Recently, a meta-analysis by Kovalic et al. reported that chronic liver disease was significantly associated with severe coronavirus disease 2019 (COVID-19) and mortality [1]. This is an interesting study. However, the pooled effect on the association between chronic liver disease and severe COVID-19 was estimated based on un-adjusted effect sizes in Kovalic et al.’s study [1]. It has been considered that several factors such as gender, age and certain comorbidities significantly influenced COVID-19 outcomes [2–5]. This suggests that these factors might modulate the relationship between chronic liver disease and COVID-19 severity. Therefore, it is urgently required to clarify this association by performing a quantitative meta-analysis based on adjusted effect estimates.
Electronic databases including PubMed, Web of Science and EMBASE were searched up to December 10, 2020 using the terms: “SARS-CoV-2”, “COVID-19”, “chronic liver disease”, “cirrhosis”, “hepatitis”, “liver cancer” and “nonalcoholic fatty liver disease”. Only studies reporting the relationship between chronic liver disease and COVID-19 severity by adjusted effect estimates were included. Case reports, reviews, duplicate publications, errata and studies without sufficient data were excluded. The heterogeneity was detected by I2 statistics. The pooled effect sizes with 95% confidence interval (CI) were estimated. Publication bias was evaluated by Begg’s test and Egger’s test. Sensitivity analysis, subgroup analysis and meta-regression analysis were also performed. All data were analyzed using Stata 12.1. p < 0.05 was considered statistical significance.
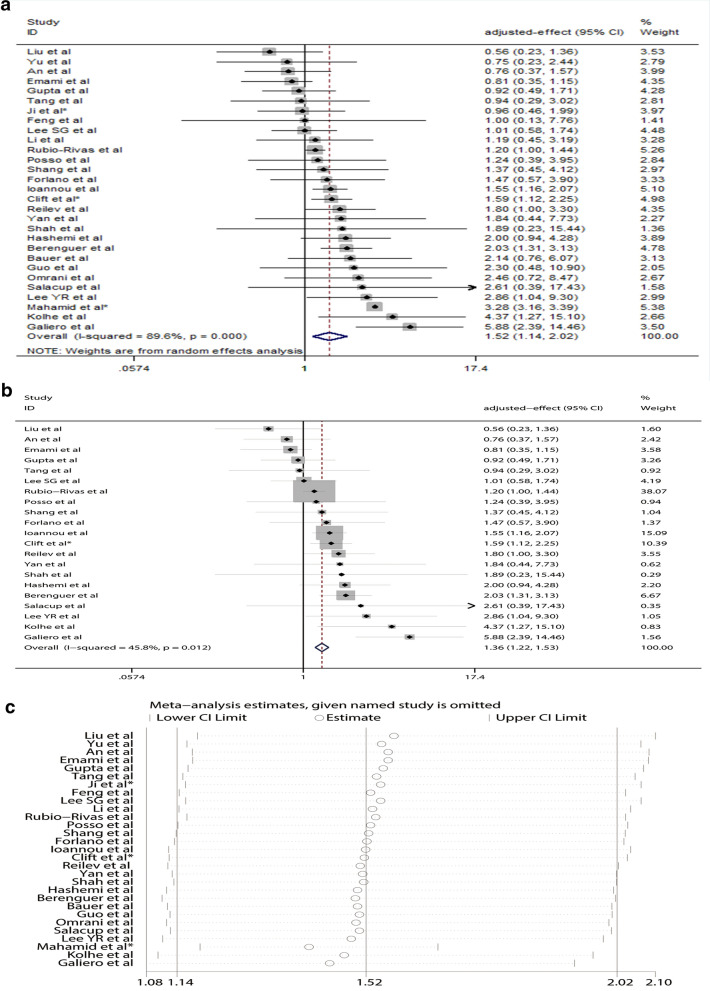
Figure S1 shows the flow diagram of study selection. 29 articles with 90,095 confirmed COVID-19 patients were included. The characteristics of the included studies are summarized in Table 1. Our meta-analysis based on adjusted effect estimates demonstrated that COVID-19 patients with chronic liver disease tended to develop severe outcome compared to those without (pooled effect size = 1.52, 95% CI: 1.14–2.02, Fig. 1a) and had a significantly increased risk for mortality compared to those without (pooled effect size = 1.36, 95% CI: 1.22–1.53, Fig. 1b). Sensitivity analysis exhibited that our findings were stable (Fig. 1c). Subgroup analyses by sample size and study design exhibited consistent results (Table S1 and Figure S2–3). But inconsistent results were observed in subgroup analyses by age, male percentage, effect estimate and region (Table S1 and Figure S4–7). Meta-regression analysis showed that the tested variables such as sample size, age, male percentage, effect estimate, study design and region might not be the source of heterogeneity (Table S1). Begg’s test and Egger’s test suggested that there might be potential publication bias (Figure S8).
Table 1.
Main characteristics of the studies included in this meta-analysis
| Author | Country | No. of cases | Male percentage (%) | Age§ | Study design | Adjusted-effect size (95% CI) | Adjusted risk factors |
|---|---|---|---|---|---|---|---|
|
Hashemi et al. (PMID: 32585065) |
USA | 363 | 55.4 | 63.4 ± 16.5 | Retrospective study | OR: 2.00 (0.94–4.28) | Age, obesity, male, cardiac diseases, hypertension, diabetes, hyperlipidemia, pulmonary disorders |
|
Ji et al. (PMID: 32597048) |
Korea | 7341 | 40.5 | 47.05 ± 19.0 | Retrospective study | OR: 1.031 (0.469–2.265) | Endocrinopathy, cardiac disease, chronic respiratory disease, renal disease, disease of digestive system, chronic neurologic disease, malignancy, musculoskeletal and rheumatologic disease, hematologic disease, obesity, nutritional deficiency, mental and behavioral disorders, immune deficiency |
| OR: 0.592 (0.082–4.289) | |||||||
|
Salacup et al. (PMID: 32617986) |
USA | 242 | 50.8 | 66 ± 14.75 | Retrospective study | OR: 2.605 (0.389–17.428) | Age, BMI, sex, ethnicity, COPD or asthma, diabetes mellitus, hypertension, heart failure, CKD |
|
Shah et al. (PMID: 32620056) |
USA | 522 | 41.8 | 63 (50–72) | Retrospective study | OR: 1.89 (0.23–15.44) | Age, BMI, gender, race, all the baseline comorbidities |
|
Shang et al. (PMID: 32653423) |
China | 584 | 47.4 | 59 (25–75) | Retrospective study | HR: 1.365 (0.452–4.119) | Sex, age, hypertension, CVD, diabetes, chronic respiratory diseases, CKD, acute kidney injury, acute liver injury, respiratory failure, acute cardiac injury |
|
Berenguer et al. (PMID: 32758659) |
Spain | 3979 | 61 | 70 (56–80) | Retrospective study | HR: 2.03 (1.31–3.13) | Sex, age, arterial hypertension, obesity, dementia, chronic neurological disorder, active cancer, dyspnoea, confusion, low age-adjusted SaO2 on room air, higher white cell blood count, higher neutrophil-to-lymphocyte ratio, lower platelet count, international normalized ratio, estimated glomerular filtration rate, concentrations of C-reactive protein |
|
Yu et al. (PMID: 32777639) |
China | 1561 | 50 | 62 (50–70) | Retrospective study | OR: 0.75 (0.23–2.44) | Age, sex, smoking history, COPD, hypertension, CVD, cerebrovascular disease, diabetes, tuberculosis, malignant tumor, CKD, prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, fibrin(ogen) degradation products, d-dimer |
|
Posso et al. (PMID: 32782092) |
Spain | 834 | 46.5 | 78.2 ± 9.8 | Retrospective study | OR: 1.24 (0.39–3.95) | CKD, heart failure, malignancy, obesity, diabetes, hypertension, chronic respiratory disease, age, gender |
|
Gupta et al. (PMID: 32818209) |
USA | 2626 | 57 | 65.35 ± 17.59 | Retrospective study | OR: 0.917 (0.492–1.709) | Age, sex, first BMI assessment, race and ethnicity, insurance, New York City borough of residence, history of hypertension, diabetes, coronary artery disease, heart failure, stroke or transient ischemic attack, atrial arrhythmias, chronic lung disease, CKD, outpatient use of beta-blockers, ACEi, ARBs, oral anticoagulants, P2Y12 receptor inhibitors |
|
Emami et al. (PMID: 32835530) |
Iran | 1239 | 55.9 | 51.48 ± 19.54 | Not clearly reported | HR: 0.807 (0.35–1.15) | Age, diabetes mellitus, CVD, CKD, cancer, HIV, smoking, asthma, immunodeficiency disease |
|
Feng et al. (PMID: 32850926) |
China | 114 | 62.3 | 63.96 ± 13.41 | Prospective study | HR: 0.997 (0.128–7.760) | Age, sex |
|
Li et al. (PMID: 32855361) |
China | 104 | 62.5 | 59 ± 12.9 | Retrospective study | RR: 1.19 (0.45–3.19) | Not explicitly reported |
|
Mahamid et al. (PMID: 32868652) |
Israel | 71 | 38.2 | 51.0 ± 21.7 | Retrospective study | OR: 3.29 (3.28–3.58) | Obesity, metabolic syndrome, diabetes, smoking |
| OR: 3.25 (3.09–3.47) | |||||||
| Reilev et al. (PMID: 32887982) | Denmark | 11,122 | 42.2 | 48 (33–62) | Population-based study | OR: 1.8 (1.0–3.3) | Age, sex |
|
Yan et al. (PMID: 32949175) |
China | 1103 | 48.6 | 63 (51–71) | Retrospective study | HR: 1.84 (0.44–7.73) | Age, male, diabetes, hypertension, COPD, chronic heart diseases, CKD, cerebrovascular diseases, hyperlipidemia, tumor, C-reactive protein, d-dimer |
|
Ioannou et al. (PMID: 32965502) |
USA | 10,131 | 91 | 64.86 ± 17.26 | Longitudinal cohort study | HR: 1.55 (1.16–2.07) | All sociodemographic characteristics, comorbid conditions, symptoms |
|
Forlano et al. (PMID: 33031439) |
UK | 193 | 62.7 | 65.97 ± 18.64 | Retrospective study | OR: 1.47 (0.57–3.9) | Male gender, presence of type-2 diabetes, hypertension, dyslipidemia |
|
Lee YR et al. (PMID: 33053932) |
Korea | 1005 | 35.9 | 61 (48–72) | Retrospective study | HR: 2.86 (1.04–9.30) | Age, BMI, smoking history, diabetes mellitus, hypertension, CVD, COPD, chronic renal disease, fever/chill, cough, shortness of breath |
|
Clift et al. (PMID: 33082154) |
UK | 10,776 | 55.3 | 69.63 ± 17.91 | Population-based cohort study | HR: 1.85 (1.15–2.29) | Age, BMI, townsend score (linear), ethnic group, domicile (residential care, homeless, neither), and a range of conditions and treatments |
| HR: 1.29 (0.83–2.02) | |||||||
|
Omrani et al. (PMID: 33076848) |
Qatar | 1409 | 82.8 | 39 (30–50) | Retrospective study | OR: 2.463 (0.716–8.465) | Age, sex, BMI (defined as body weight in kilograms divided by squared height in meters), and co-existing diabetes mellitus, systemic hypertension, coronary artery disease, CKD |
|
Kolhe et al. (PMID: 33125416) |
UK | 1161 | 56.6 | 72.10 ± 16.01 | Retrospective study | OR: 4.37 (1.27–15.1) | Age, sex, ethnicity, myocardial infarction, congestive cardiac failure, peripheral vascular disease, cerebrovascular disease, chronic lung disease, connective tissue disorder, diabetes with complications, paraplegia, CKD, dementia, cancer |
|
An et al. (PMID: 33127965) |
Korea | 10,237 | 39.9 | 44.97 ± 19.79 | Retrospective study | HR: 0.76 (0.37–1.57) | Age, sex, income level, residence, household type, disability, symptom, infection route |
|
Liu et al. (PMID: 33141117) |
China | 744 | 58.4 | 64 (54–73) | Retrospective study | HR: 0.56 (0.23–1.36) | Age, sex, APACHE II score, COPD, diabetes, hypertension, chronic cardiac disease, CKD, immunosuppression, stroke, malignancy, fever at admission, systolic pressure at admission, leukocytes, hemoglobin, platelets, lymphocytes, d-dimer, total bilirubin, serum creatinine, procalcitonin, corticosteroids, antiviral, human immunoglobulin |
|
Rubio-Rivas et al. (PMID: 33137919) |
Spain | 12,066 | 58.5 | 67 ± 16 | Retrospective study | OR: 1.20 (1.00–1.44) | Age, gender, BMI, clusters, arterial hypertension, diabetes mellitus, hyperlipidemia, COPD, ischemic cardiopathy, chronic heart failure, CKD, active cancer, charlson's index, heart rate upon admission, respiratory rate upon admission > 20 bpm, PaO2/FiO2 upon admission, laboratory test upon admission, treatments during admission |
|
Guo et al. (PMID: 33154656) |
China | 350 | 49.4 | 43 (32–56) | Retrospective study | OR: 2.30 ( 0.48–10.90) | Age, sex, Wuhan exposure, family cluster case, smoking, comorbidity, hypertension, diabetes, CVD, CKD, cerebral infarction |
|
Tang et al. (PMID: 33153910) |
USA | 752 | 39.9 | 71.16 ± 51.68 | Retrospective study | HR: 0.94 (0.29–3.02) | Age, sex, race, facility |
|
Lee SG et al. (PMID: 33218161) |
Korea | 7399 | 40.1 | 47.1 ± 19.0 | Retrospective study | OR: 1.01 (0.58–1.74) | Influenza, tuberculosis, COPD, pneumonia, asthma, diabetes mellitus, CKD, hypertension, CVDs, malignancies, HIV infection, lopinavir/ritonavir, hydroxychloroquine, ribavirin, type I interferon, human immunoglobulin G, oseltamivir, antibiotics, age, male, DG area |
|
Bauer et al. (PMID: 33220171) |
USA | 1449 | 36.5 | 54.7 ± 22.5 | Retrospective study | OR: 2.14 (0.76–6.07) | Hypertension, diabetes, chronic respiratory disease, arterial disease, congestive heart failure, CKD, cancer, immunosuppression |
|
Galiero et al. (PMID: 33301529) |
Italy | 618 | 61.3 | 65 ± 15.2 | Retrospective study | OR: 5.88 (2.39–14.46) | Age, sex, GCS/15, respiratory severity scale, chronic cardiac disease, CKD, chronic respiratory disease, malignancies |
ACEi angiotensin-converting enzyme inhibitors, APACHE acute physiology and chronic health evaluation, ARBs angiotensin receptor blockers, BMI body mass index, CI confidence interval, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, CVDs cardiovascular/cerebrovascular diseases, DG Daegu city and Gyeongsangbuk-do province, FiO2 fraction of inspired oxygen, GCS Glasgow Coma Score, HIV human immunodeficiency virus, HR hazard ratio, OR odds, ratio, PaO2 arterial partial pressure of oxygen, RR relative ratio, SaO2 arterial oxygen saturation. §The values of age are presented as mean ± standard deviation (SD) or median (interquartile range, IQR)
Fig. 1.
a The forest plot on the association between chronic liver disease and severe coronavirus disease 2019 (COVID-19) on the basis of 29 studies with 90,095 cases reporting adjusted effect estimates; b The forest plot on the association between chronic liver disease and COVID-19 mortality; c Leave-one-out sensitivity analysis indicated that our results were stable and robust. *Indicates that the combined value was calculated on the basis of subgroups
This meta-analysis has several limitations. First, inconsistent results were observed in subgroup analyses by age, male percentage and region. Thus, the findings should be cautiously extrapolated to whole population. Second, most of the included studies are retrospective, further well-designed studies with more prospective literatures are warranted to confirm our findings. Third, publication bias might exist although we tried to search potential articles in electronic databases.
In summary, our study indicated that chronic liver disease was independently associated with COVID-19 severity and mortality, especially among aged individuals, male-dominated population, USA and Europe. Proper management of COVID-19 patients with chronic liver disease is highly recommended to prevent severe situations and mortality.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Ying Wang, Hongjie Hou, Peihua Zhang, Yang Li, Jian Wu and Wenwei Xiao (All are from Department of Epidemiology, School of Public Health, Zhengzhou University, Zhengzhou 450001, China) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis.
Author contributions
Haiyan Yang and Yadong Wang designed this study. Jie Xu and Xuan Liang performed literature search. Haiyan Yang and Jie Xu performed data extraction. Jie Xu, Haiyan Yang, Xuan Liang and Li Shi performed statistical analyses. Haiyan Yang, Jie Xu and Yadong Wang wrote and reviewed the manuscript. All the authors approved the final version of this manuscript.
Funding
This study was supported by grants from National Natural Science Foundation of China (Grant Number 81973105), Key Scientific Research Project of Henan Institution of Higher Education (Grant Number 21A330008) and Joint Construction Project of Henan Medical Science and Technology Research Plan (Grant Number LHGJ20190679). The funders have no role in the data collection, data analysis, preparation of manuscript and decision to submission.
Data availability
All data relevant to this study are included in this article or uploaded as supplementary information.
Compliance with ethical standards
Conflict of interest
The authors Haiyan Yang, Jie Xu, Xuan Liang, Li Shi and Yadong Wang have no any potential conflict of interest regarding this submitted manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haiyan Yang, Email: yhy@zzu.edu.cn.
Yadong Wang, Email: wangyd76@163.com.
References
- 1.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hep Intl. 2020;14(5):612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang X, Shi L, Wang Y, Xiao W, Duan G, Yang H, et al. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infect. 2020;81(3):e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020;9:1–12. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Xiao W, Liang X, Zhang P, Shi L, Wang Y, et al. The association of cerebrovascular disease with adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. J Stroke Cerebrovasc Dis. 2020;29(11):105283. doi: 10.1016/j.jstrokecerebrovasdis.2020.105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15(12):e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this study are included in this article or uploaded as supplementary information.