MG cells were divided into three groups: the CTHRC1-siRNA group (treated with CTHRC1-siRNA and Lipofectamine® 2000), the vector group (treated with control siRNA and Lipofectamine® 2000), and the control group (treated with Lipofectamine® 2000 only). The sequence of the human CTHRC1-siRNA was GAAATGAATTCAACAATTA (sense) (Genechem Co., Shanghai, P.R. China) (13). Cells were transfected with CTHRC1-siRNA, vector siRNA, and control siRNA using the X-tremeGENE siRNA transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions (14).
Cell Proliferation
Cells were plated in 96-well plates (1 × 105 cells per well) in 100 μl of complete medium and allowed to attach overnight. After incubation for 24, 48, or 72 h, 20 μl of MTT (Sigma-Aldrich, St. Louis, MO, USA) was added and then incubated for 4 h. The supernatant was discarded, the precipitate was dissolved in 200 μl of dimethyl sulfoxide (DMSO), and plates were read with a microplate reader at 570 nm (15).
Cell Migration
In vitro Transwell migration assays were performed in Boyden chambers with 8-mm pore filter inserts in 24-well plates (Pierce, Rockford, IL, USA). Briefly, the lower chamber was filled with complete medium. Cells were collected after trypsinization, resuspended in 200 ml of DMEM, and transferred to the upper chamber at a density of 1 × 105 cells/well in 100 μl of migration buffer. After 24 h of incubation, the filter was gently removed from the chamber and the cells on the upper surface were trypsinized and counted with a CASY 1 counter (Merek, Whitehouse Station, NJ, USA). Cells that had migrated to the lower surface of the filter were also trypsinized and counted. The migration rate was obtained by dividing the cell number in the lower chamber by the sum of the cell numbers found in both the lower chamber and the upper chamber × 100. Three independent experiments were performed (16).
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA from tissues and cells was extracted using the TRIzol reagent (Invitrogen) and reverse transcribed using the First-Strand Synthesis Kit (Gibco BRL, Carlsbad, CA, USA). The primer sequences used for qRT-PCR were as follows: CTHRC1, 5′-TGGACACCCAA CTACAAGCA-3′ (sense) and 5′-GAACAAGTGCCAACCCAGAT-3′ (antisense) (17); E-cadherin, 5′-GCTCGGCCTGAAGTGACTCG-3′ (sense) and 5′-CCGCTTCCTTCATAGTCAAACAC-3′ (antisense) (7); N-cadherin, 5′-CTCCATGTGCCGGATAGC-3′ (sense) and 5′-CGATTTCACCAGAAGCCTCTAC-3′ (antisense); SNAIL, 5′-GCTGCAGGACTCTAATCCAGA-3′ (sense) and 5′-ATCTCCGGAGGTGGGATG-3′ (antisense); Slug, 5′-TGGTTGCTTCAAGGACACAT-3′ (sense) and 5′-GTTGCAGTGAGGGCAAGAA-3′ (antisense) (18). The gene-specific primers were amplified with a denaturation step (95°C for 2 min), followed by 35 cycles of denaturation (95°C for 30 s), annealing (55°C for 30 s), and extension (72°C for 50 s). Samples from three separate experiments were analyzed in duplicate. The results from the RT-PCR were expressed using β-actin as a reference (17).
Western Blot
Western blot analysis was performed as previously described (19). Protein concentrations of tissue sample or cell lysates were measured by the BCA protein assay kit (Gibco, Rockville, MD, USA), and proteins were electrophoresed on SDS-PAGE gels. Separated proteins were then transferred onto PVDF membranes (Millipore, Boston, MA, USA) followed by the incubation with specific antibodies against CTHRC1, E-cadherin, N-cadherin, SNAIL, Slug, and β-catenin, and immunoblotting with the appropriate secondary antibody. Chemiluminescence detection was performed using an ECL system (Sigma-Aldrich).
Luciferase Reporter Gene Assay
For the reporter gene assay, cells seeded in 24-well plates were transfected with the firefly luciferase reporter gene construct (TOP 200 ng) and 1 ng of pRL-SV40 Renilla luciferase (as an internal control). Cell extracts were prepared 24 h after transfection, and luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) (6).
Statistical Analysis
The SPSS 19.0 software was used to analyze the related data with a t-test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
CTHRC1 mRNA and Protein Expression in Glioblastoma Tissues and Glioblastoma Cells
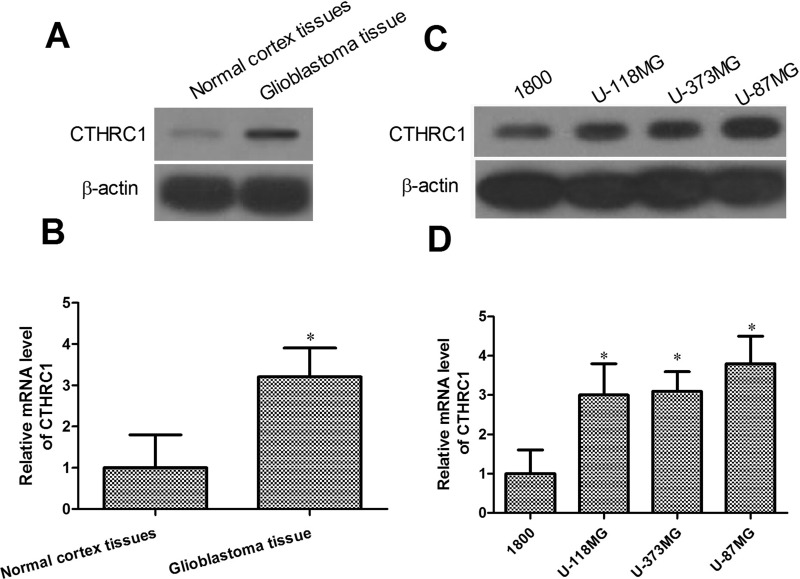
To confirm the expression of CTHRC1 in glioblastoma, we determined the mRNA and protein levels of CTHRC1 in glioblastoma tissues and in a human glioblastoma cell line array. As indicated in Figure 1A and B, the mRNA and protein expression levels of CTHRC1 in glioblastoma tissues were significantly higher than in normal cortex tissues (p < 0.05). Next we investigated the mRNA and protein expression in U-118MG, U-373MG, U-87MG, and the normal human astrocyte cell line 1800 using qRT-PCR and Western blot. Our results indicated that the CTHRC1 mRNA and protein expression levels were higher in the glioblastoma cells U-118MG, U-373MG, and U-87MG than in the normal human astrocyte cell line 1800 (Fig. 1C and D). These results demonstrate that CTHRC1 might be a critical molecule in glioblastoma.
Figure 1.
CTHRC1 is overexpressed in glioblastoma tissues and glioblastoma cells. (A, C) Western blot analysis was performed to examine CTHRC1 expression in glioblastoma tissues, normal cortex tissues, several glioblastoma cell lines, and normal human astrocyte cells 1800. β-Actin was used as a loading control. (B, D) CTHRC1 mRNA expression was determined in glioblastoma tissues, normal cortex tissues, several glioblastoma cell lines, and normal human astrocyte cells 1800. *p < 0.05 versus control. All experiments were repeated at least three times.
Determination of Transfection Effects
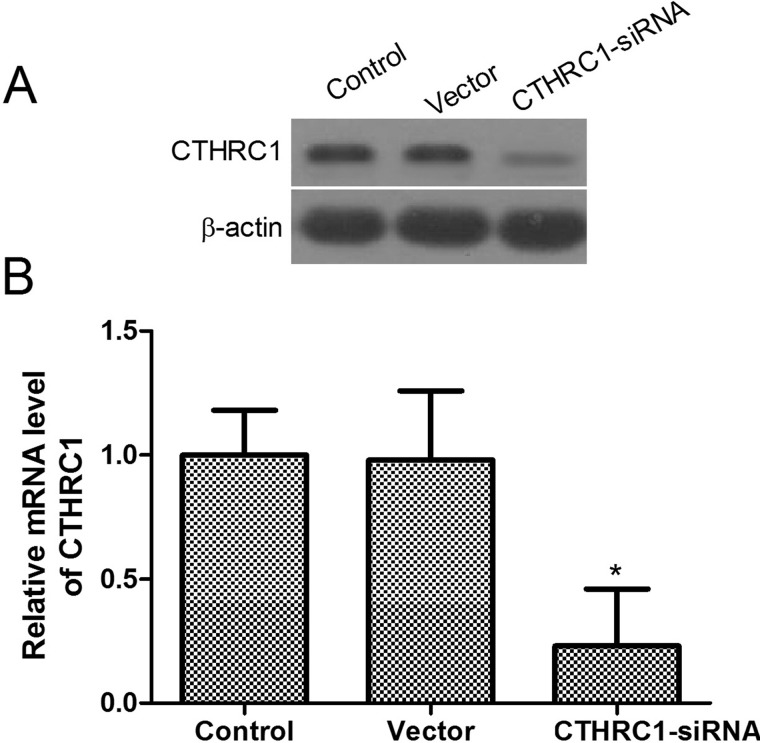
On the basis of our observations, we hypothesized that CTHRC1 may affect the tumorigenic properties in glioblastoma cells. Therefore, we generated the stable knockdown CTHRC1 U-87MG line. To explore the efficiency of CTHRC1 transfection, qRT-PCR and Western blot were employed to determine the expression levels of the mRNA and protein. As shown in Figure 2, the mRNA and protein expression levels of CTHRC1 were significantly decreased in the CTHRC1-siRNA-transfected group. There was no significant difference in the expression level of CTHRC1 mRNA and protein between the vector group and the control groups.
Figure 2.
The expression of CTHRC1 in U-87MG cells. (A) Western blot analysis of CTHRC1 expression 48 h after transfection with CTHRC1-siRNA and control siRNA. β-Actin was used as a loading control. (B) CTHRC1 mRNA expression was detected by qRT-PCR 48 h after transfection with CTHRC1-siRNA and control siRNA. All experiments were repeated at least three times, and all data are reported as means ± SD (n = 3) (*p < 0.05 vs. control).
Effect of CTHRC1 on U-87MG Cell Viability
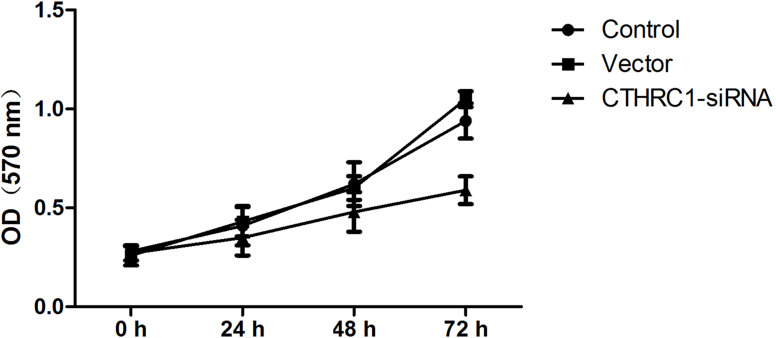
The impact of CTHRC1 on U-87MG cell proliferation was determined via an MTT assay every 24 h after transfection, for up to 72 h. The results revealed that the viability of U-87MG cells was, to some extent, inhibited by CTHRC1-siRNA in a time-dependent manner. As shown in Figure 3, the CTHRC1-siRNA-transfected group grew more slowly than the control group and vector group.
Figure 3.
Effect of CTHRC1 on U-87MG cell proliferation. MTT assay was conducted to assess the effect of CTHRC1 on cell proliferation every 24 h after transfection, for up to 72 h. All experiments were repeated at least three times. All data are means ± SD (n = 3).
Effect of CTHRC1 on U-87MG Cell Migration
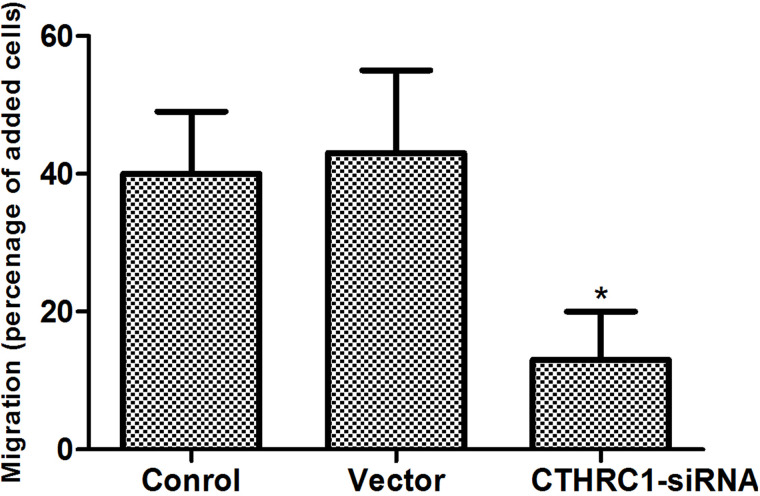
We determined the effect of decreased CTHRC1 expression on glioblastoma cell migration after 48 h of transfection. U-87MG cell migration was significantly reduced by CTHRC1-siRNA according to the migration assay (Fig. 4). There was no significant difference in U-87MG cell migration rate between the vector group and the control group.
Figure 4.
Effect of CTHRC1 on U-87MG cell migration. Cell migration was measured with a Transwell assay 48 h after CTHRC1-siRNA transfection. All experiments were repeated at least three times. All data are means ± SD (n = 3) (*p < 0.05 vs. control).
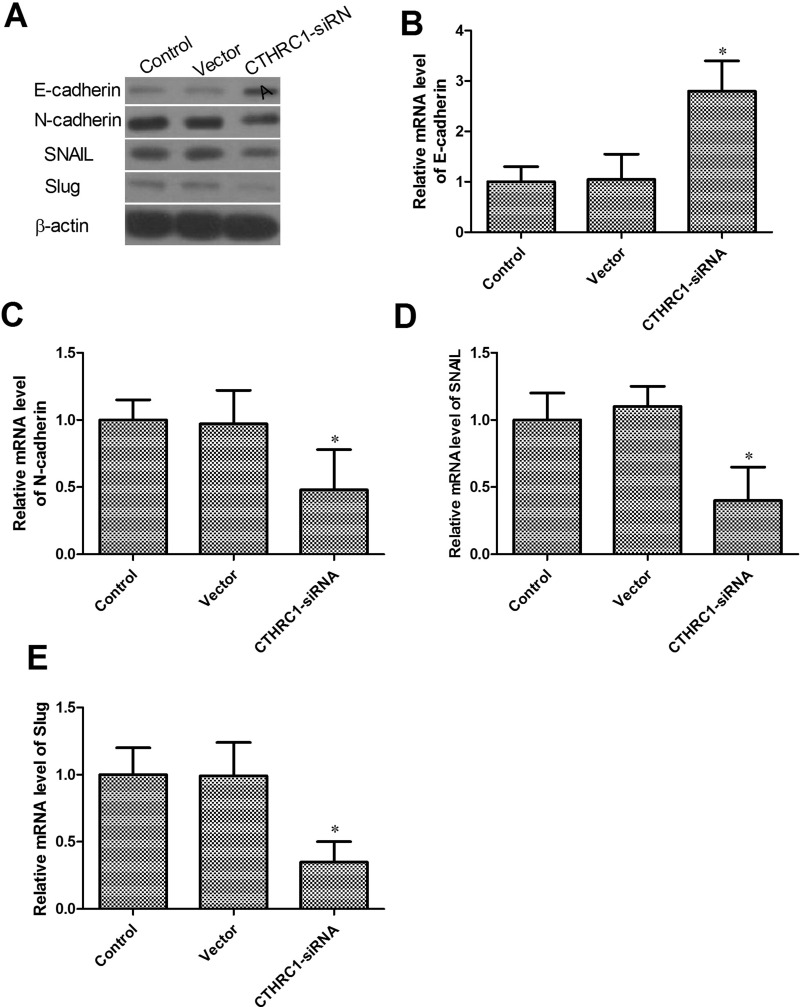
Effect of CTHRC1 on EMT of U87-MG Cells
The epithelial-to-mesenchymal transition (EMT) has been proven to play a critical role in driving tumor migration and metastasis (20). To investigate whether CTHRC1 decreased cancer migration by inhibiting EMT, we analyzed the mRNA and protein level of several EMT markers. As shown in Figure 5A–E, Western blot and qRT-PCR results revealed increased protein and mRNA levels of E-cadherin and decreased protein and mRNA levels of N-cadherin, SNAIL, and Slug in the CTHRC1-siRNA-transfected group. There was no significant difference in the expression level of E-cadherin, N-cadherin, SNAIL, or Slug mRNA and protein level between the vector group and the control group.
Figure 5.
Effect of CTHRC1 on EMT of U-87MG cells. (A) Effects of CTHRC1 knockdown on protein expression of EMT markers by Western blot. (B–E) Effects of CTHRC1 knockdown on mRNA expression of EMT markers by qRT-PCR. β-Actin was used as an internal loading control. All data are means ± SD (n = 3) (*p < 0.05 vs. control).
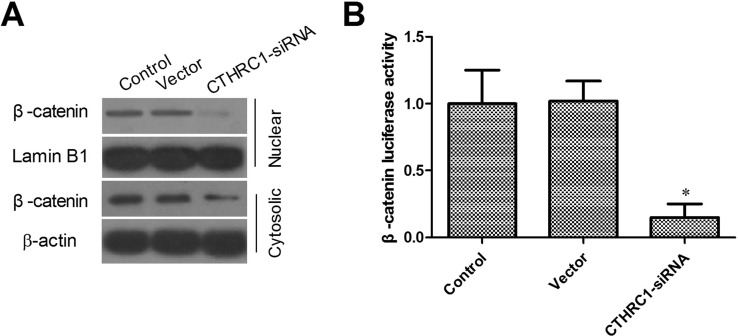
Effect of CTHRC1 on Wnt/β-Catenin Signaling Pathway
Overexpression of CTHRC1 led to Wnt/β-catenin pathway activation (21). The expression of β-catenin was examined in the U87-MG cell line by Western blotting. After downregulating CTHRC1 expression in U-87MG cells, we observed that the protein expression of β-catenin was strongly downregulated in the CTHRC1-siRNA group, suggesting that CTHRC1 might contribute to the inhibition of Wnt signaling (Fig. 6A). As expected, luciferase assays also demonstrated that CTHRC1-siRNA reduced the β-catenin/TCF transcriptional activity in U-87MG cells (Fig. 6B).
Figure 6.
Effects of CTHRC1 on Wnt/β-catenin signaling pathway. (A) The subcellular localization of β-catenin was observed through Western blot. (B) The Dual Luciferase Reporter Assay showed that knockdown of CTHRC1 inhibited Wnt/β-catenin signaling. Relative luciferase activity is presented as the mean ± SD from each sample after normalizing to the control (*p < 0.05 vs. control).
DISCUSSION
Glioblastoma, the most common primary malignant brain tumor in humans, exhibits a high rate of recurrence and a poor prognosis due to the invasive nature of the tumor (22). Although there have been great advancements in the treatment of glioblastoma to date, the median survival of glioblastoma patients is still far from satisfactory (23). Therefore, the development of new methods of glioblastoma therapy and prognosis is a key task for many researchers around the world.
CTHRC1 was initially found in a screen for differentially expressed genes in balloon-injured versus normal rat arteries (24). Aberrant CTHRC1 expression is detected in several malignant tumors, such as breast cancer, ovarian cancer, thyroid cancer, and liver cancer (15). A previous study showed that CTHRC1 overexpression promotes human colorectal cancer cell migration, invasion, and proliferation (9). CTHRC1 increases cellular motility to repair the injury by limiting the deposition of collagen matrix and promoting cell migration (5). Hou et al. (21) found that ectopic transfection of CTHRC1 in epithelial ovarian cancer cells upregulated the expression of EMT markers such as vimentin and N-cadherin, and EMT-associated transcriptional factor Snail. However, the clinicopathological significance and the biological role of CTHRC1 in glioblastoma remain unclear.
The present study demonstrated that CTHRC1 is overexpressed in glioblastoma tissues and cell lines. To study the function of CTHRC1, we generated a stable CTHRC1 knockdown U-87MG cell line and evaluated the effects of the downregulation of CTHRC1 on the proliferation and migration in the U-87MG cell line. Our results showed that the knockdown of CTHRC1 inhibited the proliferation and migration of the U-87MG cell line. These results suggested that CTHRC1 plays a crucial role in glioblastoma carcinogenesis.
Notably, EMT has been recognized to be vital in promoting cancer metastasis (25). It is increasingly acknowledged that EMT plays an important role in the metastasis of many types of carcinomas (26). On the basis of this knowledge and our results above, we hypothesized that CTHRC1 can induce EMT during its promotion of migration. In CTHRC1-siRNA-transfected U-87MG cells, we detected an increased expression of E-cadherin, which is a hallmark of EMT and often lost in metastatic tumors (27), while the expression of N-cadherin, SNAIL, and Slug was downregulated, suggesting that CTHRC1 inhibits glioblastoma cell migration by suppressing EMT.
Next, to further elucidate the molecular mechanism of how CTHRC1 mediates the migration of U87-MG cells, we analyzed its effects on the Wnt/β-catenin signaling pathway. Moreover, in most settings, the canonical Wnt/β-catenin signaling pathway has been extensively implicated as the regulator of tumor cell migration and metastasis (28). Moreover, in most conditions, a group of transcription factors downstream of Wnt/β-catenin signaling pathway plays a significant role in the regulation of EMT and cancer metastasis. Knockdown of CTHRC1 led to a significant decrease in Wnt/β-catenin transcriptional activity and a downregulation of nuclear β-catenin. These data suggest that the downregulation of CTHRC1 might be associated with the downregulation of Wnt/β-catenin and that the downregulation of Wnt/β-catenin might be further responsible for the regulation of EMT. However, its exact mechanism needs further research.
These results may suggest that the knockdown of CTHRC1 might play an important role in carcinogenesis and the development of glioblastoma. Additional studies are needed to investigate the specific mechanisms underlying the effects of glioblastoma in the EMT and metastasis. In short, CTHRC1 plays an important role in regulating the biological behavior of glioblastoma cells.
ACKNOWLEDGMENT
This research was supported by Jilin Provincial Science and Technology Department of China (20130101149JC).
REFERENCES
- 1. Sun L.; Zhang C.; Yang Z.; Wu Y.; Wang H.; Bao Z.; Jiang T. KIF23 is an independent prognostic biomarker in glioma, transcriptionally regulated by TCF-4. Oncotarget 7(17):24646–24655; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z.; Chen Q. β-Catenin knockdown inhibits the proliferation of human glioma cells in vitro and in vivo. Exp. Ther. Med. 11(3):1059–1064; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X.; Wu C.; Chen N.; Gu H.; Yen A.; Cao L.; Wang E.; Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 7(22):33440–33450; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayashi K.; Michiue H.; Yamada H.; Takata K.; Nakayama H.; Wei F. Y.; Fujimura A.; Tazawa H.; Asai A.; Ogo N.; Miyachi H.; Nishiki T.; Tomizawa K.; Takei K.; Matsui H. Fluvoxamine, an anti-depressant, inhibits human glioblastoma invasion by disrupting actin polymerization. Sci. Rep. 6:23372; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu L.; Liu L.; Zhong L.; Bai Y.; Sui H.; Wei X.; Zhang W.; Huang P.; Gao D.; Kong Y.; Lou G. Cthrc1 overexpression is an independent prognostic marker in gastric cancer. Hum. Pathol. 45(5):1031–1038; 2014. [DOI] [PubMed] [Google Scholar]
- 6. Ke Z.; He W.; Lai Y.; Guo X.; Chen S.; Li S.; Wang Y.; Wang L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget 5(19):9410–9424; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding C.; Luo J.; Li L.; Li S.; Yang L.; Pan H.; Liu Q.; Qin H.; Chen C.; Feng J. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J. Exp. Clin. Cancer Res. 35(1):5; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma M. Z.; Zhuang C.; Yang X. M.; Zhang Z. Z.; Ma H.; Zhang W. M.; You H.; Qin W.; Gu J.; Yang S.; Cao H.; Zhang Z. G. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 16(3):265–278; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X. M.; You H. Y.; Li Q.; Ma H.; Wang Y. H.; Zhang Y. L.; Zhu L.; Nie H. Z.; Qin W. X.; Zhang Z. G.; Li J. CTHRC1 promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling. Int. J. Clin. Exp. Pathol. 8(10):12793–12801; 2015. [PMC free article] [PubMed] [Google Scholar]
- 10. Tang L.; Dai D. L.; Su M.; Martinka M.; Li G.; Zhou Y. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin. Cancer Res. 12(12):3716–3722; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Thurnher M. M. 2007 World Health Organization classification of tumours of the central nervous system. Cancer Imaging 9(Spec No A):S1–S3; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang R.; Qian D.; Hu M.; Zhang X.; Song J.; Li L.; Chen H.; Wang B. Association between human cytomegalovirus infection and histone acetylation level in various histological types of glioma. Oncol. Lett. 10(5):2812–2820; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tameda M.; Sugimoto K.; Shiraki K.; Yamamoto N.; Okamoto R.; Usui M.; Ito M.; Takei Y.; Nobori T.; Kojima T.; Suzuki H.; Uchida M.; Uchida K. Collagen triple helix repeat containing 1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation and motility. Int. J. Oncol. 45(2):541–548; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu R. R.; Lv Y. S.; Tang Y. X.; Wang Y. F.; Chen X. L.; Zheng X. X.; Xie S. Z.; Cai Y.; Yu J.; Zhang X. N. Eukaryotic translation initiation factor 5A2 regulates the migration and invasion of hepatocellular carcinoma cells via pathways involving reactive oxygen species. Oncotarget 26;7(17):24348–60; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou M.; Cheng Z.; Shen H.; He S.; Li Y.; Pan Y.; Feng C.; Chen X.; Zhang Y.; Lin M.; Wang L.; Ke Z. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget 6(34):35813–35829; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meng X.; Kong D. H.; Li N.; Zong Z. H.; Liu B. Q.; Du Z. X.; Guan Y.; Cao L.; Wang H. Q. Knockdown of BAG3 induces epithelial-mesenchymal transition in thyroid cancer cells through ZEB1 activation. Cell Death Dis. 5:e1092; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ke Z.; He W.; Lai Y.; Guo X.; Chen S.; Li S.; Wang Y.; Wang L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget 5(19):9410–9424; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishioka M.; Venkatesan N.; Dessalle K.; Mogas A.; Kyoh S.; Lin T. Y.; Nair P.; Baglole C. J.; Eidelman D. H.; Ludwig M. S.; Hamid Q. Fibroblast-epithelial cell interactions drive epithelial-mesenchymal transition differently in cells from normal and COPD patients. Respir Res. 16:72; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu D.; Li L.; Yan W. Knockdown of TC-1 enhances radiosensitivity of non-small cell lung cancer via the Wnt/beta-catenin pathway. Biol. Open 5(4):492–498; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayer C.; Darb-Esfahani S.; Meyer A. S.; Hubner K.; Rom J.; Sohn C.; Braicu I.; Sehouli J.; Hansch G. M.; Gaida M. M. Neutrophil granulocytes in ovarian cancer—Induction of epithelial-to-mesenchymal-transition and tumor cell migration. J. Cancer 7(5):546–554; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hou M.; Cheng Z.; Shen H.; He S.; Li Y.; Pan Y.; Feng C.; Chen X.; Zhang Y.; Lin M.; Wang L.; Ke Z. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget 6(34):35813–35829; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olar A.; Aldape K. D. Using the molecular classification of glioblastoma to inform personalized treatment. J. Pathol. 232(2):165–177; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Y. C.; Hueng D. Y.; Huang H. Y.; Chen J. Y.; Chen Y. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 7(20):29116–29130; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma M. Z.; Zhuang C.; Yang X. M.; Zhang Z. Z.; Ma H.; Zhang W. M.; You H.; Qin W.; Gu J.; Yang S.; Cao H.; Zhang Z. G. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 16(3):265–278; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ke L.; Xiang Y.; Guo X.; Lu J.; Xia W.; Yu Y.; Peng Y.; Wang L.; Wang G.; Ye Y.; Yang J.; Liang H.; Kang T.; Lv X. c-Src activation promotes nasopharyngeal carcinoma metastasis by inducing the epithelial-mesenchymal transition via PI3K/Akt signaling pathway: A new and promising target for NPC. Oncotarget 7(19):28340–28355; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiappetta G.; Valentino T.; Vitiello M.; Pasquinelli R.; Monaco M.; Palma G.; Sepe R.; Luciano A.; Pallante P.; Palmieri D.; Aiello C.; Rea D.; Losito S. N.; Arra C.; Fusco A.; Fedele M. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration. Oncotarget 6(7):5310–5323; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang F.; Zhang X.; Meng J.; Zhao Y.; Liu X.; Liu Y.; Wang Y.; Li Y.; Sun Y.; Wang Z.; Mei Q.; Zhang T. ING5 inhibits cancer aggressiveness via preventing EMT and is a potential prognostic biomarker for lung cancer. Oncotarget 6(18):16239–16252; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chien A. J.; Conrad W. H.; Moon R. T. A Wnt survival guide: From flies to human disease. J. Invest. Dermatol. 129(7):1614–1627; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]