Abstract
The CKLF-like MARVEL transmembrane domain-containing 3 (CMTM3), a member of the CMTM family, was found in several human tumors and plays an important role in the development and progression of tumors. However, the role of CMTM3 in hepatocellular carcinoma (HCC) remains largely unknown. Thus, in the present study, we explored its expression pattern in human HCC cell lines, as well as its functions in HCC cells. Our results demonstrated that the expression of CMTM3 is lowly expressed in HCC cell lines. In vitro, we found that overexpression of CMTM3 obviously inhibited the proliferation, invasion, and EMT process in HCC cells. Furthermore, overexpression of CMTM3 significantly downregulated the expression levels of phosphorylation of JAK2 and STAT3 in HepG2 cells. In vivo, overexpression of CMTM3 attenuated the tumor growth in Balb/c nude mice. In conclusion, we demonstrated that CMTM3 could play an important role in HCC metastasis by EMT induction via, at least partially, suppressing the JAK2/STAT3 signaling pathway. Therefore, CMTM3 may serve as a potential molecular target in the prevention and/or treatment of HCC invasion and metastasis.
Key words: The CKLF-like MARVEL transmembrane domain-containing 3 (CMTM3), Hepatocellular carcinoma (HCC), Metastasis, JAK2/STAT3 pathway
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world and accounts for 90% of cases of primary liver cancer1. There are about 600,000 new-onset patients with this disease every year2. In spite of improvements in clinical therapeutic strategy, the clinical outcome of patients with HCC remains unsatisfactory because of tumor recurrence and metastasis of the primary tumor3–5. Therefore, it is critical to discover the mechanisms underlying HCC metastasis.
CKLF-like MARVEL transmembrane domain-containing family (CMTM) consists of nine genes, CKLF and CMTM1 to CMTM8. Previous studies have demonstrated that CMTMs play a major role in immune and inflammatory responses as well as tumorigenesis6–8. CMTM3 is a member of the CMTM family that is located at 16q22.1. Downregulation of CMTM3 has been found in several human tumors and plays an important role in the development and progression of tumors9–11. For example, one study showed that the expression of CMTM3 was significantly reduced in oral squamous cell carcinoma (OSCC) cell lines and primary tumor specimens, and overexpression of CMTM3 inhibited the growth and migration of OSCC cells, as well as attenuated the growth of OSCC xenografts in nude mice12. However, the role of CMTM3 in HCC remains largely unknown. Thus, in the present study, we explored its expression pattern in human HCC cell lines as well as its functions in HCC cells. We found that CMTM3 is lowly expressed in HCC cell lines, and it promoted proliferation, invasion, and in vivo tumorigenesis, suggesting that CMTM3 may play an important role in HCC.
MATERIALS AND METHODS
Cell Culture
Four human HCC cell lines (HepG2, 97H, Hep3B, and HCCLM3) and a hepatocyte cell line (HL-7702) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Abcam, Cambridge, UK), penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), and sodium pyruvate (1 mM) in 5% CO2 at 37°C.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from HCC cells using TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA using a PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara, Dalian, P.R. China). The RT-PCRs were performed on the basis of the thermal cycler dice real-time system. The primer sequences used for PCR amplification were as follows: CMTM3, 5′-TTTTATCTGCTATGTGGCGTCC-3′ (forward) and 5′-TGTCTTGTGGGCTGTGGTCTC-3′ (reverse); GAPDH, 5′-GCACCGTCAGGCTGAGAAC-3′ (forward) and 5′-ATGGTGGTGAAGACGCCAGT-3′ (reverse). The PCR procedure was as follows: 94°C for 3 min; 94°C for 20 s, 59°C for 30 s, and 72°C for 20 s; 2 s for plate reading for 40 cycles; and melt curve from 65°C to 95°C. Quantitative measurements were determined using the comparative ΔCt method.
Western Blot Analysis
Total protein was extracted from HCC cells using RIPA lysis buffer (Beyotime, Nantong, P.R. China) according to the operating instructions. A total of 30 μg of protein was separated by 10% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes (Amersham, Little Chalfont, UK). After being blocked in Tris-buffered saline containing 5% nonfat milk powder, the membranes were incubated with primary antibodies: anti-CMTM3, anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-p-JAK2, anti-JAK2, anti-p-STAT3, anti-STAT3, and anti-GAPDH (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, TX, USA). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:2,000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h at room temperature. Signals were detected on x-ray film using an electrochemiluminescence detection system (Pierce, Rockford, IL, USA).
Construction of the pcDNA3.1-CMTM3 and Cell Transfection
The plasmid pcDNA3.1-CMTM3 and control vector pcDNA3.1 were purchased from GenePharma (Shanghai, P.R. China). Cells grew to 70% to 90% confluence before transfection. The pcDNA3.1-CMTM3 and empty vector were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Stable cell lines were selected in complete medium containing 1 μg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA) for 48 h after transfection.
Cell Proliferation Assay
Cell proliferation was evaluated using the cell counting kit-8 (CCK-8; Sigma-Aldrich) assay according to the manufacturer’s instructions. Briefly, infected cells (3 × 105 cells/ml) were cultured in DMEM containing 10% FBS and cultured for 24, 48, 72, or 96 h. Then 10 μl of CCK-8 was added to each well. After incubating for 2 h, the absorbance of each well was measured at 450 nm using a microplate spectrophotometer (BioTek Instrument Inc., Winooski, VT, USA).
Cell Migration and Invasion Assays
Cell migration and invasion were assayed using Transwell chambers (8 μm, 24-well format; Corning Co., Corning, NY, USA). Briefly, infected cells (3 × 105 cells/ml) were added to the bottom part of the chamber, whereas 600 μl of DMEM containing 10% FBS was added to the upper well of the chamber. The cells were incubated for 24 h at 37°C in a 5% CO2 humidified atmosphere. After incubation, nonmigrating cells in the upper chamber were scraped off, and the migrated cells on the bottom part of the filter were washed, fixed, stained with crystal violet, and quantified by counting six high-powered fields in the center of each well. The invasion assay was performed using the same procedure, except that the membrane was coated with Matrigel to form a matrix barrier.
In Vivo Xenograft Tumor Assay
Balb/c nude mice (female, 5 weeks old) were purchased from the Laboratory for Animals of The First Affiliated Hospital of Xi’an Medical University (P.R. China), and this study was performed with approval from the Animal Ethics Committee of The First Affiliated Hospital of Xi’an Medical University (P.R. China). For the in vivo assay, Balb/c nude mice were inoculated subcutaneously with 3 × 106 HepG2-CMTM3 cells (n = 5) or HepG2-vector cells (n = 5), used as a negative control. Tumor size was measured every 5 days using a caliper and calculated using the formula: volume = length × width2 × π/6. About 25 days after inoculation, mice were euthanized and the tumors were weighed.
Statistical Analysis
Data are expressed as the mean ± SD. Statistical analysis was carried out with Student’s t-test. A value of p < 0.05 was considered as a statistically significant difference.
RESULTS
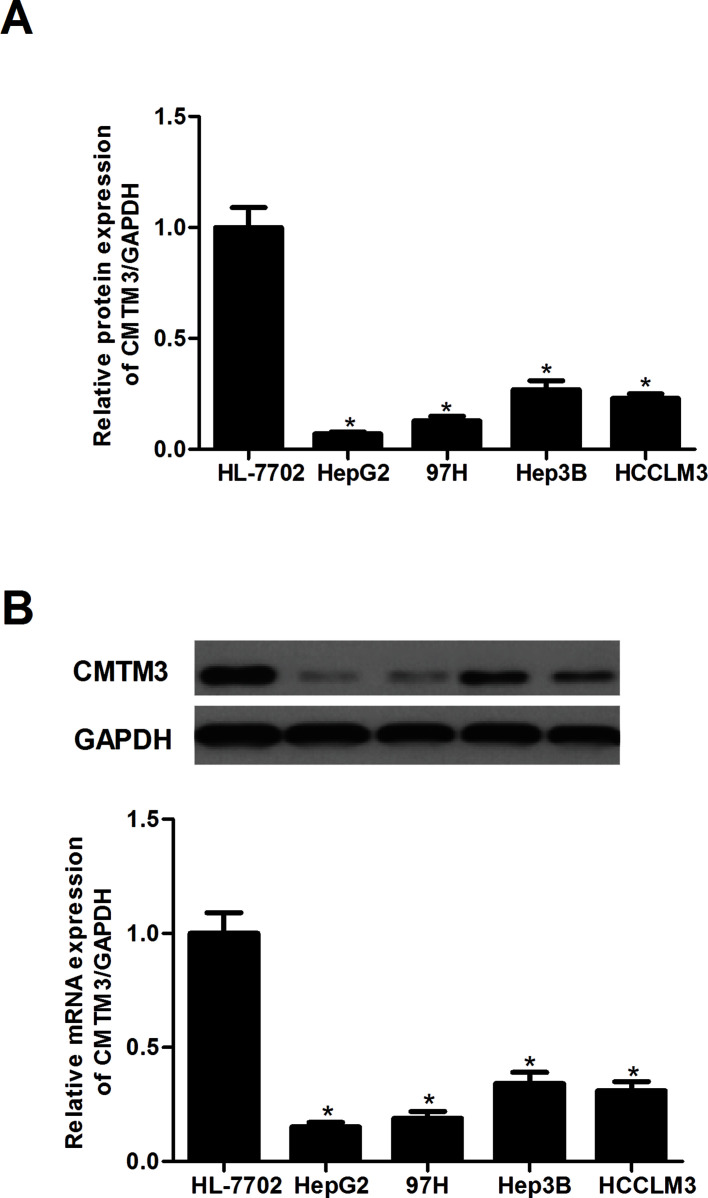
CMTM3 Is Lowly Expressed in Human HCC Cells
First, we performed a qRT-PCR assay to examine the mRNA expression of CMTM3 in human HCC cell lines. We found that the mRNA expression levels of CMTM3 were significantly decreased in human HCC cell lines, compared with the control group (Fig. 1A). Similarly, the results of the Western blot analysis demonstrated that a significant decrease in CMTM3 protein level was observed in human HCC cell lines compared with the HL-7702 cell line (Fig. 1B).
Figure 1.
CMTM3 is lowly expressed in human HCC cells. (A) The mRNA expression of CMTM3 in human HCC cell lines was determined by the qRT-PCR assay. (B) The protein expression of CMTM3 in human HCC cell lines was determined by the Western blot assay. These data are from three independent experiments and presented as the mean ± SD. *p < 0.05.
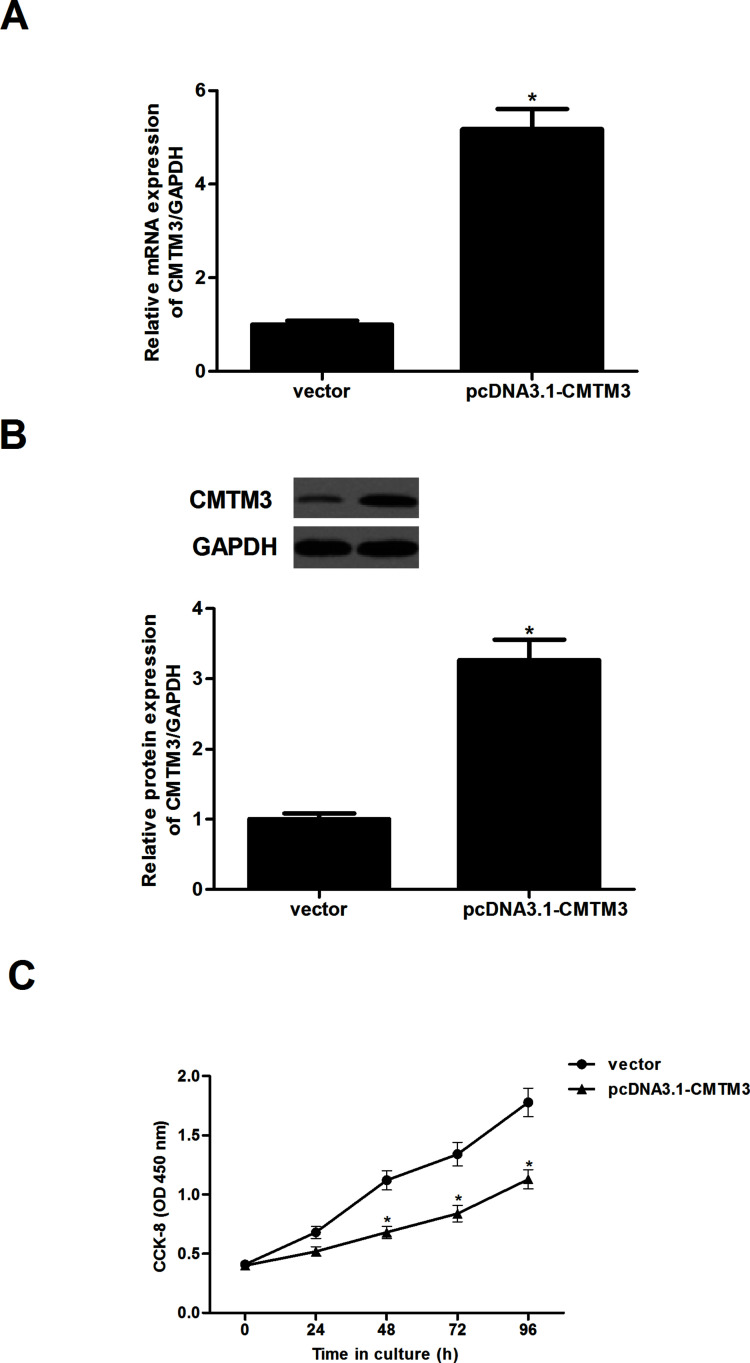
CMTM3 Inhibits the Proliferation of HCC Cells
To clarify the effect of CMTM3 on cell proliferation, we established which HepG2 cells were stably transfected with pcDNA3.1-CMTM3 in the HepG2-CMTM3 cell line. The gene (Fig. 2A) and protein expression levels (Fig. 2B) of CMTM3 in the HepG2-CMTM3 cell line were confirmed by qRT-PCR and Western blot assays.
Figure 2.
CMTM3 inhibits the proliferation of HCC cells. HepG2 cells were transfected with pcDNA3.1-CMTM3 or empty vector for 48 h. The gene (A) and protein expression levels (B) of CMTM3 in the HepG2-CMTM3 cell line were confirmed by qRT-PCR and Western blot assays. (C) Cell proliferation was evaluated using the cell counting kit-8 assay. These data are from three independent experiments and presented as the mean ± SD. *p < 0.05.
We then used the CCK-8 assay to measure the effect of CMTM3 on HCC cell proliferation. At the indicated time points, HepG2 cells that were overexpressing CMTM3 exhibited reduced incorporation compared with vector controls (Fig. 2C).
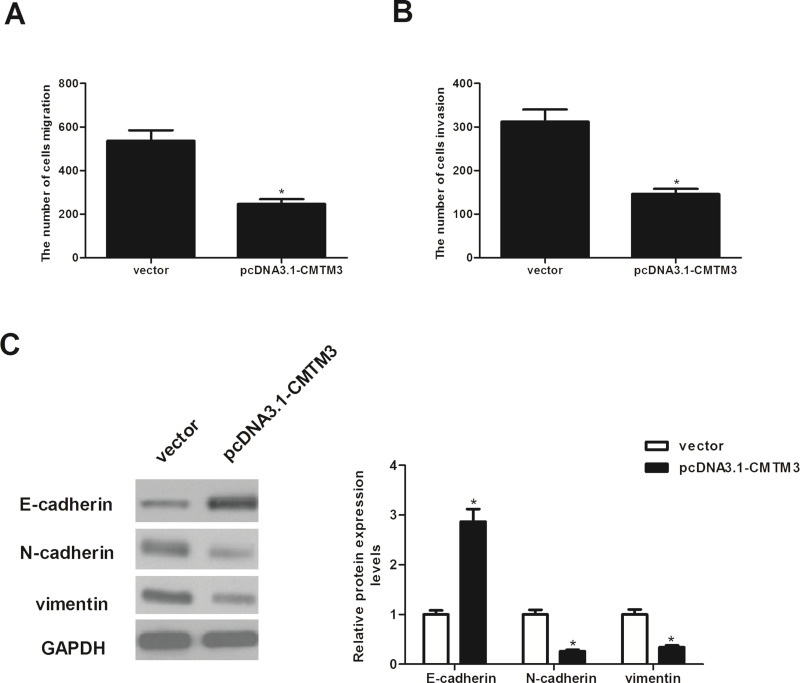
CMTM3 Inhibits the Invasion and Migration of HCC Cells
We examined the effect of CMTM3 on the migration of HepG2 cells using the Transwell migration assay. As shown in Figure 3A, the migration of CMTM3-transfected HepG2 cells was remarkably reduced compared with that of vector-transfected cells. Moreover, the effect of CMTM3 on HCC cell invasion was assessed by Matrigel invasion assay. The Matrigel invasion assay showed that the number of invading cells was significantly decreased in CMTM3-transfected cells than that in the vector-transfected cells (Fig. 3B). In addition, we investigated the effect of CMTM3 on the expression of EMT-related markers. As shown in Figure 3C, the expression of the epithelial marker E-cadherin was increased at the protein level, while protein expression of the mesenchymal markers N-cadherin and vimentin was decreased in CMTM3-transfected HepG2 cells.
Figure 3.
CMTM3 inhibits the migration and invasion of HCC cells. HepG2 cells were transfected with pcDNA3.1-CMTM3 or empty vector for 48 h. (A) Cell migration was evaluated using the Transwell migration assay. (B) Cell invasion was assessed by the Matrigel invasion assay. (C) E-cadherin, N-cadherin, and vimentin protein levels were determined by Western blot analysis. Quantification analysis of E-cadherin, N-cadherin, and vimentin was performed using the Gel-Pro Analyzer version 4.0 software. GAPDH was used as loading control. These data are from three independent experiments and presented as the mean ± SD. *p < 0.05.
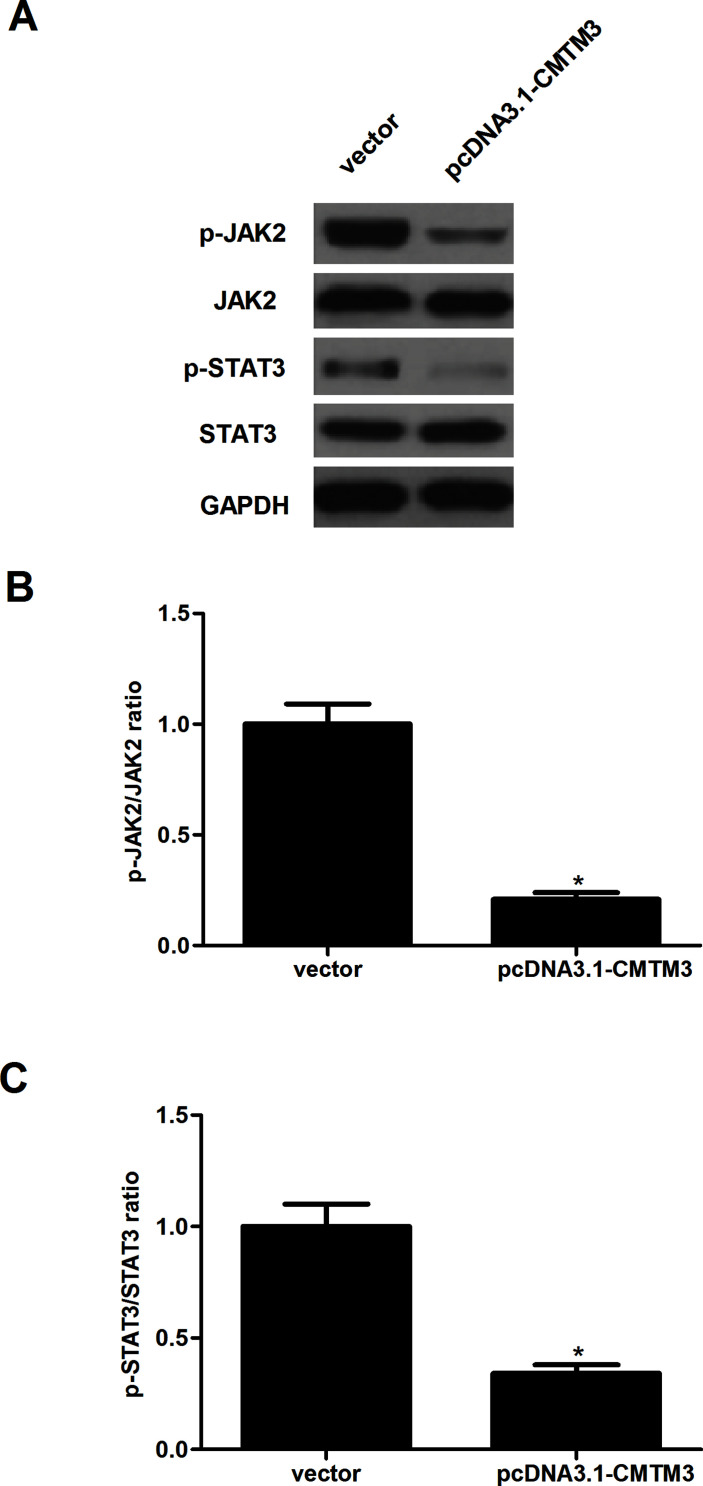
CMTM3 Inhibits the Activation of JAK2/STAT3 Signaling in HCC Cells
To further investigate the underlying mechanism of how CMTM3 inhibited the proliferation and invasion of HCC cells, we examined the effect of CMTM3 on the activity of the JAK2/STAT3 signaling pathway. The results of the Western blot analysis indicated that overexpression of CMTM3 significantly downregulated the expression levels of the phosphorylation of JAK2 and the downstream JAK substrate STAT3 in HepG2 cells, compared with the vector group (Fig. 4).
Figure 4.
CMTM3 inhibits the activation of JAK2/STAT3 signaling in HCC cells. HepG2 cells were transfected with pcDNA3.1-CMTM3 or empty vector for 48 h. (A) p-JAK2, JAK2, p-STAT3, and STAT3 protein levels were determined by Western blot analysis. GAPDH was used as loading control. (B, C) Quantification analysis of p-JAK2/JAK2 and p-STAT3/STAT3 was performed using the Gel-Pro Analyzer version 4.0 software. These data are from three independent experiments and presented as the mean ± SD. *p < 0.05.
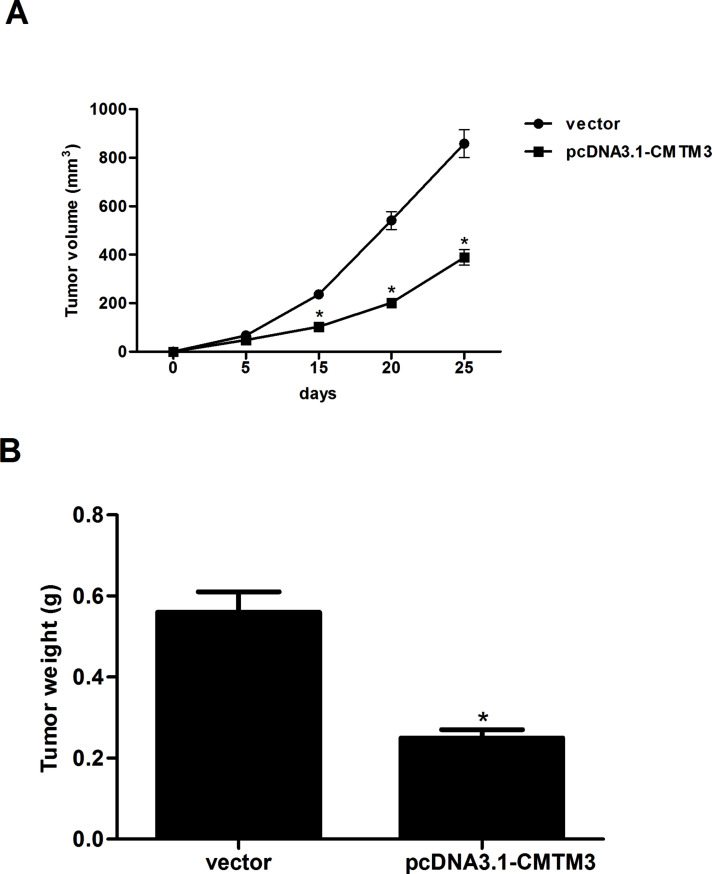
CMTM3 Inhibits Tumorigenicity In Vivo
Next, to evaluate the effect of CMTM3 on tumorigenesis in vivo, HepG2-CMTM3 cells or HepG2-vector cells were introduced subcutaneously to Balb/c nude mice. As shown in Figure 5A, the average tumor volumes of HepG2-CMTM3 cell-grafted mice were obviously decreased, compared with the vector group. Moreover, overexpression of CMTM3 sharply reduced the weight of tumors 25 days after inoculation (Fig. 5B).
Figure 5.
CMTM3 inhibits the tumorigenicity in vivo. HepG2-CMTM3 cells or HepG2-vector cells were introduced subcutaneously to Balb/c nude mice. (A) The tumor volumes were calculated in each group every 5 days. (B) The tumors were weighed at 25 days after inoculation. These data are from three independent experiments and presented as the mean ± SD. *p < 0.05.
DISCUSSION
In the present study, our results demonstrated that the expression of CMTM3 is lowly expressed in HCC cell lines. In vitro, we found that overexpression of CMTM3 obviously inhibited the proliferation, invasion, and EMT process in HCC cells. Furthermore, overexpression of CMTM3 significantly downregulated the expression levels of the phosphorylation of JAK2 and STAT3 in HepG2 cells. In vivo, overexpression of CMTM3 attenuated the tumor growth in Balb/c nude mice.
Previous studies demonstrated that CMTM3 plays a critical role in tumorigenesis. Li et al. confirmed that CMTM3 was frequently downregulated or silenced in testicular cancer cell lines and tumor tissues, and the reexpression of CMTM3 sharply suppressed the colony formation, proliferation, and migration capacity of testicular cancer cells10. Xie et al. reported that CMTM3 was downregulated in clear cell renal cell carcinoma tissues, and its overexpression significantly suppressed the anchorage-independent growth and proliferation of 786-0 cells13. In accordance with previous studies, we found that the expression of CMTM3 is lowly expressed in HCC cell lines. Overexpression of CMTM3 obviously inhibited the proliferation and the tumor growth in Balb/c nude mice. These data suggest that CMTM3 may act as a putative tumor suppressor in the development and progression of HCC.
High invasion and metastasis are the major factors causing poor prognosis of patients with HCC14. EMT is believed to be a major mechanism by which cancer cells become migratory and invasive. It is characterized by a loss of E-cadherin and enhanced cell motility as well as the acquisition of N-cadherin and vimentin15. In this study, we found that overexpression of CMTM3 obviously inhibited the migration and invasion of HCC cells. Moreover, we observed that overexpression of CMTM3 increased the expression of the epithelial marker E-cadherin, while it decreased the expression of the mesenchymal marker N-cadherin in HepG2 cells. These results suggest that CMTM3 inhibits the migration and invasion of HCC cells by repressing the EMT phenotype.
The JAK2/STAT3 signaling pathway plays an important role in the development of HCC16–18. JAK2, a member of the Janus (JAK) family, regulates signaling via multiple cytokine receptors19. STAT3 is constitutively activated in human HCC tissues and controls cancer cell proliferation, invasion, and angiogenesis20,21. Recently, the activation of STAT3 has been linked to the EMT program in HCC22. It was reported that STAT3 knockdown significantly increased E-cadherin and β-cadherin, and it decreased N-cadherin and vimentin expression in human HCC cells. STAT3 may cooperate with Twist to mediate EMT and induce HCC invasion and metastasis23. Thus, inactivation of the JAK2/STAT3 signaling pathway may be a good way to inhibit HCC metastasis. For example, Kang et al. confirmed that B7 homolog 3 promotes the invasion of HCC by targeting EMT via the JAK2/STAT3/Slug signaling pathway24. Most recently, a study by Yuan et al. revealed that CMTM3 dramatically suppresses the phosphorylation of STAT3 in gastric cancer cells25. Similarly, we observed that overexpression of CMTM3 significantly downregulated the expression levels of phosphorylation of JAK2 and STAT3 in HepG2 cells. These data suggest that CMTM3 inhibits proliferation and tumorigenesis by suppressing the JAK2/STAT3 signaling pathway in HCC cells.
In summary, we demonstrated that CMTM3 could play an important role in HCC metastasis by EMT induction via, at least partially, suppressing the JAK2/STAT3 signaling pathway. Therefore, CMTM3 may serve as a potential molecular target in the prevention and/or treatment of HCC invasion and metastasis.
REFERENCES
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 3. Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Prognosis of patients with intrahepatic recurrence after hepatic resection for hepatocellular carcinoma: A retrospective study. Eur J Surg Oncol. 2009;35:174–9. [DOI] [PubMed] [Google Scholar]
- 4. Lee SC, Tan HT, Chung M. Prognostic biomarkers for prediction of recurrence of hepatocellular carcinoma: Current status and future prospects. World J Gastroenterol. 2014;20:3112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poon RT-P, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang M, Xu Y, Liu Y, Cheng Y, Zhao P, Liu H, Wang Y, Ma X. Chemokine-like factor 1 (CKLF-1) is overexpressed in keloid patients: A potential indicating factor for keloid-predisposed individuals. Medicine 2016;95:e3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao D, Hu H, Wang Y, Yu W, Zhou J, Wang X, Wang W, Zhou C, Xu K. CMTM8 inhibits the carcinogenesis and progression of bladder cancer. Oncol Rep. 2015;34:2853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H, Nan X, Li X, Chen Y, Zhang J, Sun L, Han W, Li T. CMTM5 exhibits tumor suppressor activity through promoter methylation in oral squamous cell carcinoma. Biochem Biophys Res Commun. 2014;447:304–10. [DOI] [PubMed] [Google Scholar]
- 9. Su Y, Lin Y, Zhang L, Liu B, Yuan W, Mo X, Wang X, Li H, Xing X, Cheng X. CMTM3 inhibits cell migration and invasion and correlates with favorable prognosis in gastric cancer. Cancer Sci. 2014;105:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Xie J, Wu J, Li W, Nie L, Sun X, Tang A, Li X, Liu R, Mei H. CMTM3 inhibits human testicular cancer cell growth through inducing cell-cycle arrest and apoptosis. PLoS One 2014;9:e88965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu F, Yuan W, Wang X, Sheng Z, Yuan Y, Qin C, He C, Xu T. CMTM3 is reduced in prostate cancer and inhibits migration, invasion and growth of LNCaP cells. Clin Transl Oncol. 2015;17:632–9. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Zhang J, Nan X, Li X, Qu J, Hong Y, Sun L, Chen Y, Li T. CMTM3 inhibits cell growth and migration and predicts favorable survival in oral squamous cell carcinoma. Tumor Biol. 2015;36:7849–58. [DOI] [PubMed] [Google Scholar]
- 13. Xie J, Yuan Y, Liu Z, Xiao Y, Zhang X, Qin C, Sheng Z, Xu T, Wang X. CMTM3 is frequently reduced in clear cell renal cell carcinoma and exhibits tumor suppressor activities. Clin Transl Oncol. 2014;16:402–9. [DOI] [PubMed] [Google Scholar]
- 14. Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–7. [DOI] [PubMed] [Google Scholar]
- 15. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. [DOI] [PubMed] [Google Scholar]
- 16. Fu X-T, Dai Z, Song K, Zhang Z-J, Zhou Z-J, Zhou S-L, Zhao Y-M, Xiao Y-S, Sun Q-M, Ding Z-B. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46:587–96. [DOI] [PubMed] [Google Scholar]
- 17. Gu F-M, Li Q-L, Gao Q, Jiang J-H, Zhu K, Huang X-Y, Pan J-F, Yan J, Hu J-H, Wang Z. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 2011;10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou B, Chen H, Wei D, Kuang Y, Zhao X, Li G, Xie J, Chen P. A novel miR-219-SMC4-JAK2/Stat3 regulatory pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu H, Jove R. The stats of cancer-new molecular targets come of age. Nat Rev Cancer 2004;4:97–105. [DOI] [PubMed] [Google Scholar]
- 20. Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002;21:2000–8. [DOI] [PubMed] [Google Scholar]
- 21. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. [DOI] [PubMed] [Google Scholar]
- 22. Zhang C, Guo F, Xu G, Jia W, Ge Y. Stat3 activation mediates epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. Hepatogastroenterology 2014;61:1082–9. [PubMed] [Google Scholar]
- 23. Zhang C, Guo F, Xu G, Ma J, Shao F. Stat3 cooperates with twist to mediate epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Rep. 2015;33:1872–82. [DOI] [PubMed] [Google Scholar]
- 24. Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan W, Li T, Mo X, Wang X, Liu B, Wang W, Su Y, Xu L, Han W. Knockdown of cmtm3 promotes metastasis of gastric cancer via the stat3/twist1/emt signaling pathway. Oncotarget 2016;7(20):29507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]