Abstract
MicroRNAs (miRNAs) are small conserved RNAs regulating specific target genes in posttranscriptional levels. They have been involved in multiple processes of tumor progression, including cell proliferation. miR-214-5p (also miR-214*) is a newly identified miRNA, and its functions are largely unknown. In this study, we explore the role of miR-214-5p in the proliferation and invasion of human osteosarcoma (OS) cells. The results showed that miR-214-5p was sharply reduced in OS tissues and cell lines, compared with normal tissues and cell lines. In addition, the miR-214-5p mimic greatly increased the miR-214-5p level and significantly decreased the proliferation and invasion of HOS and G293 OS cells. In contrast, the miR-214-5p inhibitor had a completely opposite effect on the miR-214-5p level, cell proliferation, and cell invasion. Moreover, bioinformatics and luciferase reporter gene assays confirmed that miR-1908 targeted the mRNA 3′-UTR region of ROCK1, a characterized tumor promoter in OS. In conclusion, miR-214-5p was identified as a new tumor suppressor, which directly targeted ROCK1 and suppressed proliferation of human OS cells.
Key words: miR-214-5p, Cell proliferation, Human osteosarcoma (OS), ROCK1
INTRODUCTION
Osteosarcoma (OS) is a common primary bone cancer, which is most prevalent in children and young adults (1,2). OS commonly occurs in the long bones of the extremities near the metaphyseal growth plates. Although death rates for OS have been declining in recent years, the overall 5-year survival rate for OS is below 70%, even with a high proportion of amputation (2–4). Therefore, it is still urgent to explore reliable therapeutic targets and approaches for OS treatment.
Increasing evidence shows that microRNAs (miRNAs) are involved in nearly all types of cancers by posttranscriptionally regulating specific tumor suppressors or promoters. Aberrant expression and dysregulation of miRNAs have also been shown to significantly contribute to cancer growth and metastasis in OS patients (5–9). For example, the expression level of miR-191 was increased in OS tissues in comparison with the adjacent normal tissues, and overexpression of miR-191 promoted cell proliferation in Saos-2 and MG62 cells by directly suppressing the tumor-suppressor checkpoint kinase 2 (5). miR-301a was induced by doxorubicin in a time-dependent manner in OS cells, which modulated doxorubicin resistance by targeting AMP-activated protein kinase α1 (8). Even though miRNAs have been extensively investigated in cancers, their roles and mechanisms in OS progression are not thoroughly known.
In this study, we explore the role of miR-214-5p (also miR-214*), a very recently identified miRNA, in the progression of human OS. miR-214-5p expression was first detected in OS and normal adjacent tissues, as well as in normal human osteoblast and OS cell lines. We found miR-214-5p to be sharply reduced in OS tissues and cell lines. Then either miR-214-5p mimic or miR-214-5p inhibitor was transfected into the HOS and G293 OS cell lines, and cell proliferation was detected. Moreover, bioinformatics and luciferase reporter gene assays were used to confirm its target gene.
MATERIALS AND METHODS
Ethical Statements
This study protocol was approved by the Ethics Committee of the China–Japan Union Hospital (China). The subjects and their guardians were previously informed about the experimental details, and they gave written consent.
Tissue Samples
OS and matched normal adjacent tissues were isolated from a total of 48 adolescent OS patients who underwent tumor resection during 2012 to 2015 in our hospital. Patients who received chemo- or radiotherapy were excluded from this study.
Cell Culture and Transfection
Human normal osteoblast cell line hFOB and human OS cell lines, including HOS, MG63, G293, SAOS2, and U2OS, were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin (Gibco), and 100 U/ml streptomycin (Gibco) at 37°C in a humidified 5% CO2 incubator.
For transfection, HOS and G293 cells were subcultured in six-well plates. On reaching 70% of confluence, 60 μM of the oligo miR-214-5p mimic, 60 μM of the miR-214-5p inhibitor, or 60 μM of the oligo negative control (NC) was transfected into the cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The miR-214-5p mimic, inhibitor, and NC were designed and synthesized by Ribobio Co. Ltd. (Guangzhou, P.R. China).
Cell Proliferation
The cells were seeded in 12-well culture plates at 5 × 104/well posttransfection for 48 h. Then cell proliferation was evaluated using the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer’s instructions.
In Vitro Invasion Assay
Transwell invasion assay was used to detect cell invasion. Cells (5 × 104) with serum-free medium were plated in the top chamber with a noncoated membrane (24-well insert; 8 μm; Corning). Medium containing 10% serum was used as a chemoattractant in the lower chamber. After incubation for 24 h, a cotton swab was used to remove the nonmigrated cells in the upper chamber, and the filters were individually stained with 2% crystal violet. The invasive cells were captured under a light microscope (100×; Olympus IX70; Olympus Corporation, Osaka, Japan) and counted.
Real-Time Quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and then applied to synthesize cDNA. Real-time quantitative polymerase chain reactions (qPCRs) were carried on in a 25-μl system, using SYBR Premix Ex Taq (TaKaRa, Dalian, P.R. China), 0.4 mM of primers, and 200 ng of cDNA template. The primers of miR-214-5p and U6 RNA (as internal reference) were designed and produced by Ribobio. PCR amplification cycles were performed using the SYBR Premix Ex Taq II Kit (Invitrogen). The reactions were initially denatured at 95°C for 1 min and followed by 35 cycles of 95°C for 15 s and 60°C for 1 min. The transcript abundance was calculated using the 2−ΔΔCt method.
Western Blotting
Thirty micrograms of total protein from each sample was separated by 12% SDS-PAGE. The protein was electrotransferred onto a PVDF membrane (Millipore, Billerica, MA, USA) for immunoblotting analysis. The primary antibodies anti-Rho-associated, coiled coil-containing protein kinase 1 (ROCK1) (1:300; Abcam) and anti-GAPDH (1:500; Abcam) were used to incubate on the PVDF membrane overnight and then incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. Proteins were detected with an ECL kit (Millipore) and analyzed with Quantity One Analysis Software (Bio-Rad).
3′-UTR Luciferase Gene Reporter Assay
The 3′-UTR region of human ROCK1 mRNA was amplified by PCR, and the sequences were purified and inserted into the psiCHECK™-2 Vector (Promega, Madison, WI, USA). The NC or miR-214-5p mimic was respectively cotransfected with psiCHECK-ROCK1 3′-UTR into the HEK293 cells using the X-tremeGENE 9 DNA Transfection Reagent (Roche, Basel, Switzerland). The cells were then incubated for 48 h, and luciferase activity was measured using a Microplate Reader (Berthold Technologies, Berlin, Germany).
Statistical Analysis
All data were obtained from at least three independent experiments. The data were expressed as means ± SEM. Statistics were calculated with SPSS 22.0. Multiple comparisons were assessed by one-way ANOVA followed by Dunnett’s tests. The difference between groups was considered statistically significant if p < 0.05.
RESULTS
miR-214-5p Was Markedly Reduced in Human OS Tissues and Cell Lines
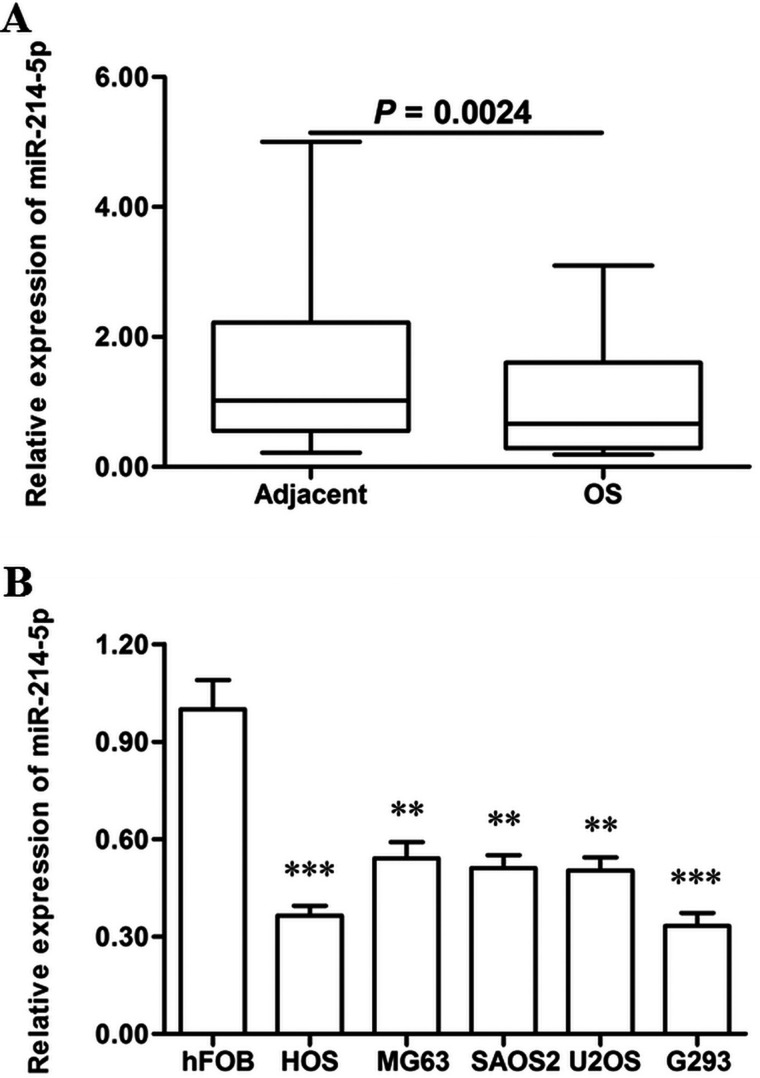
The expression levels were detected in OS tissues and matched normal adjacent tissues. Real-time qPCR analysis showed that miR-214-5p was reduced by about 40% in the OS tissues (p < 0.01) (Fig. 1A). Then the miR-214-5p levels in hFOB human normal osteoblast cell line and five OS cell lines were also detected, including HOS, MG63, G293, SAOS2, and U2OS. The results showed that miR-214-5p was reduced by more than 40% in all the OS cell lines (p < 0.01), and by more than 60% in HOS and G293 cells (p < 0.001) (Fig. 1B). These data on miR-214-5p level changes suggested that miR-214-5p might have an effect on the progression of human OS.
Figure 1.
miR-214-5p was significantly downregulated in human OS tissues and cell lines. OS tissues and matched normal adjacent tissues were sampled from 48 adolescent OS patients. The expression levels of miR-214-5p were detected in these tissues with real-time qPCR. (A) miR-214-5p was significantly downregulated in OS tissues compared with the matched normal adjacent tissues. The expression levels of miR-214-5p were detected in hFOB human normal osteoblast cell line and five OS cell lines, including HOS, MG63, G293, SAOS2, and U2OS, with real-time qPCR. (B) miR-214-5p was significantly downregulated in OS cell lines compared with normal osteoblast cell line. **p < 0.01, ***p < 0.001 versus hFOB.
miR-214-5p Mimic Transfection Suppressed Proliferation and Invasion of Human OS Cells
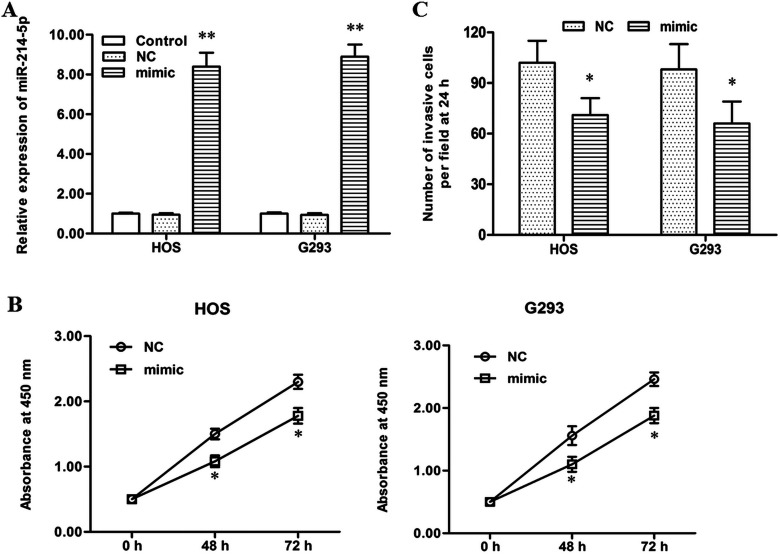
To explore the role in progression of human OS, the NC or miR-214-5p mimic was transfected into HOS and G293 cells. After incubation for 48 h, miR-214-5p expression, cell proliferation, and cell invasion were detected. Real-time qPCR analysis showed that miR-214-5p expression was increased by more than sevenfold by the miR-214-5p mimic transfection (p < 0.01) (Fig. 2A). CCK-8 and Transwell invasion assays displayed that the miR-214-5p mimic significantly suppressed proliferation and migration of HOS and G293 cells (p < 0.05) (Fig. 2B and C).
Figure 2.
miR-214-5p mimic transfection suppressed proliferation and invasion of HOS and G293 human OS cell lines. The NC or miR-214-5p mimic was transfected into HOS and G293 human OS cell lines. After incubation for 48 h, miR-214-5p expression was detected with real-time qPCR, cell proliferation was detected with the CCK-8 method, and cell invasion was detected with the Transwell invasion assay. (A) miR-214-5p expression was increased greatly by the miR-214-5p mimic transfection. (B) The miR-214-5p mimic significantly suppressed the proliferation of HOS and G293 cells. (C) The miR-214-5p mimic significantly suppressed the migration of HOS and G293 cells. *p < 0.01 versus Control.
miR-214-5p Inhibitor Transfection Promoted Proliferation and Invasion of HOS and G293 Cells
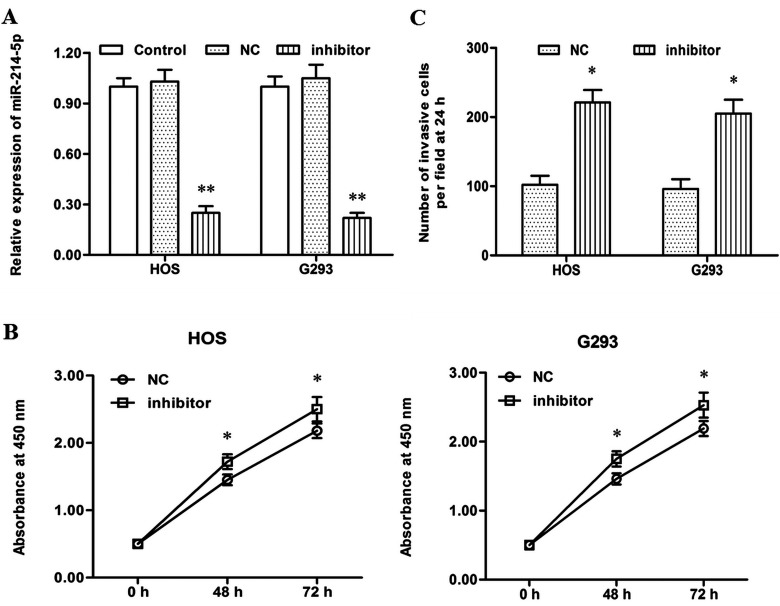
Then the NC or miR-214-5p inhibitor was transfected into HOS and G293 cells. After incubation for 48 h, miR-214-5p expression, cell proliferation, and cell invasion were detected. In contrast to the results from the miR-214-5p mimic transfection, miR-214-5p expression was sharply decreased by miR-214-5p inhibitor transfection (p < 0.01) (Fig. 3A). The miR-214-5p inhibitor significantly promoted proliferation and migration of HOS and G293 cells (p < 0.05) (Fig. 3B and C). These data on the mimic and inhibitor transfection indicated that miR-214-5p had a negative effect on the proliferation and migration of OS cells.
Figure 3.
miR-214-5p inhibitor transfection increased proliferation and invasion of HOS and G293 human OS cell lines. The NC or miR-214-5p inhibitor was transfected into HOS and G293 human OS cell lines. After incubation for 48 h, miR-214-5p expression was detected with real-time qPCR, cell proliferation was detected with the CCK-8 method, and cell invasion was detected with the Transwell invasion assay. (A) The miR-214-5p level was sharply decreased by the miR-214-5p inhibitor transfection. (B) The miR-214-5p inhibitor significantly promoted the proliferation of HOS and G293 cells. (C) The miR-214-5p inhibitor significantly promoted the migration of HOS and G293 cells. *p < 0.01 versus Control.
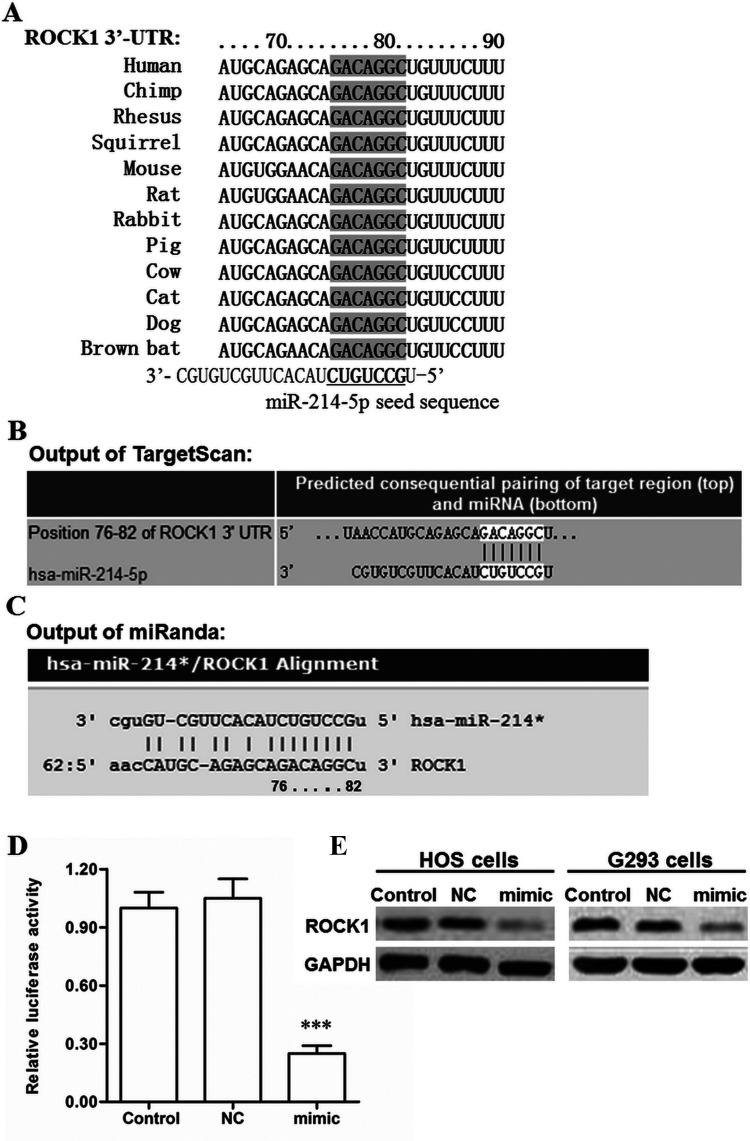
miR-214-5p Directly Targeted the ROCK1 mRNA and Suppressed Expression of the ROCK1 Protein
Next, we investigated the potential molecular mechanism from which miR-214-5p suppressed proliferation and migration of OS cells. Bioinformatics analysis was used to evaluate the target relationship between miR-214-5p and ROCK1. The sequence analysis showed that the 3′-UTR region of the ROCK1 mRNA sequences was conserved in humans and in mammals, and seven consecutive bases completely matched with the seed of miR-214-5p (Fig. 4A). The output of the TargetScan and miRanda online servers showed that miR-214-5p potentially targeted this region (Fig. 4B and C). Then a 3′-UTR luciferase reporter gene assay was used to validate their relationship. The results revealed that miR-214-5p markedly weakened the luciferase activity (Fig. 4D). To further validate that ROCK1 was a target of miR-214-5p, the NC or miR-214-5p mimic was transfected into HOS and G293 cells. After incubation for 48 h, expression of the ROCK1 protein was detected with Western blotting. The results indicated that the ROCK1 protein level was sharply reduced by the miR-214-5p mimic transfection (Fig. 4E). These data suggest that miR-214-5p suppressed proliferation and migration of OS cells by directly targeting the tumor promoter ROCK1.
Figure 4.
miR-214-5p suppressed ROCK1 expression by directly targeting the 3′-UTR of ROCK1 mRNA. (A) Alignment of hsa-miR-214-5p and ROCK1 mRNA sequences among various mammals. The outputs of the (B) TargetScan and (C) miRanda online servers on the potential targeting relationship between miR-214-5p and 3′-UTR region of ROCK1 mRNA. The NC mimic and miR-214-5p mimic were respectively cotransfected with the 3′-UTR dual-luciferase vector into HEK293 cells. After incubation for 48 h, the luciferase activity in each group was measured using a Microplate Reader. (D) The miR-214-5p mimic suppressed the luciferase activity. The miR-1908 mimic or inhibitor was transfected into HOS and G293 cells. After incubation for 48 h, the ROCK1 protein expression was detected with Western blotting. (E) The miR-214-5p mimic sharply suppressed the ROCK1 protein expression. ***p < 0.001 versus Control.
DISCUSSION
The precursor miR-214 was cleaved into two independent mature miR-214s, miR-214-5p, and miR-214-3p, by the endoribonuclease Dicer. miR-214-3p (also miR-214) is a well-characterized miRNA, which has been shown to induce cell survival and drug resistance in several cancers and play an important role in the determination of cell fate in multiple cell lineages (10–14). However, miR-214-5p was quite poorly reported, which was much less expressed than miR-214*. Until very recently, miR-214-5p was found to be upregulated in human and rodent fibrotic livers, compared with normal livers (15). In this study, we explored the role of miR-214-5p in the proliferation and invasion of human OS cells. We first found that miR-214-5p was sharply reduced in the OS tissues and cell lines compared with the normal tissues and cell lines, suggesting that miR-214-5p might have an effect on the progression of OS. Then the miR-214-5p mimic or miR-214-5p inhibitor was transfected into HOS and G293 OS cell lines. The MiR-214-5p mimic significantly decreased the proliferation and invasion of HOS and G293 OS cells. In contrast, the miR-214-5p inhibitor increased cell proliferation and invasion. Therefore, miR-214-5p played a negative role in the OS progression in vitro.
The human ROCK1 gene is located on human chromosome 18 with a specific location of 18q11.1, which translated a 1,354-amino acid, RhoA-activating serine/threonine kinase (16,17). ROCK1 has been proven to play key roles in cancer progression and, in particular, cell motility, metastasis, and angiogenesis (18–20). ROCK1 was recently identified as a therapeutic target in OS, for it was highly expressed in various OS tissues and cell lines, its knockdown decreased cell proliferation, and its high levels were strongly associated with poor outcomes of OS (21,22). Some miRNAs targeting ROCK1 have been reported to inhibit OS tumorigenesis. For instance, miR-340 was frequently downregulated in OS tumors and cell lines, and its downregulation in tumor tissues contributed to the OS progression and metastasis by targeting ROCK1 (23). Other miRNAs, like miR-335 and miR-145, have also been shown to inhibit the proliferation, migration, and invasion of OS cells (24–26). In the current study, our data on bioinformatics and luciferase reporter gene assays confirmed that miR-214-5p was also a ROCK1-targeting miRNA, which could be a rationale for miR-214-5p suppressing OS cell proliferation and invasion.
In conclusion, miR-214-5p was identified as a new tumor suppressor, which directly targeted ROCK1 and suppressed proliferation of human OS cells.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ottaviani G.; Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 152:3–13; 2009. [DOI] [PubMed] [Google Scholar]
- 2. Duchman K. R.; Gao Y.; Miller B. J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) program database. Cancer Epidemiol. 39:593–599; 2015. [DOI] [PubMed] [Google Scholar]
- 3. Flores R. J.; Kelly A. J.; Li Y.; Nakka M.; Lau C. C.; Hicks J.; Man T. K. A high level of circulating CXCL10 at initial diagnosis is associated with poor prognosis of osteosarcoma patients. Cancer Res. 75:5163–5163; 2015. [Google Scholar]
- 4. Qi Y.; Zhao C.; Li H.; Zhang B.; Tada K.; Abe H.; Tada M. Genetic variations in interleukin-6 polymorphism and the association with susceptibility and overall survival of osteosarcoma. Tumor Biol. 1–5; 2016. [DOI] [PubMed] [Google Scholar]
- 5. Huang Y. Z.; Zhang J.; Shao H. Y.; Chen J. P.; Zhao H. Y. MicroRNA-191 promotes osteosarcoma cells proliferation by targeting checkpoint kinase 2. Tumor Biol. 36:6095–6101; 2015. [DOI] [PubMed] [Google Scholar]
- 6. Grilli A.; Sciandra M.; Terracciano M.; Picci P.; Scotlandi K. Integrated approaches to miRNAs target definition: Time-series analysis in an osteosarcoma differentiative model. BMC Med. Genomics 8:34; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han K.; Chen X.; Bian N.; Ma B.; Yang T.; Cai C.; Fan Q.; Zhou Y.; Zhao T. MicroRNA profiling identifies MiR-195 suppresses osteosarcoma cell metastasis by targeting CCND1. Oncotarget 6:8875–8889; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y.; Duan G.; Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem. Biophys. Res. Commun. 459:367–373; 2015. [DOI] [PubMed] [Google Scholar]
- 9. Nugent M.: MicroRNA and bone cancer. Adv. Exp. Med. Biol. 889:201–230; 2015. [DOI] [PubMed] [Google Scholar]
- 10. Yang H.; Kong W.; He L.; Zhao J. J.; O’Donnell J. D.; Wang J.; Wenham R. M.; Coppola D.; Kruk P. A.; Nicosia S. V. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68:425–433; 2008. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X. J.; Ye H.; Zeng C. W.; He B.; Zhang H.; Chen Y. Q. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J. Hematol. Oncol. 3:46; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan Q.; Wang X.; Gong W.; Ni L.; Chen C.; He X.; Chen F.; Yang L.; Wang P.; Wang D. W. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS One 7:e31518; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X.; Guo B.; Li Q.; Peng J.; Yang Z.; Wang A.; Li D.; Hou Z.; Lv K.; Kan G. MiR-214 targets ATF4 to inhibit bone formation. Nat. Med. 19:93–100; 2013. [DOI] [PubMed] [Google Scholar]
- 14. Flynt A. S.; Li N.; Thatcher E. J.; Solnica-Krezel L.; Patton J. G. Zebrafish miR-214 modulates hedgehog signaling to specify muscle cell fate. Nat. Genet. 39:259–263; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iizuka M.; Ogawa T.; Enomoto M.; Motoyama H.; Yoshizato K.; Ikeda K.; Kawada N. Induction of microRNA-214-5p in human and rodent liver fibrosis. Fibrogenesis Tissue Repair 5:12; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dennehey B. K.; Gutches D. G.; McConkey E. H.; Krauter K. S. Inversion, duplication, and changes in gene context are associated with human chromosome 18 evolution. Genomics 83:493–501; 2004. [DOI] [PubMed] [Google Scholar]
- 17. Shimokawa H.; Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol. Sci. 28:296–302; 2007. [DOI] [PubMed] [Google Scholar]
- 18. Zheng B.; Liang L.; Wang C.; Huang S.; Cao X.; Zha R.; Liu L.; Jia D.; Tian Q.; Wu J. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin. Cancer Res. 17:7574–7583; 2011. [DOI] [PubMed] [Google Scholar]
- 19. Lochhead P.; Wickman G.; Mezna M.; Olson M. Activating ROCK1 somatic mutations in human cancer. Oncogene 29:2591–2598; 2010. [DOI] [PubMed] [Google Scholar]
- 20. Vigil D.; Kim T. Y.; Plachco A.; Garton A. J.; Castaldo L.; Pachter J. A.; Dong H.; Chen X.; Tokar B.; Campbell S. L. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 72:5338–5347; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu X.; Choy E.; Hornicek F. J.; Yang S.; Yang C.; Harmon D.; Mankin H.; Duan Z. ROCK1 as a potential therapeutic target in osteosarcoma. J. Orthop. Res. 29:1259–1266; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Zhou W.; Hao M.; Du X.; Chen K.; Wang G.; Yang J. Advances in targeted therapy for osteosarcoma. Discov. Med. 17:301–307; 2014. [PubMed] [Google Scholar]
- 23. Zhou X.; Wei M.; Wang W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem. Biophys. Res. Commun. 437:653–658; 2013. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y.; Zhao W.; Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol. Cell. Biochem. 384:105–111; 2013. [DOI] [PubMed] [Google Scholar]
- 25. Lei P.; Xie J.; Wang L.; Yang X.; Dai Z.; Hu Y. MicroRNA-145 inhibits osteosarcoma cell proliferation and invasion by targeting ROCK1. Mol. Med. Rep. 10:155–160; 2014. [DOI] [PubMed] [Google Scholar]
- 26. Li E.; Zhang J.; Yuan T.; Ma B. MiR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumor Biol. 35:7645–7650; 2014. [DOI] [PubMed] [Google Scholar]