Abstract
Aberrantly expressed microRNAs (miRNAs/miRs) and their role in cancer development have recently gained more attention. However, the potential role of miRNAs in hepatocellular carcinoma (HCC) remains largely unknown. In this study, we demonstrated that miR-377 was markedly downregulated in HCC cell lines and primary human HCC tissues. The decreased expression of miR-377 contributes to the upregulation of Bcl-xL expression by targeting its 3′-untranslated region (3′-UTR). Functionally, knockdown of miR-377 noticeably increased HCC cell growth and colony formation and inhibited apoptosis. In contrast, overexpression of miR-377 suppressed cell proliferation and increased apoptosis. This study provides new insights for the use of miR-377 as a potential molecular target in HCC therapy.
Key words: miR-377, Hepatocellular carcinoma (HCC), Apoptosis, Bcl-xL
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and is the third leading cause of cancer-related deaths. HCC, which accounts for 70% to 85% of primary liver cancers, is rarely detected early and often results in a short survival time1. Because of our poor understanding of the mechanism underlying HCC progression, it is difficult to diagnose and treat the disease at an early stage2.
MicroRNAs (miRNAs/miRs), a group of noncoding small RNAs that are approximately 22–25 nucleotides in length, function as key posttranscriptional regulators of gene expression3. Growing evidence suggests that miRNAs can inhibit gene expression, either at the level of protein translation or degradation, by binding the 3′-untranslated region (3′-UTR) of messenger RNA (mRNA)4. Studies have shown that miRNAs are involved in many cancers, including colon, breast, lung, and liver. miRNAs are involved in many biological events, including cell cycle, proliferation, differentiation, apoptosis, and metabolism5. Therefore, miRNAs may play a critical role in HCC progression by regulating a variety of gene expression profiles.
Apoptosis, in addition to dysregulated aberrant differentiation and proliferation, is an important characteristic of cancer cells6. Apoptosis is regulated by a careful balance of Bcl-2 family proteins, such as proapoptotic proteins Bak and Bax, and the antiapoptotic proteins Bcl-xL and Mcl-17. Previous studies have reported that Bcl-xL is overexpressed in one third of human HCC tissues and confers resistance of hepatoma cells to apoptosis8. HCC patients with high Bcl-xL expression were shown to have a significantly shorter disease-free survival period after surgery8. The mechanisms underlying Bcl-xL overexpression in HCC are not clearly understood.
Recently, there has been an increasing interest in the role of miR-377 in tumorigenesis and cancer treatment9. Studies have shown that upregulation of miR-377 can lead to increased fibronectin production in diabetic nephropathy9. Furthermore, miR-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1 (Sp1)10. However, the function of miR-377 in HCC has not been reported. In this study, we demonstrated that Bcl-xL, an antiapoptotic gene that has a key role in tumor progression, is a direct target of miR-377. Moreover, ectopic expression of miR-377 can suppress cancer cell proliferation and apoptosis by inhibiting Bcl-xL expression.
MATERIALS AND METHODS
Cell and Tissue Culture
Primary human hepatocytes were purchased from ATCC and cultured with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated serum (Sigma-Aldrich, St. Louis, MO, USA). All of the human hepatoma cell lines were cultured with DMEM containing 10% heat-inactivated serum. HCCs and their adjacent nontumor counterparts were obtained at the time of surgical resection. The surgically removed tissues were quickly frozen and stored in liquid nitrogen until the time of use. All of the samples were collected with the informed consent of each patient, and the experiments were approved by the ethics committee.
RNA Extraction and qRT-PCR
The total RNA, which includes the miRNAs, was extracted from cell lines and tissues using the miRNeasy kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. To test the expression of the miRNA, real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis was performed using the SYBR RT-PCR kit (Takara) and LightCycler (Roche). The relative expression of miR-377 was normalized to the internal control, U6, by using the 2−ΔΔCt (cycle threshold) method.
Transfections
All of the transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The miR-377 mimic, miR-377 inhibitor, and the negative control were purchased from GenePharma (Shanghai, P.R. China). The miR-377 mimic and miR-377 inhibitor were used at a final concentration of 40 nM.
Western Blot
Cells were lysed using RIPA buffer containing a protease inhibitor cocktail (Roche). Protein extracts were subjected to an 8% SDS-PAGE and transferred onto PVDF membranes. Primary antibodies specific to Bcl-xL or β-actin and the horseradish peroxidase-coupled secondary antibodies were purchased from Cell Signaling Technology.
Cell Proliferation Assays
Five thousand cells transfected with the miR-377 mimic, miR-377 inhibitor, or a scramble control were seeded into 96-well plates and cultured at different time points. The cells were treated with Cell Counting Kit-8 (CCK-8) (DOJINDO, Tabaru, Japan) for 2 h. The optical density was measured at an absorbance of 450 nm. The proliferation rate was calculated as the experimental OD value divided by the control OD value.
Colony Formation Assay
Five hundred cells transfected with the miR-377 mimic, miR-377 inhibitor, or a scramble control were seeded into a six-well plate and cultured in DMEM containing 10% FBS for 10 days. Colonies were fixed and stained with 0.1% crystal violet in 20% methanol for 30 min.
Detection of Apoptosis
Cells were transfected with a scramble control, miR-377 mimic, or miR-377 inhibitor. At 48 h posttransfection, cells were harvested, washed, resuspended in the staining buffer, and examined with the Vybrant Apoptosis Assay Kit (Invitrogen). Stained cells were detected by FACSCalibur. The annexin V-positive cells were regarded as apoptotic cells.
Luciferase Reporter Assay
For the luciferase reporter assay, HEK 293T cells (3 × 104) were seeded in a 24-well plate and grown to 80%–90% confluence. To explore the binding of miR-377 to the Bcl-xL 3′-UTR, the cells were cotransfected with 20 nM of either the scramble control or miR-377 mimic as well as 40 ng of either pmiRGLO-Bcl-xL-3′-UTR-WT or pmiRGLO-Bcl-xL-3′-UTR-MUT using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The cells were collected 48 h after transfection and analyzed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). A plasmid constitutively expressing Renilla luciferase was cotransfected as an internal control to correct for differences in both transfection and harvesting efficiencies. The transfections were performed in duplicate, and at least three independent experiments were performed.
Statistical Analysis
Data are presented as the mean ± standard error from at least three independent experiments. Statistical analysis was performed with SPSS software. Student’s t-tests were used to determine the statistical significance. The significance level was set at p < 0.05 for all analyses.
RESULTS
miR-377 Is Downregulated in Hepatocellular Carcinoma
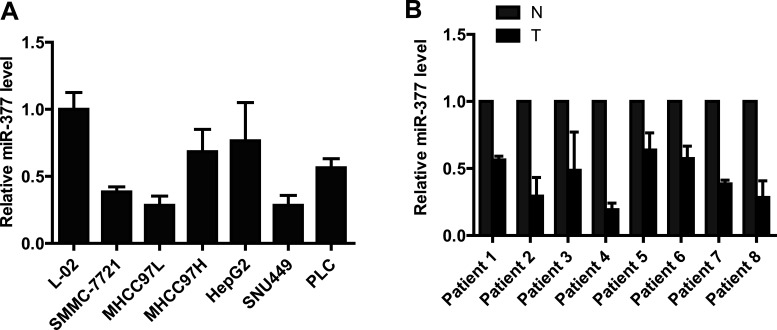
To test the role of miR-377 in HCC, we compared the miR-377 expression level in human hepatocytes and HCC cell lines using qRT-PCR. We found that the expression of miR-377 is substantially lower in HCC cells than in hepatocyte cells (Fig. 1A). We also examined the expression of miR-377 in eight paired samples from HCC tumors and adjacent normal tissues (Fig. 1B). The results showed that miR-377 was significantly downregulated among these eight matched sample pairs.
Figure 1.
miR-377 is downregulated in hepatocellular carcinoma. (A) miR-377 is significantly downregulated in HCC cell lines compared with human hepatocyte cells. (B) Real-time PCR analysis shows that miR-377 is noticeably reduced in hepatocellular carcinoma tissues compared with adjacent normal tissues.
miR-377 Inhibits HCC Cell Proliferation In Vitro
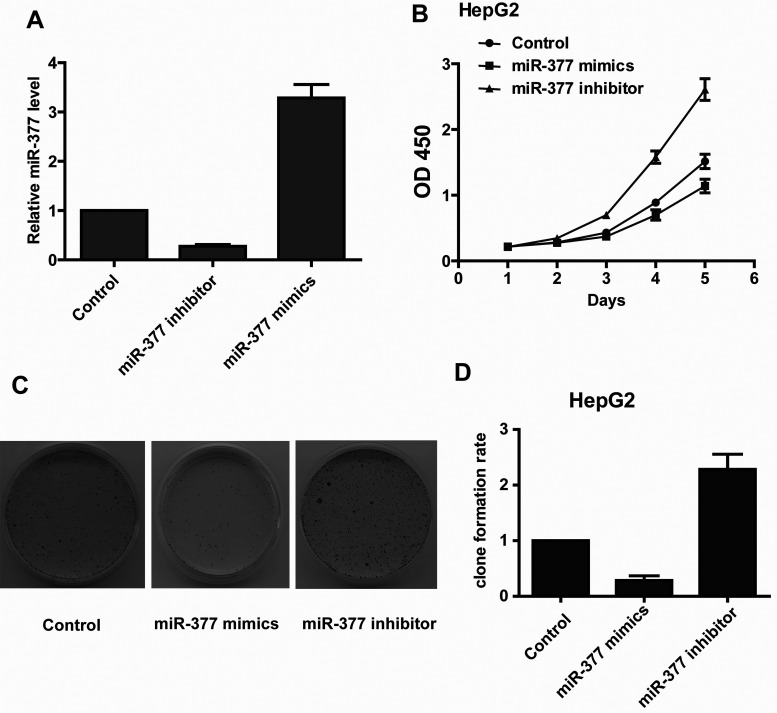
To explore the role of miR-377 on cancer cell growth, we transfected HepG2 cells with 40 nM aliquots of the miR-377 mimic or 40 nM aliquots of the miR-377 inhibitor. The qRT-PCR analysis revealed that the expression level of miR-377 was dramatically and specifically decreased in the miR-377 inhibitor-transfected cells and increased in the miR-377 mimic-transfected cells (Fig. 2A). The CCK-8 assay demonstrated that the cell proliferation rate was higher in the miR-377 inhibitor-transfected cells than in the control cells (Fig. 2B). As expected, a gain of miR-377 function attenuated cell proliferation (Fig. 2B). The colony formation assay also showed that a loss of miR-377 function increased HepG2 colony formation, while a gain of miR-377 function inhibited colony formation (Fig. 2C and D). Taken together, these results suggested that miR-377 plays a critical role as a tumor suppressor in HCC cell growth.
Figure 2.
miR-377 suppresses the growth of hepatocellular carcinoma cells. (A) Real-time PCR analysis of miR-377 expression levels in HepG2 cells transfected with the miR-377 inhibitor or mimic. (B) The CCK-8 assay results show that the growth rate of miR-377-overexpressing HepG2 cells is lower than in control cells. Conversely, knockdown of miR-377 in HepG2 cells increases cell growth. (C and D) The colony formation assays show that knockdown of miR-377 enhances colony formation in HepG2 cells, while overexpression of miR-377 inhibits colony formation in HepG2 cells.
miR-377 Induces HCC Cell Apoptosis
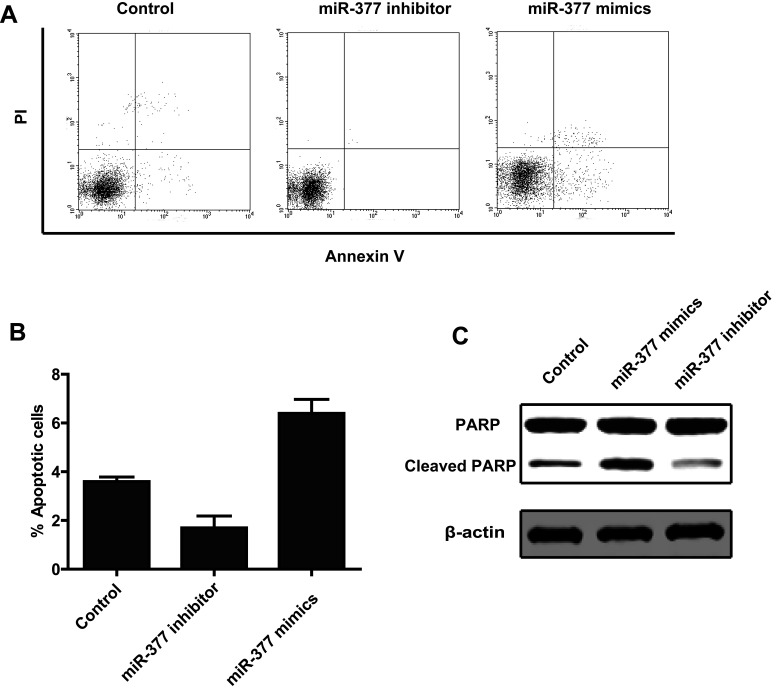
To test the effect of miR-377 on apoptosis, we analyzed the apoptosis of HepG2 cells at 48 h posttransfection with either the miR-377 mimic, the miR-377 inhibitor, or the scramble control by annexin V staining using flow cytometry. The results showed that the apoptotic rate in miR-377 mimic-transfected cells was significantly higher than in the control cells (Fig. 3A and B). As expected, miR-377 knockdown suppressed apoptosis (Fig. 3A and B). To further confirm the role of miR-377 in apoptosis in HCC cells, we performed a Western blot to detect the level of cleaved PARP, a well-known marker of apoptosis. We found that the miR-377 mimic increased the level of cleaved PARP, while the miR-377 inhibitor decreased the level of cleaved PARP (Fig. 3C). In conclusion, these data indicated that miR-377 induced apoptosis in HCC cells.
Figure 3.
miR-433 promotes cell apoptosis. (A) HepG2 cells were transfected with a scramble control, miR-377 inhibitor, or miR-377 mimic. Cells were stained with PI and annexin V and then analyzed by FACS. (B) The annexin V-positive populations of HepG2 cells transfected with the scramble control, miR-433 inhibitor, or miR-433 mimic. (C) Cleaved PARP was detected by Western blot in HepG2 cells transfected with the scramble control, miR-433 mimic, or miR-433 inhibitor.
miR-377 Induces Apoptosis by Directly Suppressing Bcl-xL Expression
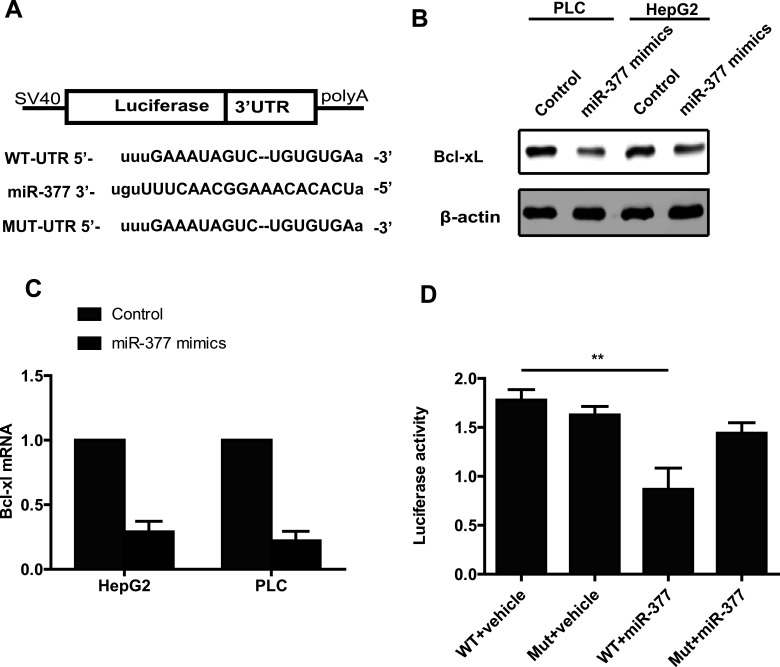
To further examine the mechanism by which miR-377 regulates apoptosis in HCC cells, we utilized the miRanda and TargetScan software to predict potential downstream targets of miR-377. We were particularly interested in the candidates associated with apoptosis. For further study, we chose to focus on Bcl-xL, a well-known prosurvival protein that is well characterized in many tumors. The 3′-UTR of Bcl-xL has a potential binding region for miR-377 (Fig. 4A). To validate whether Bcl-xL is a direct target of miR-377, we performed a Western blot to examine the protein level of Bcl-xL in HepG2 cells transfected with the miR-377 mimic. We found a decreased Bcl-xL protein level in HepG2 cells transfected with the miR-377 mimic compared with control cells (Fig. 4B). The mRNA level of Bcl-xL in miR-377-overexpressing cells was also decreased. These results indicated that miR-377 downregulated Bcl-xL expression at both the mRNA and protein levels (Fig. 4C). To further test whether Bcl-xL is a direct downstream target of miR-377, we constructed a wild-type dual-luciferase UTR vector that contained the 3′-UTR of Bcl-xL and a mutant vector that contained mutations in the potential miR-377 binding sites (Fig. 4A). As expected, miR-377 significantly inhibited the luciferase reporter activity of the wild-type vector group; however, this decreased luciferase activity was rescued when the cells were transfected with the mutant vector (Fig. 4D). Taken together, these results showed that miR-377 directly regulates Bcl-xL expression at both the mRNA and protein levels via binding sites in the 3′-UTR.
Figure 4.
miR-377 inhibits the expression of Bcl-xL. (A) The miR-377 sequence and the potential binding sequences in the 3′-UTR of Bcl-xL. (B) Western blot assays showed that overexpression of miR-377 decreases the expression of Bcl-xL. (C) Real-time PCR analysis showed that overexpression of miR-377 decreases the mRNA level of Bcl-xL. (D) Dual-luciferase assays showed that miR-377 suppresses luciferase activity in the 3′-UTR wild-type group but not in the 3′-UTR mutant group.
DISCUSSION
Apoptosis is essential for homeostasis and normal development11. Deregulation of apoptosis plays a key role in cancer initiation and progression12. The ability to resist apoptosis is a hallmark of cancer and is required for the development of neoplastic cells13.
Antiapoptotic members of the Bcl-2 family, which consists of five members (Bcl-xL, Mcl-1, Bcl-2, Bcl-w, and Bfl-1), are critically involved in apoptosis induced by the mitochondrial pathway14. Apoptosis induced by an anticancer agent is largely mediated by the Bcl-2 family14. Many reports have shown that Bcl-xL is overexpressed in many cancers, including HCC15.
Recently, miRNAs have attracted more attention due to their critical role in many biological events, including cell growth and differentiation16. Many aberrant miRNAs were reported in numerous cancer tissues and cancer cell lines16. Growing evidence suggests that miRNAs are required in apoptosis; however, the process to elucidate these new aberrant miRNAs and their role in apoptosis is ongoing.
In this study, we found that miR-377 was downregulated in HCC tissues compared with the adjacent normal tissues. Furthermore, we found that miR-377 was decreased in HCC cells in vitro. In combination with previous data revealing the role of miR-377 in glioblastoma, we further hypothesized that miR-377 may function as a tumor suppressor in carcinogenesis and cancer progression. The results of the CCK-8 and colony formation assays indicated that miR-377 inhibited cell proliferation. We then demonstrated for the first time that Bcl-xL is a target gene of miR-377. Moreover, we demonstrated that miR-377 suppressed cell growth by promoting cell apoptosis through the downregulation of Bcl-xL expression.
In conclusion, we explored the altered expression pattern of miR-377 in HCC tissues and cell lines. The decreased levels of miR-377 can inhibit HCC cell apoptosis through the suppression of Bcl-xL expression. These results identify miR-377 as a tumor suppressor involved in apoptosis in HCC and as a potential therapeutic target for HCC.
REFERENCES
- 1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. [DOI] [PubMed] [Google Scholar]
- 2. Sun J, Bie B, Zhang S, Yang J, Li Z. Long non-coding RNAs: Critical players in hepatocellular carcinoma. Int J Mol Sci. 2014;15:20434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101:2309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: Molecular features and role in cancer. Front Biosci. 2012;17:2508–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 2015;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shigematsu S, Fukuda S, Nakayama H, Inoue H, Hiasa Y, Onji M, Higashiyama S. ZNF689 suppresses apoptosis of hepatocellular carcinoma cells through the down-regulation of Bcl-2 family members. Exp Cell Res. 2011;317:1851–9. [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22:4126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang R, Luo H, Wang S, Chen W, Chen Z, Wang HW, Chen Y, Yang J, Zhang X, Wu W, Zhang SY, Shen S, Dong Q, Zhang Y, Jiang T, Lu D, Zhao S, You Y, Liu N, Wang H. MicroRNA-377 inhibited proliferation and invasion of human glioblastoma cells by directly targeting specificity protein 1. Neuro Oncol. 2014;16:1510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb.) 2011;3:279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med. 2004;199:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 14. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. [DOI] [PubMed] [Google Scholar]
- 15. Yang L, Si X, Wang W. Overexpression of bcl-2 protects hepatoma cell line HCC-9204 from ethanol-induced apoptosis. Chin Med J (Engl.) 2002;115:8–11. [PubMed] [Google Scholar]
- 16. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9. [DOI] [PubMed] [Google Scholar]