Abstract
miRNAs play a pivotal role in the development and progression of osteosarcoma (OS). Previous studies indicated that miR-140 acts as a tumor suppressor in many cancers. However, its accurate expression and exact function in OS cells remain unknown. Herein, we demonstrated the lower expression of miR-140 in 40 paired OS tissues. Restoring miR-140 expression in OS cells had a marked effect on inhibiting cell proliferation and invasion, inducing cell apoptosis in vitro, and suppressing tumor growth in vivo. Moreover, a bioinformatics prediction indicated that the histone deacetylase 4 (HDAC4) is a target gene of miR-140 and is involved in miR-140-mediated suppressive effects. In conclusion, our findings show that miR-140 acts as a tumor suppressor in OS by targeting HDAC4.
Key words: miR-140, Osteosarcoma, Histone deacetylase 4 (HDAC4), Hypoxia-inducible factor 1α (HIF-1α)
INTRODUCTION
Osteosarcoma, also known as osteogenic sarcoma (OS), is the most common primary bone malignancy among children and adolescents1. Because of modern radiographic methods for diagnosis and effective chemotherapy regimens, the prognosis of OS has dramatically improved from a 10-year survival rate of 10%–20% in the 1960s to a rate of 60%–78% to date. However, about 30%–40% of patients relapse within 3 years after chemotherapy2. Thus, a better understanding of the pathological mechanisms of this malignancy is of crucial importance in reducing the morbidity and mortality of this devastating disease.
MicroRNAs (miRNAs) are classical small (∼22 nt), noncoding RNA molecules that regulate target genes by both translational inhibition and mRNA destabilization. By combining them with the complementary sequences in the 3′-untranslated region (3′-UTR) of target messenger RNAs, miRNAs can induce mRNA cleavage or translational repression. Increasing evidence shows that miRNAs play an important role in regulating a number of normal physiological and pathological processes, including cancers3–5. For OS, many reports have revealed that miRNAs play a crucial role during its progression and might provide new insights for its diagnosis, prognosis, and therapy6. Among these, miRNA-140 (miR-140) is an interesting member. It was reported to be associated with chemotherapy resistance of OS and colon cancer, as miR-140 is overexpressed in OS tumor xenografts and overexpression of miR-140 induced p53 and p21 expression accompanied with G1 and G2 phase arrest7. Its effect on other malignant phenotypes of OS has not been identified yet. We detect the expression of miR-140 in a cohort of OS specimens and explore its effect on the malignant phenotype of OS and its relative mechanisms.
MATERIALS AND METHODS
Clinical Samples
Forty OS tumor samples and normal tissue samples adjacent to OS were collected with informed consents between 2009 and 2012 from the Department of Orthopedics, The First Affiliated Hospital of Nanchang University (P.R. China), which were stored in liquid nitrogen. Clinical and pathological information was accessed from patient medical records and pathology reports. No patients had any therapy performed before recruitment to this research. The study was approved by The First Affiliated Hospital of Nanchang University Institutional Ethics Committee.
Cell Lines and Cell Culture
Human osteosarcoma cell lines (HOS and U2) were purchased from the Cell Bank of the Chinese Academy of Medical Sciences (Shanghai, P.R. China). Cells were cultured in the RPMI-1640 medium (Gibco; Fisher Scientific, Hampton, NH, USA) with 10% fetal bovine serum (Biological Industries, Israel) and incubated in a humidified atmosphere of 5% CO2 at 37°C.
Cell Transfection
miR-140 mimic, scramble mimic, siRNA (specific for HDAC4), and siRNA-control oligonucleotides were all purchased from Dharmacon, Inc. (Lafayette, CO, USA). All oligonucleotides were transfected into HOS and U2 cells to a final concentration of 50 nM using Dhamafect 1 (Dharmacon) in accordance with specifications. The medium was changed after 6 h of transfection; cells were cultured for 48 h and harvested for further experiments.
RNA Extraction and RT-PCR
Total RNA was extracted from the cells, and tissues were harvested using TRIzol (Invitrogen, Carlsbad, CA, USA) in accordance with specifications. The RNA was quantified by absorbance at 260 nm. To measure the expression level of miR-140 and HDAC4, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green (TransGen Biotech, Beijing, P.R. China) in the Bio-Rad CFX96 RT-PCR system. The data were normalized to endogenous U6 snRNA and GAPDH, respectively. The forward and reverse primers used were as follows: miR-140, 5′-GCCGCAGTGGTTTTACCCT-3′ and 5′-CAGTGCAGGGTCCGAGGT-3′; U6, 5′-CTCGCTTCGGCAGCACATATACT-3′ and 5′-ACGCTTCACGAATTTGCGTGTC-3′; HDAC4, 5′-AGGAGAAGGGCAAAGAG AGT-3′ and 5′-GAGGGTCGCTGGAAATGC-3′; GAPDH, 5′-TCAACGA CCACTTTGTCAAGCTCA-3′ and 5′-GCTGGTGGTCCAGGGGTCTTACT-3′. The 2−ΔΔCt method was used in the analysis of the PCR data.
Vector Construction and Luciferase Assays
To prove that miR-140 regulates the expression of the human gene HDAC4 by directly targeting its 3′-UTR, the full-length 3′-UTR of the HDAC4 mRNA was amplified from genomic DNA using primer pairs of HDAC4-UTR-F/R (forward primer: 5′-GGAAGCCAAGCACACTCTG-3′; reverse primer: 5′-TTCACCAGGCAACAAGGGT-3′) and then cloned in between the NotI and XbaI sites of the pGL-3 vector (Promega, Madison, WI, USA). Mutations of the HDAC4 3′-UTR sequence were created using a QuickChange Site-Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA). A luciferase reporter construct containing the miR-140 consensus target sequence served as a positive control. Before transfection, about 1 × 105 cells/well were seeded into 24-well plates for 24 h. Cells were transfected with the pGL-3 firefly luciferase reporter (50 ng/well), pRL-TK Renilla luciferase reporter (10 ng/well), and the miR-140 mimic (50 nM). The pRL-TK vector served as the internal control. All transfections were carried out in triplicate with Lipofectamine 2000 (Invitrogen). Cell lysates were prepared using passive lysis buffer (Promega) 48 h after transfection, and luciferase activity was measured using the Dual-Luciferase Reporter Assay (Promega). Results were normalized to the Renilla luciferase.
Cell Proliferation Assays
To measure proliferation rates with the effect of the miR-140 mimic, cells were seeded into 96-well plates at 0.5 × 104 cells/well after transfection for 24 h. Cells were incubated in 10% cell counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) and diluted in normal culture medium at 37°C until visual color conversion occurred. The proliferation rate was determined at 0, 24, 48, and 72 h after cells were seeded into 96-well plates, and quantification was done on a microtiter plate reader according to the manufacturer’s instruction. All experiments were performed in triplicate.
Cell Apoptosis Analysis
Apoptosis assay was performed on HOS and U2 cell lines 48 h after transfection, using the Annexin-V-FITC Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction and then analyzed by fluorescence activated cell sorting (FACS). All experiments were repeated in triplicate.
Cell Invasion Assays
The Transwell migration chambers (8-mm pore filter) were coated with Matrigel (BD Biosciences), incubated at 37°C for 4 h, and inserted into 24-well plates. After 24 h of transfection, 2 × 105 cells were suspended in serum-free medium and added to the upper chamber. The medium containing 20% FBS was added to the lower chamber as a chemoattractant. After 24 h, noninvasive cells on the upper surface of the chamber were gently removed with a cotton swab. Invasive cells located on the lower surface were stained with crystal violet (0.05%), air dried, and photographed. All experiments were run in triplicate.
Western Blot
After 48 h of transfection, cells were harvested with PBS at 4°C and lysed on ice in cold-modified radioimmunoprecipitation buffer supplemented with protease inhibitors. Protein concentration was measured by the BCA Protein Assay Kit (Beyotime Biotechnology, Jiangsu, P.R. China), and equal amounts of protein were analyzed by SDS-PAGE. Gels were electroblotted onto nitrocellulose membranes (ED Millipore, Billerica, MA, USA). Membranes were blocked for 1 h with 5% fat-free dry milk in Tris-buffered saline containing 0.1% Tween 20 and incubated at 4°C overnight with primary antibody. Detection was measured using peroxidase-conjugated secondary antibodies and the enhanced chemiluminescence system (ECL) (ED Millipore). Primary antibodies used were GAPDH, HDAC4, HIF-1 (Cell Signaling Technology, Danvers, MA, USA). Experiments were repeated three times.
In Vivo Assays
Nude mice xenograft model assays were performed according to institutional guidelines. HOS viable cells (5 × 106) were transplanted subcutaneously into the posterior flanks of 5-week-old nude mice, with five mice per group. When transplantation volume reached 100 mm3, miR-140 mimic and scramble mimic were mixed with Lipofectamine 2000 (Invitrogen) solution (100 nmol mimic in 100 μl of total volume), and then intratumor injections were performed. The tumors were injected every 3 days for a total of seven times. Tumor sizes were measured after 7 days from injection and then every 3 days. Mice were euthanized 28 days after injection, and tumors were weighed after necropsy. Calculation of tumor volume was determined by length × width2 × 1/2.
Immunohistochemistry
Mice xenografts were made into paraffin sections (5 μm) and pretreated at 56°C for 2 h and then deparaffinized. Antigen recovery was carried out before application of the primary antibodies (HDAC4; 1:1,000; Boster, P.R. China) overnight at 4°C. Thereafter, slides were incubated with the secondary antibody conjugated to horseradish peroxidase for 2 h at room temperature (1:100; ZSGB, P.R. China). The activity of horseradish peroxidase was detected using the Liquid DAB+ Substrate Chromogen System (ZSGB). Finally, sections were counterstained with hematoxylin and photographed under the positive position microscope.
Statistical Analysis
Data were shown as the mean ± SD of no less than three independent experiments. Statistical analysis was carried out using the SPSS 18.0 software (IBM, North Castle, NY, USA). Student’s t-test was used for comparisons between two groups. A value of p ≤ 0.05 was considered to be significant.
RESULTS
miR-140 Was Downregulated in OS Tissues
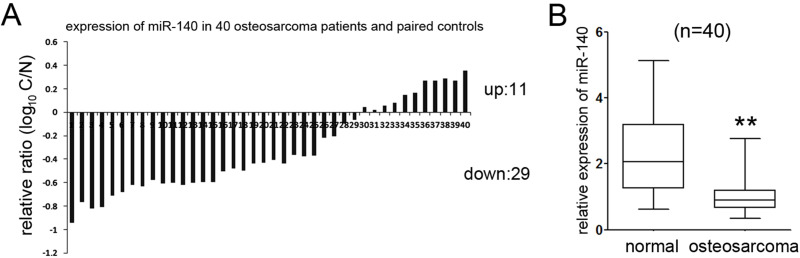
To analyze the putative effects of miR-140 on OS, we measured the level of miR-140 in 40 paired OS tissues and the adjacent nonneoplastic tissues using qRT-PCR and found that 72.5% (29 out of 40) OS tissues showed a lower level of miR-140 relative to the adjacent nontumor tissues (Fig. 1A). Moreover, statistical analysis identified that the average level of miR-140 in OS tissues was significantly attenuated compared with normal tissues (Fig. 1B).
Figure 1.
miR-140 was downregulated in OS specimens. (A) qRT-PCR analysis of the expression of miR-140 in 40 paired of OS tissues; 72.5% of the patients exhibited the underexpression of miR-140 relative to normal tissues. (B) Statistical analysis of the relative expression of miR-140 in OS tissues and adjacent normal regions. Compared with normal regions, the expression level of miR-140 in OS tissues was significantly lower. **p < 0.01.
Overexpression of miR-140 Inhibited Cell Proliferation and Invasion of HOS and U2 Cells
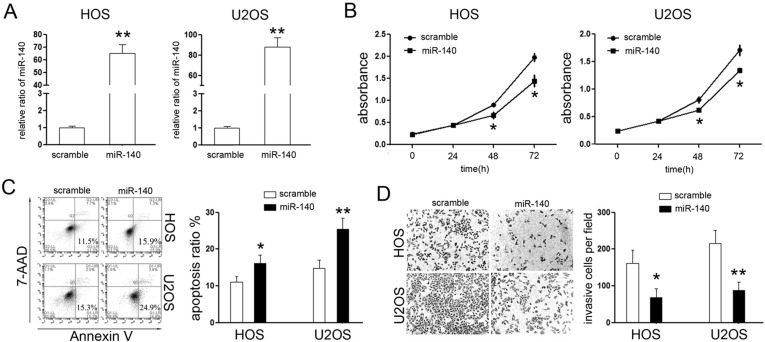
The downregulated expression of miR-140 suggested the ts-miRNA role of miR-140 in OS. Thus, we further explored its effects on OS cells. First, we sought to restore its expression in HOS and U2 cells by transient transfection with the miR-140 mimic, and cells transfected with the scramble mimic were used as a control. Upon transfection, the expression of miR-140 was about 65-fold higher in HOS cells and 85-fold higher in U2 cells compared with relative scramble control group (Fig. 2A).
Figure 2.
miR-140 suppressed the malignant phenotype of OS cells in vitro. (A) qPCR analysis of miR-140 in HOS and U2 cells 48 h after transfection of the miR-140 mimic. The expression of miR-140 in HOS and U2 cells transfected with miR-140 mimics was upregulated. (B) Cellular viability assay was detected by CCK-8 assay. (C) Analysis of apoptosis was performed by fluorescence activated cell sorting (FACS) after transfection at 48 h and showed that miR-140 induced early apoptosis of OS cells. (D) Cell invasion assay was detected by a Matrigel invasion chamber. Restoring the expression of miR-140 reduced cells invasion through the chamber (100×). *p < 0.05; **p < 0.01.
Next, CCK-8 proliferation assays were performed to detect its effect on the cell proliferative rate of OS cells. As shown in Figure 2B, overexpression of miR-140 significantly inhibited the proliferative rates of HOS and U2 cells. To demonstrate whether miR-140-induced inhibition of proliferation might be due to induction of cell death, the number of apoptotic fractions of HOS and U2 cells following transfection was examined using phycoerythrin (PE)-annexin V staining. As expected, fewer early apoptotic cells were detected in the scramble mimic group, while treatment with the miR-140 mimic increased the percentage of early apoptotic cells (Fig. 2C), which means overexpression of miR-140 induced the cell apoptosis of OS cells in vitro. Last, the Matrigel invasion chamber assays were performed to explore the effects of miR-140 on the invasive capability of OS cells. As expected, exogenetic overexpression of miR-140 can effectively suppress the invasive capacity of HOS and U2 cells (Fig. 2D). These results suggested that restoration of miR-140 markedly suppressed the proliferation and invasion of OS cells.
miR-140 Targets HDAC4 and Suppresses the Expression of HIF-1α
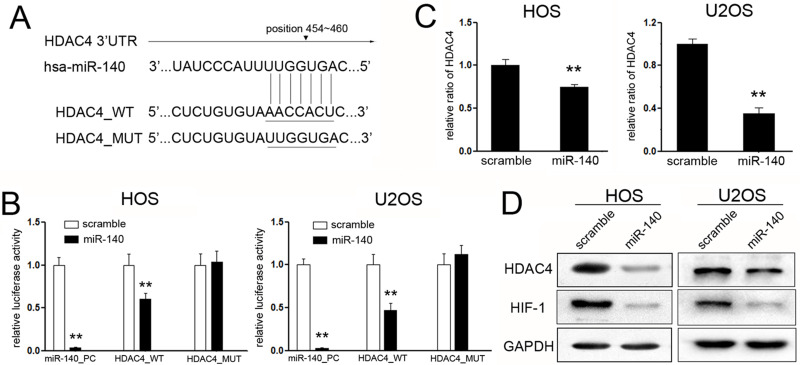
To investigate the mechanisms involved in miR-140-mediated tumor suppression of OS, putative targets of miR-140 were searched using target prediction programs, TargetScan and miRanda. Among the genes being predicted, histone deacetylase 4 (HDAC4) attracted our attention most (Fig. 3A). To identify the target role of HDAC4, dual-luciferase reporter assays were performed. The miR-140 mimic, rather than the scramble mimic, significantly suppressed the luciferase activity of the reporter gene containing the wild-type 3′-UTR of HDAC4 but did not affect the activity of the gene containing the mutant 3′-UTR in HOS and U2 cells (Fig. 3B).
Figure 3.
Histone deacetylase 4 (HDAC4) is a direct target of miR-140 in OS cells. (A) Schematic representation of HDAC4 3′-UTR showing the putative binding site of miR-140. (B) Relative luciferase activity of the indicated HDAC4 reporter construct in HOS and U2 cells is shown. The miR-140 mimics, but not the scrambled mimics, markedly suppressed the luciferase activity of the reporter gene containing wild-type 3′-UTR of HDAC4 (HDAC4 WT), but not the mutant 3′-UTR (HDAC4 MUT), in HOS and U2 cell. (C) qPCR detected the effects of miR-140 on the expression of HDAC4 in HOS and U2 cells. Exogenetic overexpression of miR-140 significantly decreased HDAC4 transcripts. (D) Western blotting was used to examine the expression of HDAC4 and its downstream targets HIF-1α upon transfection with miR-140 mimics and scramble mimics. Ectopic expression of miR-140 inhibited the expression of HDAC4 and HIF-1α in HOS and U2 cells. **p < 0.01.
To further confirm that HDAC4 is a target gene of miR-140, the mRNA and protein levels of HDAC4 were detected upon transfection with the miR-140 or scramble mimic in HOS and U2 cells. Obviously, the mRNA level of HDAC4 was significantly decreased after transfection (Fig. 3C). The same results were observed in the HDAC4 protein levels of both OS cells upon transfection (Fig. 3D). Moreover, since hypoxia-inducible factor 1α (HIF-1α) was reported to be involved in HDAC4 inhibition-mediated increase of glycolysis and resistance to docetaxel chemotherapy, this might be an important reason for OS progression8; we also detected the effects on the expression of HIF-1α. As expected, accompanied with suppressed expression of HDAC4, the expression of HIF-1α was significantly suppressed upon treatment with miR-140 (Fig. 3D), which means that HIF1α might participate in the suppressive effects of miR-140 by targeting HADC4.
miR-140 Suppresses Cell Function by Regulating HDAC4
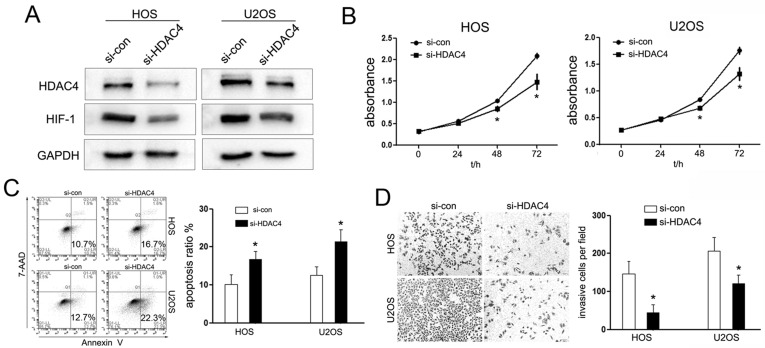
HDAC4 is a kind of class II HDACs, which play a global role in the regulation of gene transcription, cell growth, survival, and proliferation. On the basis of the research mentioned, we speculated that miR-140 might perform its biological function by downregulating HDAC4 expression. To validate this hypothesis, we knocked down the expression of HDAC4 using RNA interference and further observed its effects on OS cell growth. The protein level of HDAC4 was identified upon transfection with si-HDAC4 (Fig. 4A). Interestingly, knockdown of HDAC4 inhibited the proliferation of HOS and U2 cells, which is in accord with the effects of miR-140 overexpression (Fig. 4B). In addition, we observed that si-HDAC4 induced cell apoptosis and impaired the cell-invasive capacity of HOS and U2 cells (Fig. 4C and D). These results indicated that HDAC4 acts as an oncogene in OS carcinogenesis.
Figure 4.
HDAC4 siRNA (si-HDAC4) inhibited tumorous phenotypes of OS cells. (A) Western blot analysis indicated that the protein levels of HDAC4 and its downstream gene HIF-1α were all downregulated. GAPDH was used as a loading control. (B) Interference HDAC4 expression inhibited the proliferation of HOS and U2 cells detected by the CCK-8 assay at 72 h. (C) Cell apoptosis of HOS and U2 cells treated as described 48 h after transfection with HDAC4 siRNAs or siRNA-control; si-HDAC4 induced OS cell apoptosis. (D) Transwell assays were conducted to detect the effects on cell invasion of HOS and U2 cells treated with si-HDAC4. si-HDAC4 decreased the cell-invasive ability of HOS and U2 cells. *p < 0.05.
miR-140 Inhibited the Growth of HOS-Engrafted Tumors
The tumorigenic role of HDAC4 combined with our substantial evidence that miR-140 inversely regulates HDAC4 expression indicates the antitumor effects of miR-140. To verify the putative therapeutic effects of miR-140 in OS development in vivo, 5 × 106 HOS cells were subcutaneously transplanted in the posterior flank of BALB/c nude mice. When tumors reached 100 mm3, the miR-140 mimic or the scrambled mimic was injected into the tumors directly. The tumors were injected a total of seven times per 3 days. The tumor growth curve indicated that miR-140 inhibited the growth of OS-engrafted tumors relative to scrambled group (Fig. 5A). Nude mice were euthanized after 4 weeks, and xenografts were trimmed out. The tumor sizes of the miR-140 mimic group were much smaller than those of the scramble group (Fig. 5B). Consistent with the tumor growth curve, the tumor weights of the miR-140 mimic group were significantly decreased compared with scramble group (Fig. 5C).
Figure 5.
miR-140 inhibited OS growth in vivo. (A) Mice were divided into two groups with five mice in each group. The graph represents tumor sizes at the indicated days, and the tumor growth curve indicated that miR-140 can inhibit the OS-engrafted tumorous growth relative to the scrambled group. (B, C) The transplants were resected and weighed after 28 days of the OS cell injection. (D) In the subcutaneously transplanted tumor, the miR-140 level of the miR-140 mimic group was overexpressed compared with that of the scramble group as detected by qPCR. (E) Immunohistochemistry analysis of HDAC4 in tumors from xenograft mice, and HDAC4 was decreased in the miR-140 group compared with scramble controls. *p < 0.05, **p < 0.01.
The expression of miR-140 was significantly upregulated in the tumors of the miR-140-treated group (Fig. 5D), while the expression of HDAC4 showed the opposite trend, which was significantly suppressed in the miR-140-treated group (Fig. 5E). Last, we detected the expression of HIF-1α upon treatment of miR-140. As expected, the miR-140-treated group showed suppressed expression of HIF-1α (Fig. 5E). Taken together, we demonstrated that the miR-140 mimic could inhibit OS proliferation in vivo and might provide a novel method for OS therapy.
DISCUSSION
Accumulating evidence has identified that unrevealed molecular factors, particularly noncoding RNAs, play an important role in tumorigenesis or tumor progression by targeting various genes that were involved in the biological progression. In this article, we evaluated the expression of miR-140 in OS patients and explored the biological role and regulatory mechanisms of miR-140 in OS cells.
miR-140 is a kind of conservative gene that participates in many biological and pathological progressions, including chondrogenic differentiation, adipogenesis, cardiomyocyte apoptosis, and skeletal development, as well as cancer cell growth, migration, and invasion. It has been reported to function as a tumor suppressor in many tumors, such as in the study by Li et al., who showed that miR-140 was decreased in the esophageal tissues and overexpression of miR-140 repressed cell invasion by targeting Slug 9. In lung cancer cells, reexpressing miR-140 can significantly inhibit proliferation and promote cell apoptosis10. For OS, miR-140 was associated with the chemotherapy resistance of osteosarcoma. miR-140 induced p53 and p21 expression accompanied with G1 and G2 phase arrest of OS cells. We identified the suppressed expression of miR-140 in OS tissues. Moreover, consistent with previous results, we found that restored expression of miR-140 inhibited the cell proliferation of HOS and U2 cells. Combined with suppressed cell proliferation, miR-140 induced cell apoptosis and inhibited cell invasion of OS at the same time, which identified the tumor suppressor role of miR-140 in OS. Our study further concludes that the effects of miR-140 on the biological functions of OS cells all suggest the tumor-suppressive role of miR-140 in OS.
To find the putative mechanisms involved in miR-140-mediated suppressive effects, the putative target genes of miR-140 were searched. Among these genes, HDAC4 attracted our attention most. Transfection with miR-140 caused a marked reduction in luciferase activity by the luciferase expression constructs, which carry the target HDAC4 fragment compared with the mutant constructs that lack the binding site. HDAC4 is a kind of class II HDACs, which play a global role in the regulation of gene transcription, and thus their aberrant expression will lead to a series of pathological progression, including cancer development11,12. Chen et al. demonstrated that HDAC5 acts as an oncogene in OS and promotes OS development by upregulating the expression of Twist 1 13. Another study showed that HDAC9 is overexpressed in OS and that overexpression of HDAC9 promoted cell malignant phenotypes in U2OS and MG63 cells by repressing p53 transcription activity directly14. The function of HDAC4 in OS was further identified by the observation that HDAC4 knockdown induced cell growth retardation, apoptosis, and suppressed cell invasion, which paralleled the tumor-suppressive effects induced by miR-140 restoration. It suggested that HDAC4 could facilitate OS development. Combining that with previous research results, it suggested that HDAC4 partially participates in miR-140-mediated tumorigenesis.
A hypoxic microenvironment is common in many types of solid tumors, including OS15,16. Since a previous work reported that HDAC4 inhibition could reduce the hypoxia-related increase in glycolysis and resistance to docetaxel chemotherapy8, we further explored whether it performs that function in miR-140-mediated suppressive effects. We found that transfection with miR-140 or si-HDAC4 both suppressed the expression of HIF-1α, which means HIF-1α might perform a function in miR-140-mediated suppressive effects. However, more experiments are needed to prove whether it performs a function in the suppressive effect and resistance to chemotherapy.
Last, we found that miR-140 inhibited tumorigenicity in a xenograft model in OS. We also observed the tumor-suppressive role of miR-140 in vivo by injecting miRNA mimics directly and finding that miR-140 can reduce the growth of OS cells significantly. Further immunohistochemistry analysis demonstrated the negative regulation of miR-140 to HDAC4 and HIF-1α in xenograft tumor tissues, suggesting that miR-140 might be a new potential therapeutic target in OS.
Our findings strongly demonstrate that miR-140 functions as a tumor suppressor in OS cells by, at least partially, suppressing HDAC4. To the best of our knowledge, this is the first comprehensive study to explore the role of miR-140 in OS. The combined miRNA-based and epigenetic treatment may be a novel potential therapeutic target for OS.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81460405 and 81260399); Key Program of Jiangxi Provincial Department of Science and Technology (Grant No. 20152ACB21024); Young Scientist Program of Jiangxi Province (Grant No. 20133BCB23027); Program of Jiangxi Provincial Department of Science and Technology (Grant No. 20132BBG70068); and Research Program of Health and Family Planning Commission of Jiangxi Province (Grant Nos. 2015587 and 20155110).
REFERENCES
- 1. Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61–6. [DOI] [PubMed] [Google Scholar]
- 2. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–27. [DOI] [PubMed] [Google Scholar]
- 3. Ell B, Kang Y. MicroRNAs as regulators of bone homeostasis and bone metastasis. Bonekey Rep. 2014;3:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene 2006;25:6163–9. [DOI] [PubMed] [Google Scholar]
- 5. Kala R, Peek GW, Hardy TM, Tollefsbol TO. MicroRNAs: An emerging science in cancer epigenetics. J Clin Bioinforma 2013;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: Review. Front Pediatr. 2015;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, Fodstad O, Ju J. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 2009;28:4065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geng H, Harvey CT, Pittsenbarger J, Liu Q, Beer TM, Xue C, Qian DZ. HDAC4 protein regulates HIF1alpha protein lysine acetylation and cancer cell response to hypoxia. J Biol Chem. 2011;286:38095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W, Jiang G, Zhou J, Wang H, Gong Z, Zhang Z, Min K, Zhu H, Tan Y. Down-regulation of miR-140 induces EMT and promotes invasion by targeting Slug in esophageal cancer. Cell Physiol Biochem. 2014;34:1466–76. [DOI] [PubMed] [Google Scholar]
- 10. Yuan Y, Shen Y, Xue L, Fan H. miR-140 suppresses tumor growth and metastasis of non-small cell lung cancer by targeting insulin-like growth factor 1 receptor. PLoS One 2013;8:e73604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beumer JH, Tawbi H. Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr Clin Pharmacol. 2010;5:196–208. [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Qin G, Zhao TC. HDAC4: Mechanism of regulation and biological functions. Epigenomics 2014;6:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Xia J, Yu YL, Wang SQ, Wei YB, Chen FY, Huang GY, Shi JS. HDAC5 promotes osteosarcoma progression by upregulation of Twist 1 expression. Tumour Biol. 2014;35:1383–7. [DOI] [PubMed] [Google Scholar]
- 14. Zhao YX, Wang YS, Cai QQ, Wang JQ, Yao WT. Up-regulation of HDAC9 promotes cell proliferation through suppressing p53 transcription in osteosarcoma. Int J Clin Exp Med. 2015;8:11818–23. [PMC free article] [PubMed] [Google Scholar]
- 15. Koh MY, Spivak-Kroizman TR, Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15–34. [DOI] [PubMed] [Google Scholar]
- 16. Masoud GN, Li W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015;5:378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]