Abstract
Rab23, a novel member of the Rab GTPase family, was found to be implicated in the progression of some human cancers. However, what role Rab23 plays in prostate cancer (PCa) remains to be illustrated. In the present study, we investigated the expression pattern and roles of Rab23 in PCa. The study results showed that Rab23 was upregulated in PCa tissues and cell lines. Moreover, downregulation of Rab23 remarkably suppressed the proliferation, migration, and invasion of PCa cells. In addition, downregulation of Rab23 significantly downregulated the protein expression levels of Shh and Gli1. Furthermore, we found that the Gli1 inhibitor GANT-61 greatly enhanced the suppressive effect of Rab23 downregulation on PCa cells. In conclusion, we suggested Rab23 as a potential therapeutic target for PCa treatment.
Key words: Rab23, Proliferation, Migration, Invasion, Prostate cancer (PCa)
INTRODUCTION
Prostate cancer (PCa), one of the most prevalent malignancies, is a main cause of cancer-related deaths among men (1). Thanks to the improvement in PSA screening, prostate biopsies, and MRI imaging, prostate tumors have been detected and localized more accurately (2). In addition, the survival rate of PCa patients has been greatly increased due to the development of therapeutic strategies including surgery, radiotherapy, and pharmacotherapy (3,4). However, 5% of PCa patients, at the time of diagnosis, still suffer from metastatic lesions, which lead to a later stage (5,6). To achieve a satisfactory therapeutic effect for patients with advanced PCa, we desperately need to find a novel indicator for more effective treatment of PCa.
The Rab GTPase family has been reported to play a crucial role in the regulation of some important processes, such as intercellular vesicle trafficking, receptor recycling, and signal transduction (7,8). Rab23, a novel member of the Rab GTPase family, localizes to plasma membranes, cytosol, and endosomes (9). It serves as a significant regulator during the differentiation of skeletal and nervous systems (10,11). Recently, Rab23 was found to be implicated in the progression of some human cancers. For example, Liu et al. reported the overexpression of Rab23 in hepatocellular cancer and its association with tumor size (12). Similarly, Hou et al. demonstrated the upregulation of Rab23 in gastric cancer and its promoting effect on the invasion of gastric cancer cells (13). These findings suggested Rab23 as an oncogene in human cancers. On the contrary, Denning et al. reported the downregulation of Rab23 in thyroid cancer and its tumor-inhibitive effect on thyroid cancer cells (14). Despite all of the studies on the role of Rab23 in human cancers, there have been no reports on the expression and effect of Rab23 in PCa.
In this study, we investigated the clinical significance of Rab23 in PCa. The study results showed that Rab23 was upregulated in PCa tissues and cell lines. Furthermore, downregulation of Rab23 remarkably suppressed the proliferation, migration, and invasion of PCa cells as well as the protein expression levels of Shh and Gli1. We also discovered that the Gli1 inhibitor GANT-61 greatly potentiated the suppressive effect of Rab23 downregulation on PCa cells. All in all, we suggest Rab23 as a potential therapeutic target for PCa treatment.
MATERIALS AND METHODS
Clinical Samples
Sixteen patients from the Department of Urology of Huaihe Hospital of Henan University participated in the study and provided informed consent. PCa tissues and corresponding normal tissues were collected from the participants during surgery. All tissue samples were stored in liquid nitrogen before use. All experiments in the study were approved by the ethics committee of the Huaihe Hospital of Henan University.
Cell Lines and Cell Culture
Human PCa cell lines (DU145, PC3, and 22RV1) and the normal epithelial prostate cell line RWPE-1 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cell lines were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 μg/ml streptomycin (Gibco), and 100 IU/ml penicillin (Gibco) in a humidified incubator with 5% CO2 at 37°C.
Quantitative Real-Time RT-PCR
Extraction of total RNA from tissue samples or cell lines was performed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNA was carried out using a reverse transcription kit (Zhongshan Biotech, Beijing, P.R. China) according to the manufacturer’s instructions. The thermal cycling conditions for PCR were 94°C for 1 min, 95°C for 15 s, and 30 cycles of 60°C for 1 min. Primer sequences were listed as follows: Rab23, 5′-GTAGTAGCCGAAGTGGGA-3′ (forward) and 5′-CCTTTGTTTGTTGGGTCTC-3′ (reverse); GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-TGGTGAAGACGCCAGTGGA-3′ (reverse). GAPDH was used as internal control. The expression levels of genes were measured using the comparative CT method (2−ΔΔCt) (15).
Western Blot
Tissues or cells were lysed in lysis buffer. After centrifugation at 12,000 rpm for 20 min, lysates were collected, separated by 10% SDS-PAGE, and then transferred onto PVDF membranes. The membranes were blocked in Tris-buffered saline (TBS) and 5% nonfat milk and then incubated overnight at 4°C with the primary antibodies against Rab23, Shh, Gli1, or GAPDH. Subsequently, the membranes were washed three times and then incubated for 1 h at room temperature with HRP-coupled anti-mouse/rabbit IgG. All antibodies used in the study were purchased from Cell Signaling Technology (Danvers, MA, USA). The membranes were visualized using ECL reagents (Sigma-Aldrich, St. Louis, MO, USA). Band intensities were analyzed using BandScan software.
Cell Transfection
Rab23-targeting siRNA and scramble control siRNA were obtained from Dharmacon (Lafayette, CO, USA). The sequences of Rab23 siRNA and control siRNA were 5′-caaacaaaggaccaagaaaTT-3′ and 5′-uucuccgaacgugucacguTT-3′, respectively. Lipofectamine 2000 (Invitrogen) was used to transfect cells with Rab23 siRNA or control siRNA according to the manufacturer’s instructions. Twenty-four hours later, cells were harvested for the following assays. The transfection efficiency was verified via Western blot analysis.
Cell Proliferation and Colony Formation Assays
For the cell proliferation assay, transfected cells were seeded in a 96-well plate at a density of 5 × 103 cells/well. After culturing for different times, 20 μl of 5 mg/ml MTT (Sigma-Aldrich) was added to each well, and the cells were incubated at 37°C for 4 h. Subsequent to removal of the culture medium, 150 μl of dimethyl sulfoxide (DMSO; Sigma) was added to each well. The absorbance at 570 nm was measured with a microplate reader (Bio-Rad, Hercules, CA, USA).
For the colony formation assay, transfected cells were seeded in a six-well plate at a density of 300 cells/well. After 2 weeks of culturing, the cells were washed with PBS and then stained with Giemsa. The number of colonies with more than 50 cells was counted using a microscope.
Cell Migration and Invasion Assays
For the migration assay, Transwell chambers (pore size: 8 μm) with Matrigel-free membranes were used. Transfected cells were seeded in 100 μl of serum-free medium and then added to the upper chamber at a density of 2 × 105 cells/chamber. FBS was added to the lower chamber to promote cell migration. After 24 h of incubation at 37°C, cells remaining on the upper side of the membrane were wiped off, and cells passing through the membrane were fixed with methanol and stained with 0.1% crystal violet. Five fields were randomly chosen to count the number of migrated cells under a microscope (400×). The invasion assay was performed according to the same procedure as mentioned above, except that Matrigel-coated membranes were used.
Tumor Xenografts In Vivo
Female 6-week-old BALB/c nude mice were obtained from the Shanghai Laboratory Animal Center (Shanghai, P.R. China). All mice were randomly divided into two groups (seven mice/group) and handled according to the procedure approved by the Institutional Animal Care and Use Committee of the Huaihe Hospital of Henan University. Transfected cells (4 × 103) were suspended in 100 μl of PBS and then injected subcutaneously into the left flank of each mouse. Tumor size was measured every 6 days. After 30 days, all mice were sacrificed for thorough removal and weighing of tumors. Tumor volume was calculated according to the following formula: volume = (length × width2)/2.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD). Statistical comparison between different groups was made by Student’s t-tests. All experiments were performed at least three times. A value of p < 0.05 was considered statistically significant.
RESULTS
Expression of Rab23 Is Upregulated in PCa Tissues and Cell Lines
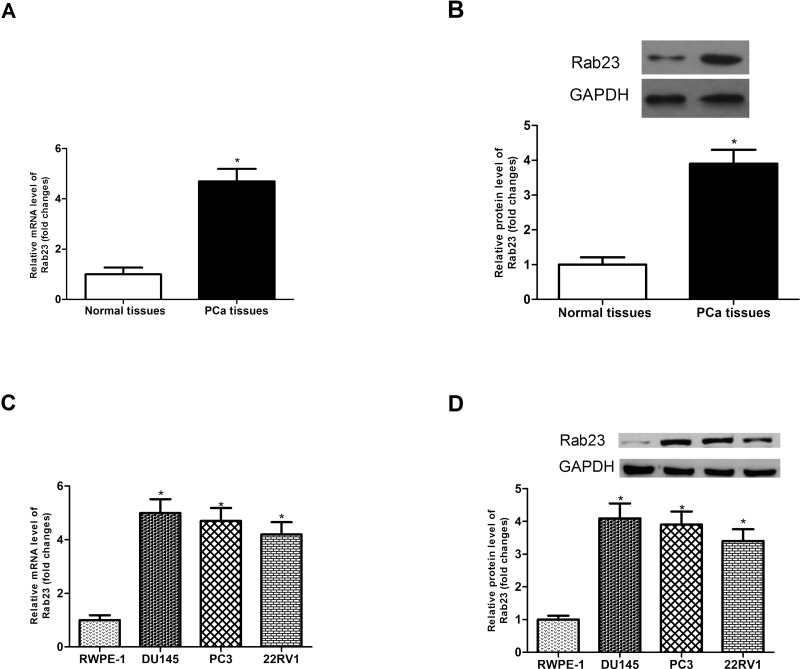
We compared the expression of Rab23 in 16 pairs of PCa tissues and corresponding normal tissues via RT-PCR and Western blot analysis. As shown in Figure 1A and B, the mRNA and protein expression levels of Rab23 were remarkably higher in PCa tissues than in corresponding normal tissues. Furthermore, we made an analysis of the mRNA and protein expression levels of Rab23 in three PCa cell lines (DU145, PC3, and 22RV1) and the normal epithelial prostate cell line RWPE-1. The results showed that the expression of Rab23 was much higher in three PCa cell lines than in the cell line RWPE-1 at the mRNA (Fig. 1C) and protein (Fig. 1D) levels. These results suggested upregulation of Rab23 in PCa tissues and cell lines.
Figure 1.
Expression of Rab23 is upregulated in PCa tissues and cell lines. (A, B) The expression of Rab23 was analyzed in PCa tissues and corresponding normal tissues via RT-PCR and Western blot. (C, D) The expression of Rab23 was measured in three PCa cell lines (DU145, PC3, and 22RV1) and the normal epithelial prostate cell line RWPE-1. *p < 0.05.
Downregulation of Rab23 Inhibits PCa Cell Proliferation In Vitro and In Vivo
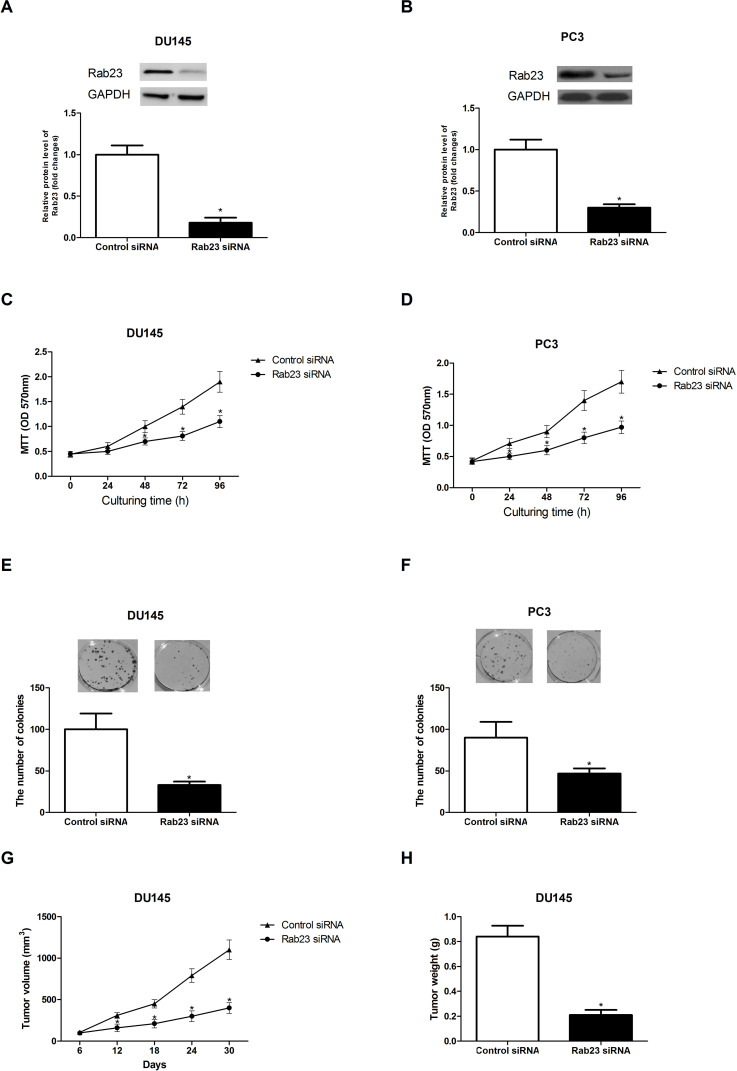
To investigate the impact of Rab23 on PCa cell proliferation, we downregulated Rab23 in DU145 and PC3 cells using siRNA transfection. As shown in Figure 2A and B, Rab23 siRNA transfection greatly reduced the protein expression of Rab23 in DU145 and PC3 cells.
Figure 2.
Downregulation of Rab23 inhibits PCa cell proliferation in vitro and in vivo. (A, B) After siRNA transfection, the protein expression of Rab23 in DU145 and PC3 cells was detected by Western blot assay. (C, D) The MTT assay showed that the proliferation rate of DU145 and PC3 cells was significantly decreased by downregulation of Rab23 in comparison with the control cells. (E, F) The colony formation assay indicated that the colony formation capability of DU145 and PC3 cells was markedly attenuated by downregulation of Rab23 in comparison with the control cells. (G, H) Downregulation of Rab23 resulted in a remarkable decrease in the volume and weight of tumors formed by DU145 cells transfected with Rab23 siRNA, compared to the control group. *p < 0.05.
Both MTT and colony formation assays were carried out to measure the effect of Rab23 downregulation on PCa cell proliferation. The MTT assay showed that the proliferation of DU145 (Fig. 2C) and PC3 (Fig. 2D) cells was remarkably suppressed by downregulation of Rab23 in comparison with the control cells. The colony formation assay indicated that the downregulation of Rab23 obstructed the formation of colonies in DU145 (Fig. 2E) and PC3 (Fig. 2F) cells in comparison with the control cells.
To further confirm the inhibitory effect of Rab23 downregulation on tumor growth, we performed in vivo experiments. In brief, DU145 cells transfected with Rab23 siRNA or control siRNA were subcutaneously injected into each nude mouse. Tumor size was measured every 6 days. After 30 days, all mice were sacrificed to weigh tumors. As shown in Figure 2G and H, downregulation of Rab23 caused a remarkable decrease in the volume and weight of tumors in nude mice compared with the control group.
Downregulation of Rab23 Inhibits PCa Cell Migration and Invasion In Vitro
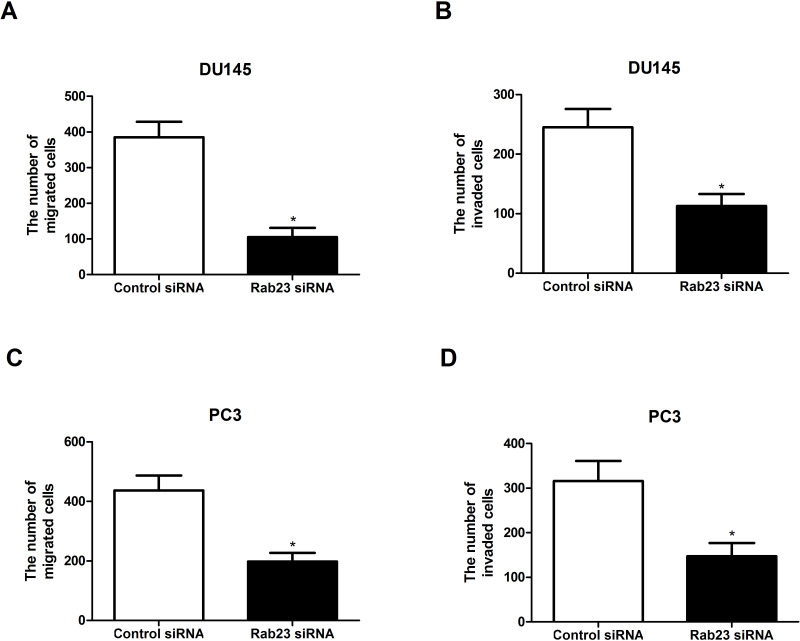
To examine the effect of Rab23 downregulation on PCa cell migration and invasion, Transwell assay was performed. As shown in Figure 3A and B, downregulation of Rab23 weakened the migratory and invasive capabilities of DU145 cells, compared with the control group. Similar results were obtained for PC3 cells (Fig. 3C and D).
Figure 3.
Downregulation of Rab23 inhibits PCa cell migration and invasion in vitro. (A, B) The Transwell assay indicated that the downregulation of Rab23 significantly reduced the number of migrated and invaded DU145 cells. (C, D) Similar results were observed in PC3 cells. *p < 0.05.
Downregulation of Rab23 Inhibits the Activity of the Shh/Gli1 Signaling Pathway
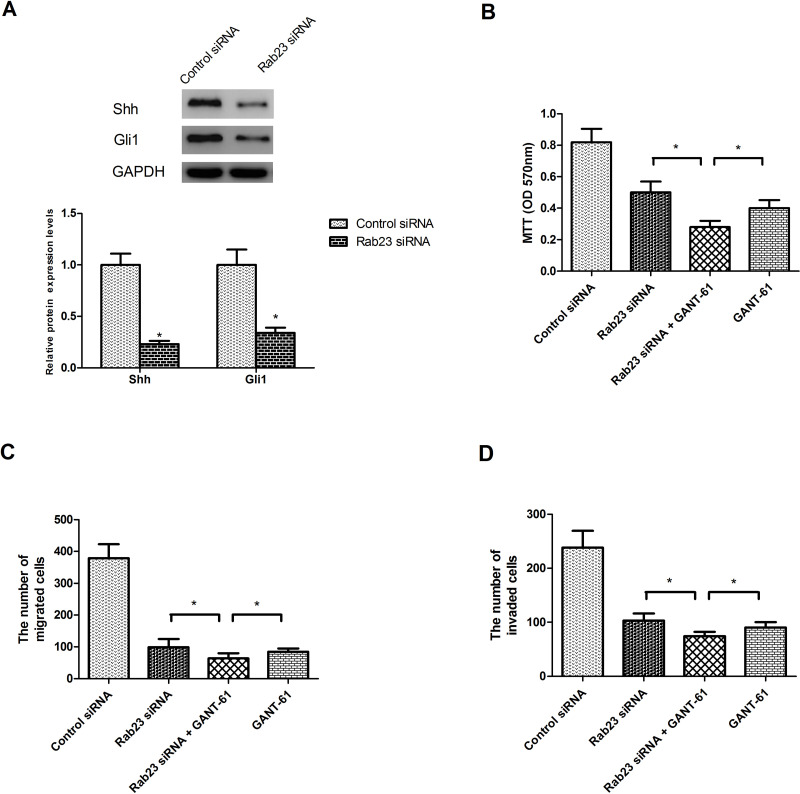
To explore the molecular mechanism underlying the suppressive effect of Rab23 downregulation on the proliferation, migration, and invasion of PCa cells, we detected the protein expression levels of Shh and Gli1, in consideration of the significant role Rab23 plays in regulating the Shh/Gli1 signaling pathway (16–21). As shown in Figure 4A, downregulation of Rab23 significantly decreased the protein expression levels of Shh and Gli1 in DU145 cells in comparison with the control cells. To validate the involvement of the Shh/Gli1 signaling pathway in Rab23-regulated proliferation, migration, and invasion of PCa cells, we employed the Gli1 inhibitor GANT-61. As shown in Figure 4B–D, GANT-61 obviously enhanced the suppressive effect of Rab23 siRNA on the proliferation (Fig. 4B), migration (Fig. 4C), and invasion (Fig. 4D) of DU145 cells.
Figure 4.
Downregulation of Rab23 inhibits the activity of the Shh/Gli1 signaling pathway. (A) The protein expression levels of Shh and Gli1 were remarkably decreased in DU145 cells by downregulation of Rab23, compared to the control group. (B–D) DU145 cells were transfected with Rab23 siRNA or control siRNA in the presence or absence of the Gli1 inhibitor GANT-61 (100 nM) for 24 h. The MTT assay was performed to measure cell proliferation. The Transwell assay was performed to measure cell migration and invasion. The results showed that GANT-61 significantly potentiated the inhibitory effect of Rab23 siRNA on the proliferation, migration, and invasion of DU145 cells. *p < 0.05.
DISCUSSION
PCa, one of the most prevalent cancers among men, is characterized by increasing morbidity and mortality (1,22). Despite the development of therapeutic strategies, 5% of PCa patients still suffer from poor quality of life owing to metastatic lesions, which lead to a later stage (3–6). Therefore, we urgently need to find a new therapeutic target for a more effective treatment of PCa, thus changing the unsatisfactory outcome of patients with advanced PCa.
Recent studies found the implication of Rab23 in the development of various human cancers such as hepatocellular cancer, gastric cancer, thyroid cancer, squamous cell cancer, and pancreatic duct adenocarcinoma (12–14,23,24). However, little is known about the specific role of Rab23 in PCa. In this study, we found that the expression of Rab23 was significantly higher in PCa tissues and cell lines than in normal prostate tissues and cell lines. This expression model of Rab23 in PCa was similar to that in hepatocellular cancer where Rab23 was found to be upregulated (12). Our study showed that downregulation of Rab23 remarkably suppressed the proliferation rate of PCa cells. Meanwhile, the migratory and invasive capabilities of PCa cells were also significantly inhibited. These results were inconsistent with those obtained from the studies performed by Jiang et al., who demonstrated overexpression of Rab23 in human bladder cancer and the promoting effect of Rab23 on bladder cancer cell proliferation and invasion (25). All these findings suggested the tumor-promoting role of Rab23 in human cancers. However, some other researchers obtained opposite results. For example, Liu et al. put forward that Rab23 suppressed growth and proliferation in breast cancer cells (26). Similar to Liu’s demonstration, Denning et al. proved the tumor-inhibitory effect on thyroid cancer cells in their studies (14). Taken together, we reasonably inferred that the role Rab23 played, an oncogene or a tumor-suppressor gene, was dependent on the type of cancer.
In our study, we demonstrated the tumor-promoting effect of Rab23 on PCa cells. However, what molecular mechanisms underlie the effect remained to be explored. Many studies have reported the key role Rab23 plays in regulating the Shh/Gli1 signaling pathway (16–21), so we detected whether downregulation of Rab23 affected this signaling pathway in PCa cells by investigating the protein expression levels of Shh and Gli1. The results showed that downregulation of Rab23 exerted a significant inhibitory effect on the protein expression levels of Shh and Gli1 in DU145 cells in comparison with the control cells. Like our study, a chondrocyte differentiation study found a similar result in that downregulation of Rab23 greatly reduced the level of Gli1 in chondrocytes (21). Conversely, Chi et al. discovered in their study that Rab23 negatively mediated the Gli1 transcription factor (20). In our study, we suggested the positive role of Rab23 in regulating Shh/Gli1. To further confirm the involvement of the Shh/Gli1 signaling pathway in Rab23-regulated proliferation, migration, and invasion of PCa cells, we employed the Gli1 inhibitor GANT-61. The related assays demonstrated that GANT-61 remarkably potentiated the suppressive effect of Rab23 downregulation on the proliferation, migration, and invasion of PCa cells.
On the basis of all the findings in our study, we concluded that Rab23 was upregulated in PCa tissues and cell lines, and downregulation of Rab23 remarkably suppressed the proliferation, migration, and invasion of PCa cells as well as the protein expression levels of Shh and Gli1. We also observed that the Gli1 inhibitor GANT-61 greatly potentiated the suppressive effect of Rab23 downregulation on PCa cells. All in all, we suggest Rab23 as a potential therapeutic target for PCa treatment.
ACKNOWLEDGMENTS
This research was funded by the Project of Science and Technology Research of Henan Province Department of Education (grant No. 15A320050).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A.; Siegel R.; Ward E.; Murray T.; Xu J.; Thun M. J. Cancer statistics, 2007. CA Cancer J. Clin. 57(1):43–66; 2007. [DOI] [PubMed] [Google Scholar]
- 2. Saad F.; Pantel K. The current role of circulating tumor cells in the diagnosis and management of bone metastases in advanced prostate cancer. Future Oncol. 8(3):321–331; 2012. [DOI] [PubMed] [Google Scholar]
- 3. Loblaw D. A.; Virgo K. S.; Nam R.; Somerfield M. R.; Ben-Josef E.; Mendelson D. S.; Middleton R.; Sharp S. A.; Smith T. J.; Talcott J. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 25(12):1596–1605; 2007. [DOI] [PubMed] [Google Scholar]
- 4. Chang S. S. Treatment options for hormone-refractory prostate cancer. Rev. Urol. 9(Suppl. 2):S13–S18; 2007. [PMC free article] [PubMed] [Google Scholar]
- 5. Nandana S.; Chung L. W. Prostate cancer progression and metastasis: Potential regulatory pathways for therapeutic targeting. Am. J. Clin. Exp. Urol. 2(2):92–101; 2014. [PMC free article] [PubMed] [Google Scholar]
- 6. Steeg P. S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 12(8):895–904; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Cheng K. W.; Lahad J. P.; Kuo W.-l.; Lapuk A.; Yamada K.; Auersperg N.; Liu J.; Smith-McCune K.; Lu K. H.; Fishman D. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 10(11):1251–1256; 2004. [DOI] [PubMed] [Google Scholar]
- 8. Caswell P. T.; Spence H. J.; Parsons M.; White D. P.; Clark K.; Cheng K. W.; Mills G. B.; Humphries M. J.; Messent A. J.; Anderson K. I. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell. 13(4):496–510; 2007. [DOI] [PubMed] [Google Scholar]
- 9. Guo A.; Wang T.; Ng E. L.; Aulia S.; Chong K. H.; Teng F. Y. H.; Wang Y.; Tang B. L. Open brain gene product Rab23: Expression pattern in the adult mouse brain and functional characterization. J. Neurosci. Res. 83(6):1118–1127; 2006. [DOI] [PubMed] [Google Scholar]
- 10. Yang L.; Clinton J. M.; Blackburn M. L.; Zhang Q.; Zou J.; Zielinska-Kwiatkowska A.; Tang B. L.; Chansky H. A. Rab23 regulates differentiation of ATDC5 chondroprogenitor cells. J. Biol. Chem. 283(16):10649–10657; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chi S.; Xie G.; Liu H.; Chen K.; Zhang X.; Li C.; Xie J. Rab23 negatively regulates Gli1 transcriptional factor in a Su (Fu)-dependent manner. Cell. Signal. 24(6):1222–1228; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y.-J.; Wang Q.; Li W.; Huang X.-H.; Zhen M.-C.; Huang S.-H.; Chen L.-Z.; Xue L.; Zhang H.-W. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J. Gastroenterol. 13(7):1010–1017; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou Q.; Wu Y. H.; Grabsch H.; Zhu Y.; Leong S. H.; Ganesan K.; Cross D.; Tan L. K.; Tao J.; Gopalakrishnan V. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 68(12):4623–4630; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Denning K. M.; Smyth P. C.; Cahill S. F.; Finn S. P.; Conlon E.; Li J.; Flavin R. J.; Aherne S. T.; Guenther S. M.; Ferlinz A. A molecular expression signature distinguishing follicular lesions in thyroid carcinoma using preamplification RT-PCR in archival samples. Mod. Pathol. 20(10):1095–1102; 2007. [DOI] [PubMed] [Google Scholar]
- 15. Brand T. M.; Iida M.; Luthar N.; Starr M. M.; Huppert E. J.; Wheeler D. L. Nuclear EGFR as a molecular target in cancer. Radiother. Oncol. 108(3):370–377; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16. Eggenschwiler J. T.; Espinoza E.; Anderson K. V. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature 412(6843):194–198; 2001. [DOI] [PubMed] [Google Scholar]
- 17. Evans T. M.; Ferguson C.; Wainwright B. J.; Parton R. G.; Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic 4(12):869–884; 2003. [DOI] [PubMed] [Google Scholar]
- 18. Eggenschwiler J. T.; Bulgakov O. J.; Li T.; Anderson K. V. Mouse Rab23 regulates hedgehog signaling from Smoothened to Gli proteins. Dev. Biol. 290(1):1–12; 2006. [DOI] [PubMed] [Google Scholar]
- 19. Boehlke C.; Bashkurov M. A.; Krick T.; John A. K.; Nitschke R.; Walz G.; Kuehn E. W. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J. Cell Sci. 123(Pt. 9):1460–1467; 2010. [DOI] [PubMed] [Google Scholar]
- 20. Chi S.; Xie G.; Liu H.; Chen K.; Zhang X.; Li C.; Xie J. Rab23 negatively regulates Gli1 transcriptional factor in a Su(Fu)-dependent manner. Cell. Signal. 24(6):1222–1228; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang L.; Clinton J., Ml; Zhang Q.; Zou J.; Zielinska-Kwiatkowska A.; Tang B.; Chansky H. Rab23 regulates differentiation of ATDC5 chondroprogenitor cells. J. Biol. Chem. 283(16):10649–10657; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clayman G. L.; Lee J. J.; Holsinger F. C.; Zhou X.; Duvic M.; Elnaggar A. K.; Prieto V. G.; Altamirano E.; Tucker S. L.; Strom S. S. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 23(4):759–765; 2005. [DOI] [PubMed] [Google Scholar]
- 23. Jian Q.; Miao Y.; Tang L.; Huang M.; Yang Y.; Ba W.; Liu Y.; Chi S.; Li C. Rab23 promotes squamous cell carcinoma cell migration and invasion via integrin β1/Rac1 pathway. Oncotarget 7(5):5342–5352; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai Z. Z.; Xu L. B.; Cai J. L.; Wang J. S.; Zhou B.; Hu H. Inactivation of Rab23 inhibits the invasion and motility of pancreatic duct adenocarcinoma. Genet. Mol. Res. 14(1):2707–2715; 2015. [DOI] [PubMed] [Google Scholar]
- 25. Jiang Y.; Han Y.; Sun C.; Han C.; Ning H.; Zhi W.; Qiao Q. Rab23 is overexpressed in human bladder cancer and promotes cancer cell proliferation and invasion. Tumour Biol. 37(6):8131–8138; 2016. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y.; Zeng C.; Bao N.; Zhao J.; Hu Y.; Li C.; Chi S. Effect of Rab23 on the proliferation and apoptosis in breast cancer. Oncol. Rep. 34(4):1835–1844; 2015. [DOI] [PubMed] [Google Scholar]