Abstract
Renal cell carcinoma (RCC) represents one of the most resistant tumors to radiation and chemotherapy. Current therapies for RCC patients are inefficient due to the lack of diagnostic and therapeutic markers. The expression of novel tumor-associated kinases has the potential to dramatically shape tumor cell behavior. Identifying tumor-associated kinases can lend insight into patterns of tumor growth and characteristics. In the present study, we investigated the receptor tyrosine kinase-like orphan receptor 2 (Ror2), a new tumor-associated kinase, in RCC primary tumors and cell lines. Knockdown of Ror2 expression in RCC cells with specific shRNA significantly reduced cell proliferation and induced apoptosis. Using in vitro migration and Matrigel invasion assays, we found that cell migration and invasive ability were also significantly inhibited. In RCC, Ror2 expression correlated with expression of genes involved at the cell cycle and migration, including PCNA, CDK1, TWIST, and MMP-2. Furthermore, in vivo xenograft studies in nude mice revealed that administration of a Ror2 shRNA plasmid significantly inhibited tumor growth. These findings suggest a novel pathway of tumor-promoting activity by Ror2 within renal carcinomas, with significant implications for unraveling the tumorigenesis of RCC.
Key words: Renal cell carcinoma (RCC), Receptor tyrosine kinase-like orphan receptor 2 (Ror2), Apoptosis, Migration, Invasion
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignant neoplasm of the kidney, accounting for 3% of all malignancies. The incidence of RCC has increased in recent years, with nearly 100,000 patients dying each year. More than a third of these patients present at advanced stages and with unresectable or metastatic disease (1,2). RCC tumors are characterized on the basis of histologic features as clear cell (80%), papillary (10%), chromophobe (<5%), or granular, spindle, or cyst-associated carcinomas (5%–15%) (3). Each of these histologic subtypes exhibits unique clinical behavior, with clear cell and granular types tending to show a more aggressive clinical phenotype (4). Among the cellular changes associated with tumor progression is tissue remodeling, compatible with the extensive migration necessary for the progression of metastatic disease. However, the mechanisms that promote tumor invasiveness or the acquisition of metastatic potential for the subtypes of RCC remain undefined.
The receptor tyrosine kinase-like orphan receptor 2 (Ror2) is a transmembrane protein that belongs to a conserved family of tyrosine kinase receptors with normal expression in the developing heart, brain, and lungs (5,6), and with the greatest expression in migrating neural crest and mesenchymal tissues (5). Recent evidence has implicated Ror2 in mediating both canonical and noncanonical signaling pathways (7). Ror2 was initially found to be highly expressed in osteosarcoma (8) and RCC (9) and has recently been found in an increasingly long list of cancers, currently including melanoma (10), colon cancer (11), head and neck squamous cell carcinoma (12), and breast cancer (13). Despite increasing evidence showing the role of the noncanonical pathways in tumorigenesis, however, the underlying molecular mechanisms are poorly understood.
In the present study, we showed upregulation of Ror2 in RCC tissues and cell lines. Knockdown of Ror2 can inhibit proliferation, migration, and invasion, and induce G1 phase cell cycle arrest and apoptosis of RCC cell lines. Furthermore, knockdown of Ror2 also inhibits tumor growth in vivo. Our data provide new insights into the molecular function of Ror2 as well as its regulatory mechanisms in RCC.
MATERIALS AND METHODS
Tissue Specimens
In the present study, a total of 21 RCC specimens were obtained from surgical tumor resections in accordance with the local Ethics Committee. The adjacent normal kidney tissue specimens were also collected from these patients.
Cell Culture and Treatments
Normal kidney cell line (HK-2) and RCC cell lines (A-498, GRC-1, 786-0, and CaKi-1) were obtained from the Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, P.R. China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies) and 100× penicillin–streptomycin solution (Invitrogen Life Technologies), and incubated in a humidified atmosphere at 37°C with 5% CO2.
The shRNA knockdown cells were generated by introducing commercial shRNA in a lentiviral vector (pLKO.1-EGFP) for Ror2 (Sino Biological Inc. Beijing, P.R. China), a control virus delivering a scramble short hairpin retrovirus. Overexpression cell lines were generated by transferring Ror2 cDNA (Sino Biological Inc.) into the pLV-IRES-eGFP plasmid (Invitrogen Life Technologies) via EcoRI/BamHI restriction enzyme sites. The infection of 786-0 and CaKi-1 RCC cells using lentivirus-expressing shRNA-Ror2 and Ror2 was prepared as previously described (14). The recombinant lentivirus pLKO.1-EGFP-scramble shRNA (shRNA-NC) and black pLV-IRES-eGFP (Vector-NC) were used as the negative control, respectively.
Reverse Transcription PCR Analysis
RNA was extracted from RCC cells and frozen RCC tumor tissues. cDNA was synthesized from RNA using a MMLV RT Reagent Kit (Thermo Fisher Scientific Inc.). The DyNAmo Flash SYBR Green qPCR kit (Finnzymes Oy, Espoo, Finland) was used and analyzed on the 7900H Fast Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions. For RT-PCR, primers targeting the Ror2 (left: 5′-TTTCAGGATGATTACCACGAG-3′, right: 5′-CTCACACTTGGGCAGCTGAA-3′), PCNA (left: 5′-GGTGTTGGAGGCACTCAAGG-3′, right: 5′-CAGGGTGAGCTGCACCAAAG-3′), CDK1 (left: 5′-ACCATACCCATTGACTAAC-3′, right: 5′-ATAAGCACATCCTGAAGAC-3′), TWIST (left: 5′-AGTCCGCAGTCTTACGAG-3′, right: 5′-GCTTGCCATCTTGGAGTC-3′), MMP-2 (left: 5′-TTGACGGTAAGGACGGACTC-3′, right: 5′-GGCGTTCCCATACTTCACAC-3′), and GAPDH (left: 5′-CACCCACTCCTCCACCTTTG-3′, right: 5′-CCACCACCCTGTTGCTGTAG-3′) were generated. Relative quantification of mRNA expression levels was determined using the 2−ΔΔCt method.
Immunoblotting
Cells were lysed in whole-cell extraction buffer containing 20 mM Tris, 100 mM NaCl, 1 mM EDTA, 1% NP-40, and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). Protein samples were separated on 8% SDS–polyacrylamide gels and transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The following antibodies were used to assess for expression: polyclonal Ror2 at 1:5,000 (Abcam, Cambridge, MA, USA), monoclonal PCNA at 1:1,000 (Cell Signaling Technology Inc., Danvers, MA, USA), monoclonal CDK1 at 1:1,000 (Abcam), polyclonal TWIST at 1:1,000 (Abcam), polyclonal MMP-2 at 1:1,000 (Abcam), and monoclonal GAPDH at 1:1,000 (Cell Signaling Technology Inc.) as a loading control. Appropriate horseradish peroxidase-conjugated secondary antibodies were used, and proteins were detected using ECL-Plus chemiluminescence reagents (GE).
Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Gaithersburg, USA) was used to evaluate the growth of RCC cells. Cells were seeded in 96-well plates at 5 × 103 cells per well in DMEM for 72 h. At specified time points, CCK-8 solution was added to each well as the manufactory protocol suggests. Plates were incubated for 4 h at the same incubator conditions, after which the absorbance was read at 450 nm using the iMark microplate absorbance reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Cell Cycle Analysis
Propidium iodide (PI) was used for cell cycle analysis. Cells were harvested and fixed with 70% ethanol and made RNA free. PI (5 μmol/L) was added and incubated for 20 min in the dark. Fluorescence intensity was measured using frequency lavatory-2 higher (FL-2H) with PI as the intercalating fluorescent dye.
Apoptosis Assays
Cell apoptosis was detected by annexin V/PI FACS analysis as previously described (15). Cells were harvested and washed twice with cold PBS and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml at a final volume of 100 μl. Annexin V-FITC (5 μl) was added to each tube for 20 min, and then 5 μl PI was added for another 10 min for incubation on ice. Finally, each sample was resuspended to a volume of 500 μl with 1× binding buffer and analyzed by flow cytometry within 30 min.
Cell Migration and Invasion Assay
Cells were trypsinized and counted with a hemocytometer using trypan blue, and viable cells were seeded in the upper chamber at 1 × 104 cells/well in serum-free DMEM. DMEM plus 10% FBS was placed in the lower chamber as a source of chemoattractant. Cells were allowed to migrate through a porous, uncoated membrane (BD Biosciences, Bedford, MA, USA) for 12 h at 37°C. Incubation was carried out for 48 h at 37°C in humidified air with 5% CO2. Nonmigratory cells in the upper chamber were then removed with a cotton-tip applicator. Migrated cells on the lower surface were fixed with methanol and stained with hematoxylin. The number of migrating cells was determined by counting five high-powered fields (200×) on each membrane and calculated as mean number of cells/field. The procedure for the cell invasion assay was similar to the cell migration assay, except that the Transwell membranes were precoated with Matrigel (BD Biosciences).
In Vivo Experiments
For tumor growth assay, 786-0 cells infected with shRNA-NC or shRNA-Ror2 were trypsinized and washed and resuspended in DMEM without FBS. Cell concentration and viability were determined using trypan blue. Twelve male athymic nude mice (SLAC Laboratory Animal Center, Shanghai, P.R. China) were randomly divided into two groups (six mice/group), and 2 × 106 786-0 cells were subcutaneously injected into the right armpit of the mice. Tumor size was determined every 3–4 days after the tumor formed (around 1–2 weeks). At 46 days after injection, the mice were euthanized, and the excised tumor tissues were formalin fixed, paraffin embedded, sectioned, and then analyzed with Ror2 immunohistochemistry. The tumors were weighed on a digital balance.
Immunohistochemistry
Immunohistochemistry assay was performed as previously described (16). RCC tissue sections were initially treated with deparaffinization and hydration, heated in EDTA (pH 8.0), and incubated with 3% hydrogen peroxide for 10 min for antigen retrieval. The reaction of Ror2 mouse polyclonal antibody (Abcam) took place for 1 h at room temperature, following incubation with goat anti-mouse horseradish peroxidase-conjugated IgG (Abcam). Slides were stained with DAB (Shanghai Long Island Biotec. Co., LTD, P.R. China) and hematoxylin (BASO, P.R. China). Immunohistochemical signals were calculated with the positive staining cells.
Coimmunoprecipitation (Co-IP)
Coimmunoprecipitation was performed as described previously (17). Both the input and IP samples were analyzed by Western blot using various antibodies at the following dilutions: Ror2 antibody (1:1,000), WNT5A antibody (1:1,000), WNT1 antibody (1:1,000), Flag-tag antibody (1:1,000), HA-tag antibody (1:1,000), and normal rabbit/mouse IgG (CST).
Statistical Analysis
Results were shown as mean ± SD. Statistical analysis was performed using the GraphPad Prism 5.0 environment (GraphPad Software, La Jolla, CA, USA). All assays were performed in triplicate and repeated at least three times. The comparison of different groups was analyzed using the unpaired, two-tailed Student’s t-test. All statistical analyses were two sided, and values of p < 0.05 were considered statistically significant.
RESULTS
Expression of Ror2 mRNA in RCC Specimens and Cell Lines
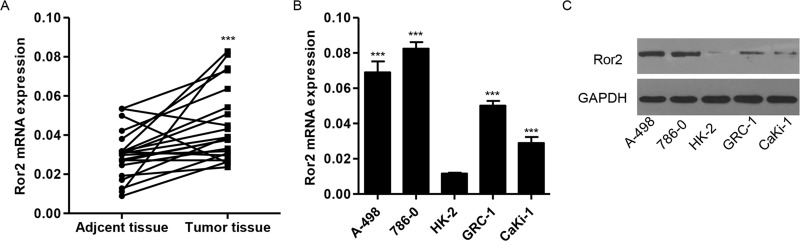
The expression of Ror2 was investigated in a panel of surgically resected RCC specimens and corresponding adjacent normal kidney specimens. Our RT-PCR analysis revealed that the expression of Ror2 was higher in RCC tissues compared with adjacent normal kidney tissues (Fig. 1). The mRNA and protein expression of endogenous Ror2 in RCC cancer cells, A-498, CaKi-1, 786-0, and GRC-1, and normal renal cells, HK-2, was confirmed. Ror2 was positively identified on multiple examinations of the RCC cell line, A-498, CaKi-1, 786-0, and GRC-1, but expressed weakly in the normal renal cells, HK-2 (Fig. 1B and C).
Figure 1.
Expression of Ror2 in RCC tissues and cell lines. (A) The expression level of Ror2 detected by RT-PCR in 21 paired RCC tissues and adjacent normal kidney tissues. (B, C) Expression of Ror2 in four RCC cell lines and a normal renal cell line was detected by RT-PCR and Western blot. ***p < 0.001 compared with adjacent normal kidney tissues or HK-2 cell lines.
shRNA-Mediated Knockdown of Ror2 in 786-0 and CaKi-1 Cells Inhibits Cell Proliferation
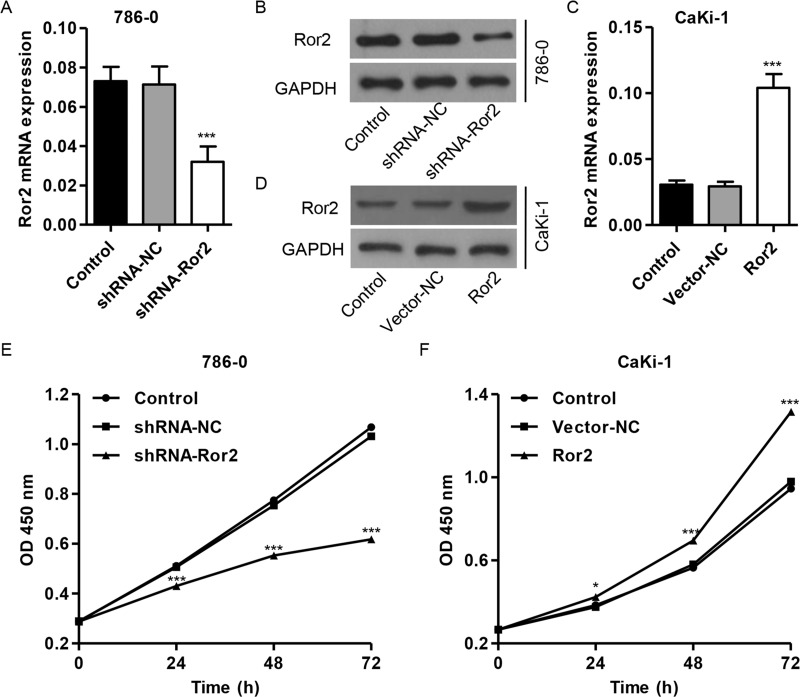
To validate whether Ror2 functions in cell growth regulation, RCC cell lines were used to examine changes in cellular phenotypes after Ror2 knockdown by RNA interference or Ror2 overexpression. In these experiments, treatment with Ror2 shRNA revealed ablation of Ror2 mRNA and protein expression (Fig. 2A and B), while Ror2 overexpression revealed abundant expression of Ror2 at both mRNA and protein levels (Fig. 2C and D). The treatment of Ror2 shRNA resulted in a gradual and significant decrease in cell proliferation in the 786-0 cell lines with highest expression of Ror2 (Fig. 2E). Meanwhile, treatment of Ror2 expression vector resulted in a gradual and significant increase in cell proliferation in the CaKi-1 cell lines with lowest expression of Ror2 (Fig. 2F). After 72 h, cell proliferation was reduced to 42% for the 786-0 cell lines and was increased to 39% for the CaKi-1 cell lines. These results suggest that suppression of Ror2 expression apparently reduces cell proliferation in RCC cells.
Figure 2.
Ror2 downregulation inhibits cell proliferation. Expression of Ror2 in 786-0 (A, B) and CaKi-1 cells (C, D) was detected by RT-PCR and Western blot. The 786-0 cells were infected with shRNA-Ror2, and the CaKi-1 cells were infected with Ror2-expressing vector; 0, 24, 48, and 72 h later, cells were collected. (E, F) Cell proliferation was detected by CCK-8 assay. *p < 0.05, ***p < 0.001 compared with shRNA-NC.
Ror2 Knockdown Induces G1 Phase Cell Cycle Arrest and Apoptosis In Vitro
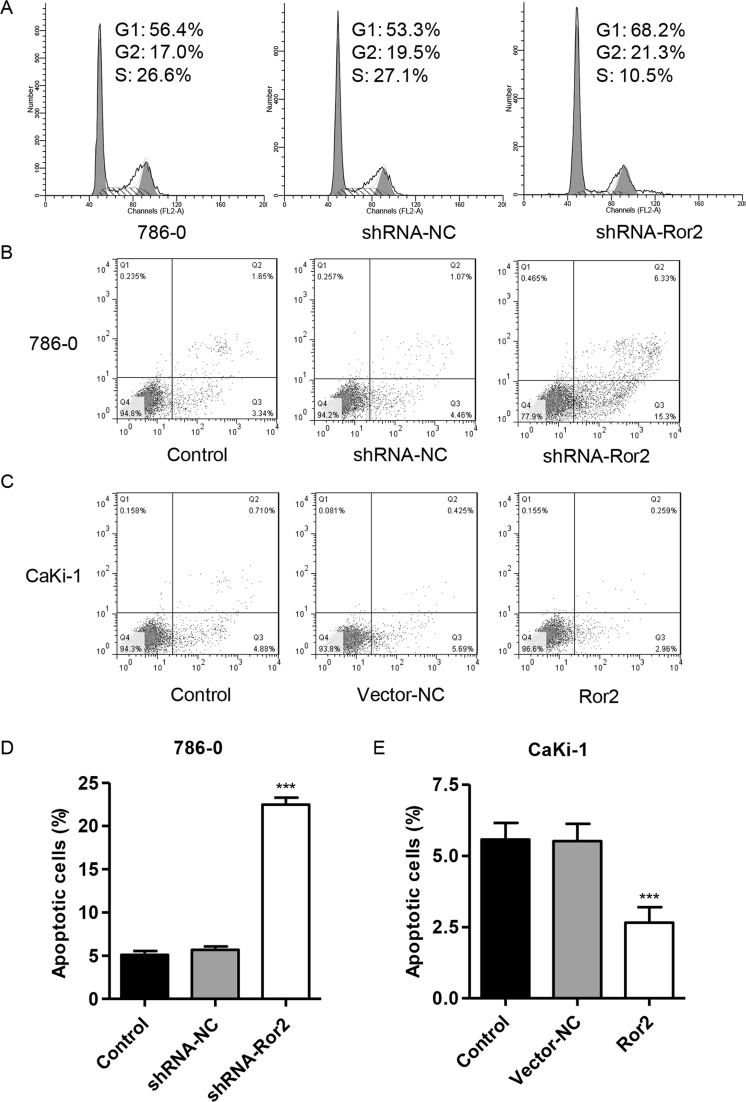
To further validate the effect of Ror2 on cell proliferation, the cell cycle was analyzed in 786-0 cell lines using flow cytometry. Cell cycle analysis showed that the knockdown of Ror2 notably increased the rate of G1 phase cells and decreased S phase cell population in 786-0 cell lines (Fig. 3A). We then evaluated the apoptotic function of Ror2 in 786-0 and CaKi-1 cells using an annexin V-FITC/PI staining and flow cytometry assay. As shown in Figure 3B and D, flow cytometry analysis revealed that the knockdown of Ror2 in 786-0 cell lines significantly induced cell apoptosis by threefold compared with control cells. Meanwhile, overexpression of Ror2 in CaKi-1 cell lines showed a significantly decreased apoptotic rate by 52% (Fig. 3C and E) compared with control cells. Taken together, these data suggest that suppression of Ror2 induces cell cycle arrest and apoptosis of RCC cells in vitro.
Figure 3.
Ror2 downregulation induces cell cycle arrest and apoptosis. The 786-0 cells were infected with shRNA-Ror2, and the CaKi-1 cells were infected with Ror2-expressing vector; 48 h later, cells were collected. (A) The 786-0 cell cycle profile was analyzed using flow cytometry and PI staining. The 786-0 (B, D) and CaKi-1 cell (C, E) apoptosis was analyzed using flow cytometry and annexin V-FITC/PI staining. ***p < 0.001 compared with shRNA-NC.
Ror2 Knockdown Suppresses Cell Migration and Invasion Properties In Vitro
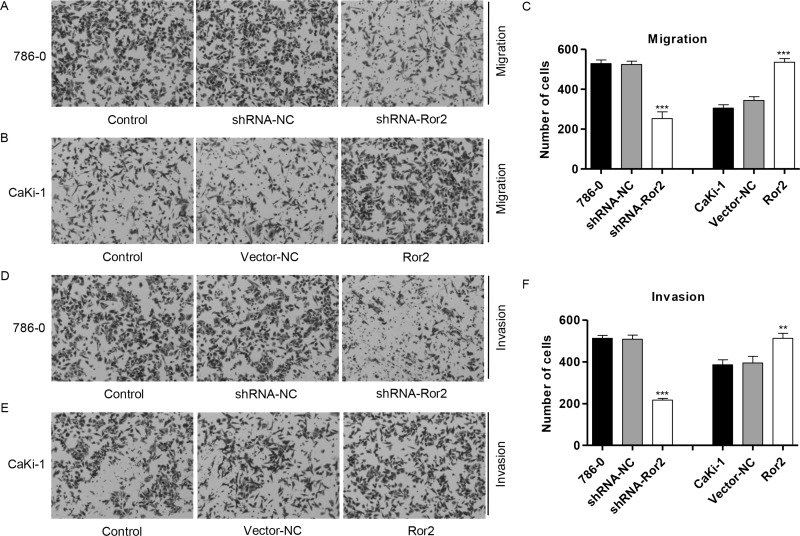
Our observation raised another question of whether substantial amounts of endogenously expressed Ror2 play any functional role(s) in cell migration and invasion. To determine if Ror2 shRNA could reduce protumorigenic cellular behaviors associated with Ror2 expression, we first determined the effect of decreased Ror2 expression on tumor cell migration. Migration assay revealed 77% inhibition in motility potential of Ror2 shRNA-infected 786-0 cell lines (Fig. 4A and C) and 1.9-fold increase in motility potential of Ror2-infected CaKi-1 cell lines (Fig. 4B and C), suggesting that the motility potential of Ror2 shRNA-infected RCC cells was severely impaired. Subsequently, we measured the capacity of RCC cells to invade through Matrigel, an artificial extracellular matrix (ECM), after infection with shRNA-NC or Ror2 shRNA. Decreased Ror2 expression led to the inhibition of invasion by 68% in 786-0 cell lines (Fig. 4D and F), and overexpression of Ror2 led to the increase in invasion by 1.1-fold in CaKi-1 cell lines (Fig. 4E and F), suggesting that the invasive potential of Ror2 shRNA-infected RCC cells was severely impaired.
Figure 4.
Ror2 downregulation inhibits cell migration and invasion. The 786-0 cells were infected with shRNA-Ror2, and the CaKi-1 cells were infected with Ror2-expressing vector; 48 h later, cells were collected. Cell migration (A–C) and invasion (D–F) were measured in 786-0 and CaKi-1 cells by Transwell assay coated without or with Matrigel.
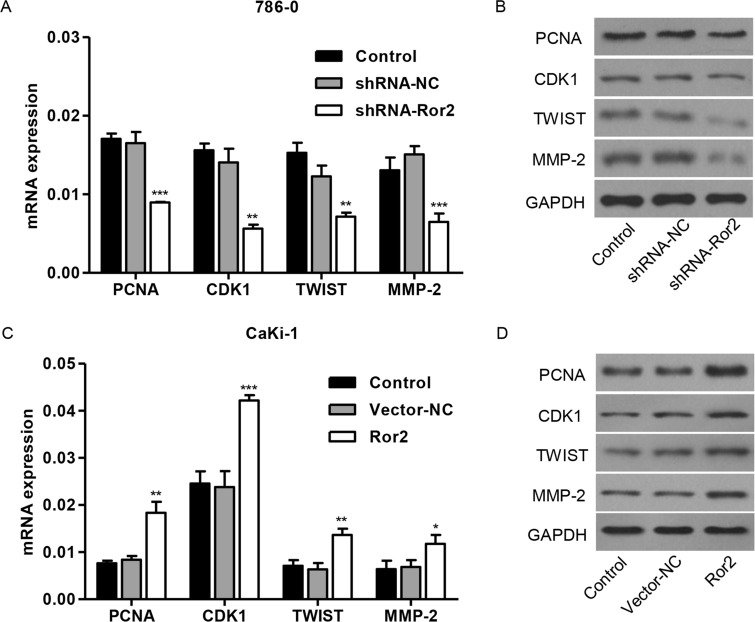
Effect of Ror2 on Expression Levels of PCNA, CDK1, TWIST, and MMP-2 In Vitro
To further explore the mechanism of Ror2 regulating cell proliferation, migration, and invasion of RCC cell lines, two cell cycle molecular markers, PCNA and CDK1, and TWIST and MMP-2 associated with migration, were predicated to be Ror2 targets. As shown in Figure 5A and B, the mRNA and protein levels of PCNA, CDK1, TWIST, and MMP-2 in 786-0 cell lines were remarkably decreased by shRNA-Ror2 in 786-0 cell lines compared with control cells, while Ror2 overexpression increased the expression of these molecules in CaKi-1 cell lines (Fig. 5C and D).
Figure 5.
Ror2 regulates proteins correlated with cell cycle and migration. The 786-0 cells were infected with shRNA-Ror2, and the CaKi-1 cells were infected with Ror2-expressing vector; 48 h later, cells were collected. (A, B) Expression of PCNA, CDK1, TWIST, and MMP-2 was evaluated in 786-0 (A, B) and CaKi-1 cells (C, D) by RT-PCR and Western blot. *p < 0.05, **p < 0.01, ***p < 0.001 compared with shRNA-NC.
Interacting Proteins for Ror2
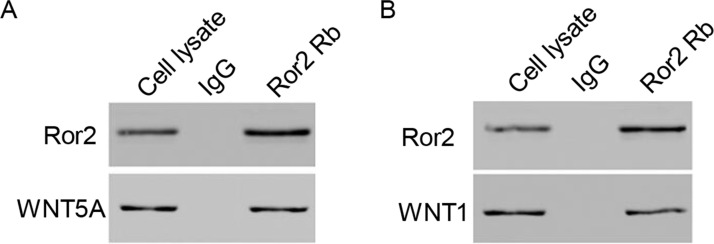
Twenty-five genes were found interacting with Ror2 by bioinformatics assay, including WNT1, WNT5A, LRP5, FZD5, FZD2, VANGL2, PRR20A, PSMC5, MAGED1, DAB1, RUVBL1, DAZAP2, MAP3K7, MAPK8, RAC1, MAPK9, MAPK10, ARRB2, CLTC, AP2M1, AP2A1, AP2A2, AP2S1, AP2B1, and CTHRC1, in which WNT5A and WNT1, which are associated with Wnt signaling, were selected to further validate our study. Our results obtained from the coimmunoprecipitation experiments indicated that WNT5A and WNT1 directly interact with Ror2 in 786-0 cell lines (Fig. 6A and B).
Figure 6.
Interacting proteins for Ror2 in vitro. (A, B) Coimmunoprecipitation showed that Ror2 interacts with WNT5A and WNT1 in the 786-0 cell lines.
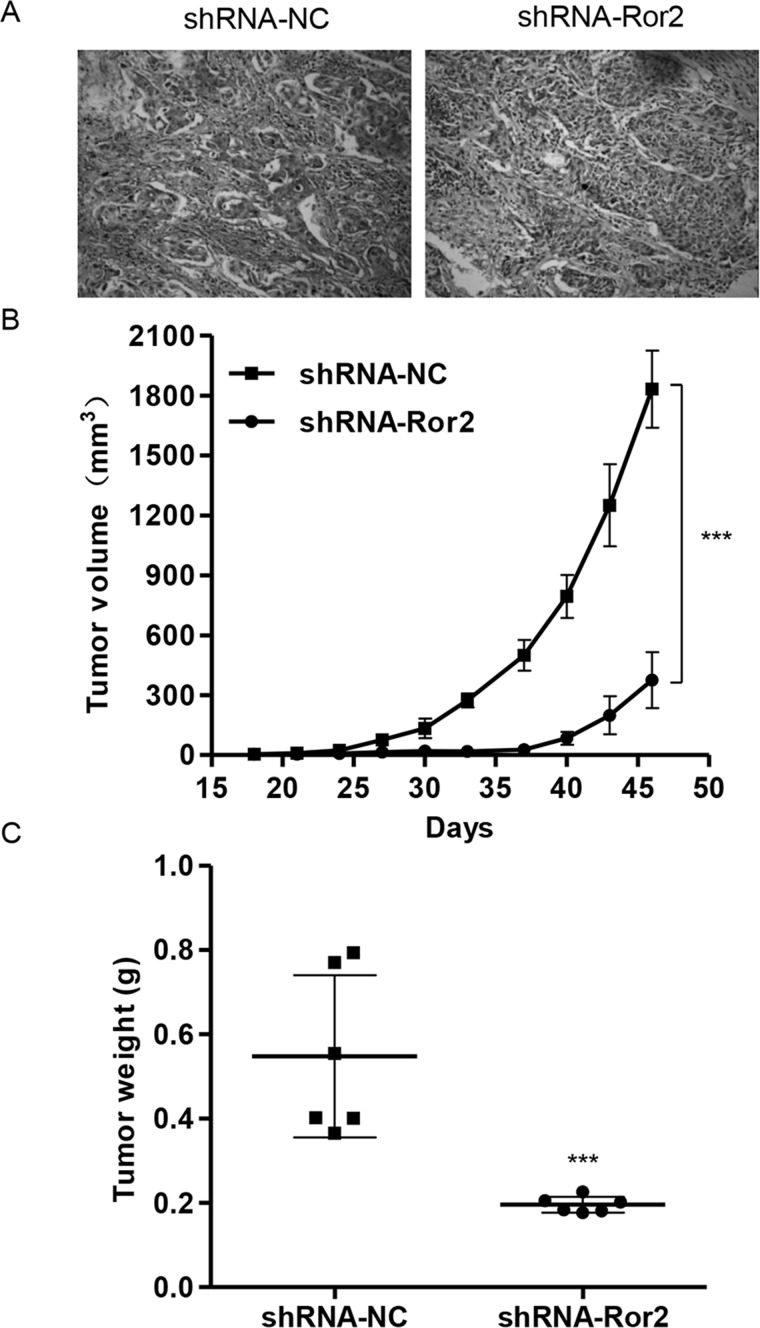
Effect of Ror2 on Tumor Growth of 786-0 Cells in Nude Mice
To further examine the effects of Ror2 on in vivo tumor growth, 786-0 cells stably expressing shRNA-NC or shRNA-Ror2 were injected subcutaneously into the right armpit of nude mice. Low expression of Ror2 (Fig. 7A) was observed in the xenograft from the nude mice injected with shRNA-Ror2-infected 786-0 cells. Moreover, shRNA-Ror2 tumors grew slower in mice, whereas shRNA-NC tumors grew faster in mice (Fig. 7B). After 46 days, tumor weights in shRNA-Ror2 mice were significantly decreased to 64% compared with those in the shRNA-NC group (Fig. 7C). These data suggest that knockdown of Ror2 inhibits tumor growth in nude mice.
Figure 7.
Ror2 downregulation in 786-0 cells reduced tumor growth in vivo. The 786-0 cells infected with shRNA-NC or shRNA-Ror2 were subcutaneously injected in nude mice. (A) Expression of CTNNB1 was determined by immunohistochemistry staining. (B) Growth curve of tumor volumes. (C) Tumor weights. ***p < 0.001 compared with shRNA-NC.
DISCUSSION
In a strategy to uncover potential signaling kinases for RCC, we have identified the developmentally regulated orphan kinase Ror2 as an activated tyrosine kinase in renal tumors and RCC cancer cell lines. The precise molecular mechanism of Ror2 involved in human cancer tumorigenesis has not be specifically described to date, although the aberrant expression of Ror2 has been observed from human glioblastoma (18), osteosarcoma (19), lung cancer (20), melanoma (21), and RCC (9). In the present study, we found that the expression of Ror2 was significantly higher in RCC tissues than in matched adjacent normal tissue in mRNA levels, which was in line with the previous study reporting that high expression of Ror2 was correlated with TNM stage, tumor status, lymph node metastasis, and overall survival of NSCLC patients (20).
Accordingly, several studies have provided in vitro evidence supporting the role of Ror2 in carcinogenesis (7,22). Ror2 silencing in cisplatin-resistant A2780-cis inhibits cell migration and invasion, whereas Ror2 overexpression in the parental cell line increases wound healing migration (23). The loss of Ror2 led to reduced RCC cell migration and anchorage-independent growth as indicated by in vitro experiments and xenograft mouse model (9). Our findings indicated that knockdown of Ror2 by RNA interference showed significant decreases in cell proliferation, migration, and invasion, and induced G1 cell cycle arrest and apoptosis. In addition, overexpression of Ror2 corrected the effects of Ror2 knockdown on tumorigenesis of RCC. Morioka et al. reported that overexpression of Ror2 in human fibroblast and kidney cells conferred increased invasive activity (8). However, in contrast to our data, overexpression of Ror2 in A2780-cis cell line had no effect on cell proliferation and cell migration and invasion through Transwells (23). Taken together, these studies suggest that Ror2 exerts oncogenic activity and may be a novel therapeutic target for some human cancers.
We investigated the correlations of Ror2 expression and cell cycle- and migration-related molecules, including PCNA, CDK1, TWIST, and MMP-2 in RCC. PCNA, CDK1 TWIST, and MMP-2 expression was significantly downregulated in the Ror2-silenced cells. However, the levels of these molecules were upregulated in the Ror2-overexpressed cells. Proper regulation of the cell cycle is a key element controlling cell division. Many proteins are involved in this process including cyclins and cyclin-dependent kinases that regulate accurate transition of the cell through subsequent phases of the cell cycle (24). PCNA has been found in the nuclei of yeast, plant, and animal cells that undergo cell division, suggesting a function in cell cycle regulation and/or DNA replication (25). According to current models, mammalian CDKs are essential for driving each cell cycle phase, so therapeutic strategies that block CDK activity are likely to selectively target tumor cells, whereas CDK1 is required for the cell cycle, and interphase CDKs are only essential for proliferation of specialized cells (26). Cell migration is a critical feature of numerous physiologic and pathologic phenomena, including development, wound repair, angiogenesis, and metastasis. TWIST plays important roles in tumor growth, progression, and survival in patients with RCC, which is significantly associated with increased cancer cell proliferation, angiogenesis, MMP-2 expression, and macrophage recruitment (27). Expression of the Ror2 kinase in human tumors was found to be tightly correlated with genes involved at the ECM, in particular MMP-2, linking this epithelially derived tumor with mesenchymal phenotypic markers and a potentially important transitioning event in the development of invasive RCC (28). These findings place Ror2 as a mediator of MMP-2 regulation in RCC and a potential regulator of ECM remodeling. The exact molecular mechanism as to how Ror2 regulates PCNA, CDK1, TWIST, and MMP-2 expression will require additional studies. However, our finding may be important for future studies on RCC invasion and metastasis.
Recently, previous data confirmed the findings of the experiments in vivo examining anchorage-independent growth, validating a role for Ror2 in promoting tumor cell growth independent of tumor cell survival in RCC (9). Similarly, in our laboratory, we have found that knockdown of Ror2 significantly inhibits xenograft tumor growth, suggesting that it may function in RCC carcinogenesis. In addition, immunohistochemistry assay revealed a humoral response against Ror2 protein in shRNA-NC and a weak response in shRNA-Ror2 mice.
Further, the regulation of Wnt signaling via Wnt receptors such as Ror2 can be greatly influenced by the availability of Wnt ligands, which may influence the activity of Ror2 in tumorigenicity (29). Wingless-type MMTV integration site family, member 5A (WNT5A) and member 1 (WNT1) are ligands for members of the frizzled family of seven transmembrane receptors regulating Wnt signaling. In the presence of Ror2, the canonical Wnt pathway is inhibited by promoting β-catenin degradation through a GSK3-independent pathway, which involves downregulation of β-catenin-induced reporter gene expression (30). Ror2 also positively modulates Wnt3A-activated canonical signaling in lung carcinoma through cooperative interactions with Fzd2, suggesting the function of Ror2 in modulating canonical Wnt signaling (31). In this study, we revealed that Ror2 directly bound to WNT5A and WNT1 in 786-0 cell lines, suggesting that signaling through Wnt pathway is a critical downstream mechanism by which Ror2 may regulate changes in the cell proliferation, cell cycle, apoptosis, and invasion.
Understanding the mechanisms involved in oncogenesis has wide-ranging implications for targeting the treatment of cancer. In particular, treatment directed at molecules such as Ror2 that are overexpressed in tumor tissues may minimize cytotoxic effects on normal cells. Furthermore, the potential use of Ror2 as a biomarker in tissue biopsies and monitoring patients after surgery should be explored. Finally, this identified kinase in RCC serves as an ideal candidate to explore as a marker of tumors with aggressive growth potential or as a putative target to disable tumor growth.
REFERENCES
- 1. Koul H.; Huh J. S.; Rove K. O.; Crompto L.; Koul S.; Meacham R. B.; Kim F. J. Molecular aspects of renal cell carcinoma: A review. Am. J. Cancer Res. 1:240–254; 2011. [PMC free article] [PubMed] [Google Scholar]
- 2. Cowey C. L.; Rathmell W. K. Using molecular biology to develop drugs for renal cell carcinoma. Expert Drug Dis. 3:311–327; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garg M.; Kanojia D.; Khosla A.; Dudha N.; Sati S.; Chaurasiya D.; Jagadish N.; Seth A.; Kumar R.; Gupta S.; Gupta A.; Lohiya N. K.; Suri A. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 68:8240–8248; 2008. [DOI] [PubMed] [Google Scholar]
- 4. Katagiri T.; Togashi A.; Ashida S.; Fujioka T.; Maruyama O.; Wakumoto Y.; Sakamoto Y.; Fujime M.; Kawachi Y.; Shuin T.; Nakamura Y. Hypoxia-inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma (RCC) and potential target for molecular therapy. Cancer Res. 66:304–304; 2006. [DOI] [PubMed] [Google Scholar]
- 5. Yoda A.; Oishi I.; Minami Y. Expression and function of the Ror-family receptor tyrosine kinases during development: Lessons from genetic analyses of nematodes, mice, and humans. J. Recept. Signal Transduct. Res. 23:1–15; 2003. [DOI] [PubMed] [Google Scholar]
- 6. Schwabe G. C.; Trepczik B.; Süring K.; Brieske N.; Tucker A. S.; Sharpe P. T.; Minami Y.; Mundlos S. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev. Dyn. 229:400–410; 2004. [DOI] [PubMed] [Google Scholar]
- 7. Debebe Z.; Rathmell W. K. Ror2 as a therapeutic target in cancer. Pharmacol. Ther. 150:143–148; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morioka K.; Tanikawa C.; Ochi K.; Daigo Y.; Katagiri T.; Kawano H.; Kawaguchi H.; Myoui A.; Yoshikawa H.; Naka N.; Araki N.; Kudawara I.; Ieguchi M.; Nakamura K.; Nakamura Y.; Matsuda K. Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer Sci. 100:1227–1233; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright T. M.; Brannon A. R.; Gordan J. D.; Mikels A. J.; Mitchell C.; Chen S.; Espinosa I.; van de Rijn M.; Pruthi R.; Wallen E.; Edwards L.; Nusse R.; Rathmell W. K. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28:2513–2523; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Connell M. P.; Fiori J. L.; Xu M.; Carter A. D.; Frank B. P.; Camilli T. C.; French A. D.; Dissanayake S. K.; Indig F. E.; Bernier M.; Taub D. D.; Hewitt S. M.; Weeraratna A. T. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29:34–44; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lara E.; Calvanese V.; Huidobro C.; Fernández A. F.; Moncada-Pazos A.; Obaya A. J.; Aguilera O.; González-Sancho J. M.; Sánchez L.; Astudillo A.; Muñoz A.; López-Otín C.; Esteller M.; Fraga M. F. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol. Cancer 9:170; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi M.; Shibuya Y.; Takeuchi J.; Murata M.; Suzuki H.; Yokoo S.; Umeda M.; Minami Y.; Komori T. Ror2 expression in squamous cell carcinoma and epithelial dysplasia of the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 107:398–406; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Henry C.; Quadir A.; Hawkins N. J.; Jary E.; Llamosas E.; Kumar D.; Daniels B.; Ward R. L.; Ford C. E. Expression of the novel Wnt receptor ROR2 is increased in breast cancer and may regulate both β-catenin dependent and independent Wnt signalling. J. Cancer Res. Clin. Oncol. 141:243–254; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H. J.; Tao J.; Sheng L.; Hu X.; Rong R. M.; Xu M.; Zhu T. Y. Twist2 promotes kidney cancer cell proliferation and invasion by regulating ITGA6 and CD44 expression in the ECM-receptor interaction pathway. OncoTargets Ther. 9:1801–1812; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mondal J.; Bishayee K.; Panigrahi A. K.; Khuda-Bukhsh A. R. Low doses of ethanolic extract of Boldo (Peumus boldus) can ameliorate toxicity generated by cisplatin in normal liver cells of mice in vivo and in WRL-68 cells in vitro, but not in cancer cells in vivo or in vitro. J. Integr. Med. 12:425–438; 2014. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X. F.; Zhu J.; Geng W. Y.; Zhao S. J.; Jiang C. W.; Cai S. R.; Cheng M.; Zhou C. Y.; Liu Z. B. Electroacupuncture at Feishu (BL13) and Zusanli (ST36) down-regulates the expression of orexins and their receptors in rats with chronic obstructive pulmonary disease. J. Integr. Med. 12:417–424; 2014. [DOI] [PubMed] [Google Scholar]
- 17. Wang S.; Chong Z. Z.; Shang Y. C.; Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of β-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr. Neurovasc. Res. 9:91–101; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stommel J. M.; Kimmelman A. C.; Ying H.; Nabioullin R.; Ponugoti A. H.; Wiedemeyer R.; Stegh A. H.; Bradner J. E.; Ligon K. L.; Brennan C.; Chin L.; DePinho R. A. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318:287–290; 2007. [DOI] [PubMed] [Google Scholar]
- 19. Zhang C.; Hu Y.; Wan J.; He H. MicroRNA-124 suppresses the migration and invasion of osteosarcoma cells via targeting ROR2-mediated non-canonical Wnt signaling. Oncol. Rep. 34:2195–2201; 2015. [DOI] [PubMed] [Google Scholar]
- 20. Lu C.; Wang X.; Zhu H.; Feng J.; Ni S.; Huang J. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget 6:24912; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai S. S.; Xue B.; Yang Y.; Zhao L.; Chu C. S.; Hao J. Y.; Wen C. J. Ror2-Src signaling in metastasis of mouse melanoma cells is inhibited by NRAGE. Cancer Genet. 205:552–562; 2012. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen N. R.; Debebe Z.; Wright T. M.; Brooks S. A.; Sendor A. B.; Brannon A. R.; Hakimi A. A.; Hsieh J. J.; Choueiri T. K.; Tamboli P.; Maranchie J. K.; Hinds P.; Wallen E. M.; Simpson C.; Norris J. L.; Janzen W. P. Rathmell WK11 Expression of Ror2 mediates invasive phenotypes in renal cell carcinoma. PLoS One 9:e116101; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henry C.; Llamosas E.; Djordjevic A.; Hacker N.; Ford C. Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis 5:e226; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strzalka W.; Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 107:1127–1140; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoimenov I.; Helleday T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 37:605–613;2009. [DOI] [PubMed] [Google Scholar]
- 26. Malumbres M.; Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 9:153–166; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Ohba K.; Miyata Y.; Matsuo T.; Asai A.; Mitsunari K.; Shida Y.; Kanda S.; Sakai H. High expression of Twist is associated with tumor aggressiveness and poor prognosis in patients with renal cell carcinoma. Int. J. Clin. Exp. Pathol. 7:3158–3165; 2014. [PMC free article] [PubMed] [Google Scholar]
- 28. Kurban G.; Hudon V.; Duplan E.; Ohh M.; Pause A. Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res. 66:1313–1319; 2006. [DOI] [PubMed] [Google Scholar]
- 29. Billiard J.; Way D. S.; Seestaller-Wehr L. M.; Moran R. A.; Mangine A.; Bodine P. V. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol. Endocrinol. 19:90–101; 2005. [DOI] [PubMed] [Google Scholar]
- 30. Topol L.; Jiang X.; Choi H.; Garrett-Beal L.; Carolan P. J.; Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 162:899–908;2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C.; Chen H.; Hu L.; Xing Y.; Sasaki T.; Villosis M. F.; Li J.; Nishita M.; Minami Y.; Minoo P. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol. Biol. 9:11; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]