Highlights
-
•
Cystic duct cancer following cholecystectomy is a very rare entity.
-
•
It is difficult to detect an early stage cystic duct cancer and confirm its superficial extension.
-
•
Preoperative diagnosis is necessary to select a curative surgical procedure.
Abbreviations: HCC, hepatic cell carcinoma; CECT, contrast enhanced computed tomography; CBD, common biliary duct; EUS, endoscopic ultrasonography; RCD, remnant cystic duct; ERCP, endoscopic retrograde cholangiopancreatography; IDUS, intraductal ultrasonography
Keywords: T1b, Remnant cystic duct, Cystic duct cancer
Abstract
Introduction
Although primary cystic duct cancer is a rare entity, remnant cystic duct cancer is even more rare. We report a case of early cystic duct cancer following cholecystectomy.
Presentation of the case
A 81 year-old man complained temporary loss of appetite. He had underwent cholecystectomy for acute cholecystitis 5 years prior. Contrast enhanced computed tomography, magnetic resonance image and endoscopic ultrasonography showed remnant cystic duct tumor with protrusion to common bile duct. Endoscopic retrograde cholangiography revealed defect of contrast medium around confluence of the remnant cystic duct and common bile duct. We performed step biopsy by using forceps which revealed adenocarcinoma. Based on these findings, extrahepatic bile duct and remnant cystic duct resection were performed. The histopathology showed adenocarcinoma, pap > tub2, filling in remnant cystic duct, 30 mm in size but showed no lymphovascular or perineural invasion, no lymph node metastasis and negative surgical margin, and was classified as pT1bN0M0.
Conclusion
This is a rare case of primary carcinoma of remnant cystic duct cancer which is detected during computed tomography follow up for hepatic cell carcinoma recurrence. We confirmed remnant cystic duct cancer and its superficial extension to common bile duct with endoscopic ultrasonography and intraductal ultrasonography. Proper curative surgery was performed.
1. Introduction
Cystic duct cancer is categorized as a type of gallbladder cancer according to the American Joint Committee on Cancer and Union for International Cancer Control manuals. Cystic duct cancer is a rare entity and remnant cystic duct cancer is even more rare. There are 9 case reports including our present case about remnant cystic duct cancer in the English literature. This report is the second case about T1b cancer.
2. Case report
We report a case of 81 year-old man who complained temporary loss of appetite. He had underwent cholecystectomy for acute cholecystitis and radiofrequency ablation for hepatic cell carcinoma(HCC) 5 years prior. The histopathology of resected gallbladder didn’t contain any malignant features. We had taken contrast enhanced computed tomography(CECT) semiannually to check for recurrence of HCC. Retrospectively, the enhanced nodule was already detected on 1 year prior CECT image on common biliary duct(CBD) wall. However, no one couldn’t mention its finding at that time. He showed no abnormal findings. Blood biochemistry revealed significant increases in the levels of transaminases and biliary enzymes (glutamate oxaloacetate transaminase: 74 U/L, glutamate pyruvate transaminase: 305U/L, γ-glutamyl transpeptidase: 648mU/mL and alkaline phosphatase: 753 U/L). The total bilirubin level was 1.9 mg/dL. Immunoglobulin G subgroup 4 was 38.7 mg/dL. Cancer antigen 19−9 (CA19−9) level was 13.1 U/mL. On CECT, we observed enhanced nodules on CBD wall (Fig. 1). On T2-weighted imaging of magnetic resonance, the nodules were shown without diffusion restriction on diffusion-weighted imaging and apparent diffusion coefficient imaging. Endoscopic ultrasonography (EUS) showed low echoic lesion in the remnant cystic duct(RCD) and its protrusion to CBD. Another nodule on opposite side wall of CBD as well (Fig. 2). Endoscopic retrograde cholangiopancreatography (ERCP) revealed defect of contrast medium around confluence of the RCD and CBD, but RCD wasn’t shown. Low echoic mass protruded to CBD from RCD in intraductal ultrasonography (IDUS) (Fig. 3). We performed step biopsy by using forceps which revealed adenocarcinoma from nearby the confluence of RCD and no malignant lesion from the hepatic hilar and the intrapancreatic bile duct. We considered to perform pancreatoduodenectomy. However, we performed distal bile duct resection in consideration for the patient’s age and comorbidities.
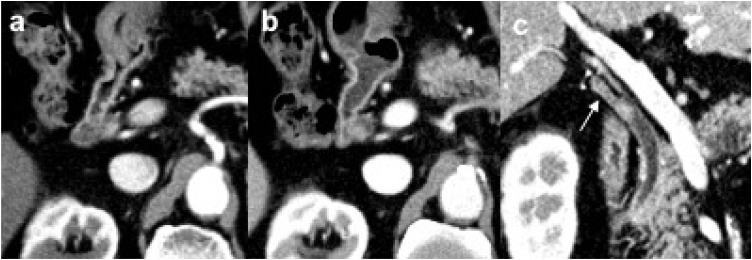
Fig. 1.
a the enhanced nodule was already detected on 1 year prior image in the common biliary duct(CBD). b the nodule expanded slightly when this patient complained sympyoms. c a multi planar reconstruction image shows the enhanced lesion in the remnant cystic duct(RCD)(arrow) and protrusion to CBD.
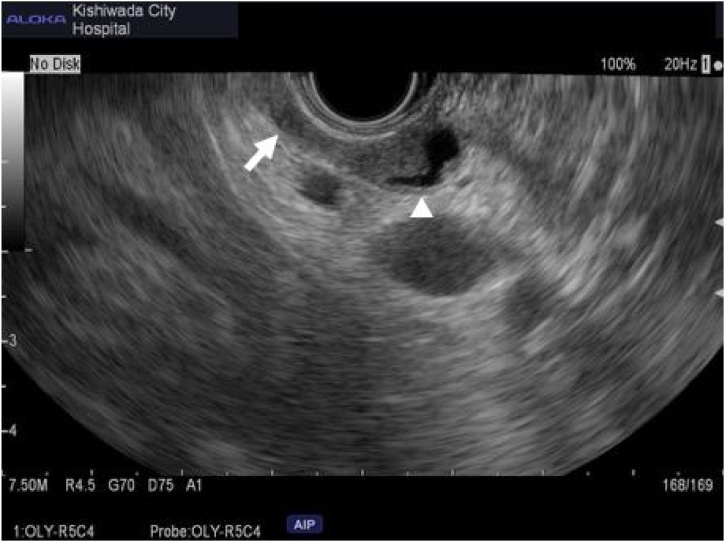
Fig. 2.
Endoscopic ultrasound sonography: Low echoic mass lesion in remnant cystic duct(arrow) protrudes to common hepatic duct(arrow head). Another nodule on opposite side wall of common hepatic duct as well.
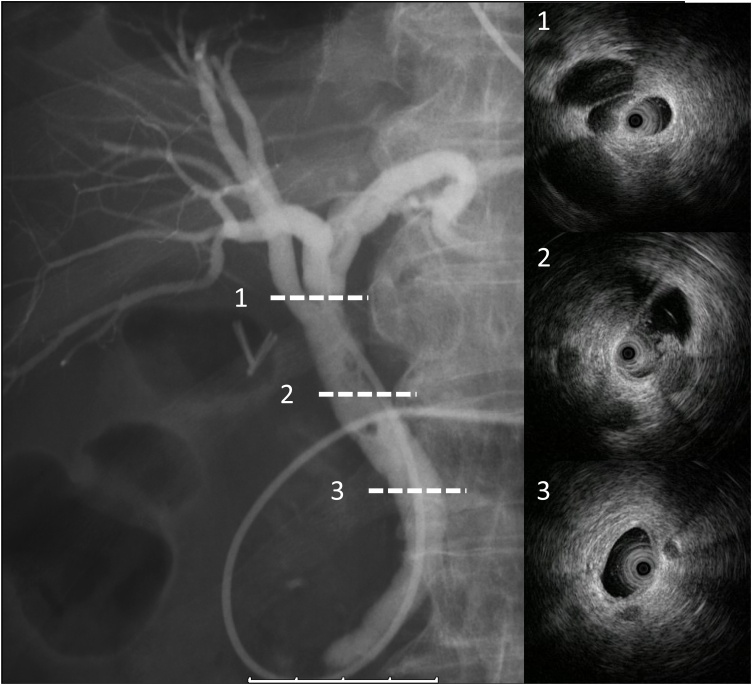
Fig. 3.
Endoscopic nasobiliary cholangiography depicts filling defect around the confluence of cystic duct and common hepatic duct, but cystic duct wasn’t shown. Intraductal ultrasound image; 1,3 no significant bile duct wall thickness. 2 low echoic mass protrudes to common hepatic duct from remnant cystic duct.
The histopathology showed adenocarcinoma, pap > tub2, filling in RCD with protrusion to CBD, 30 mm in size, that had invaded to the fibromuscular layer, but showed no lymph node metastasis, and negative surgical margin, and was classified as pT1bN0M0 according to the American Joint Committee on Cancer and Union for International Cancer Control manual, 8th edition [1]. (Fig. 4, Fig. 5) He recovered without any complication. There was no recurrence 8 months after operation.
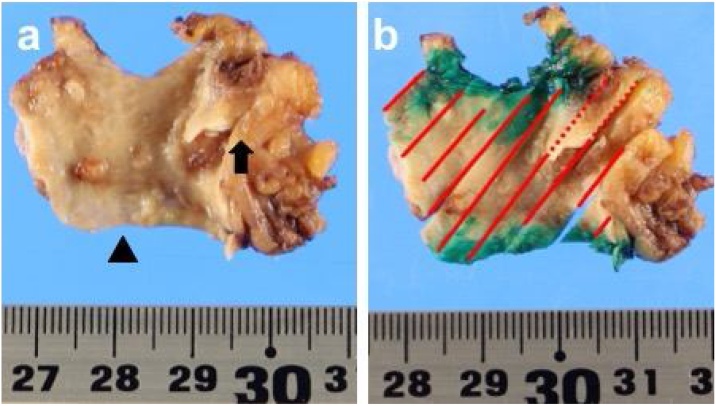
Fig. 4.
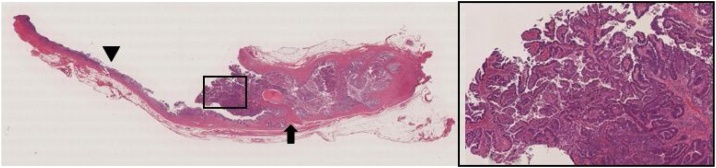
Resected specimen(arrow head is duodenal surgical margin of side common hepatic duct). a nodules on common hepatic duct wall and papillary expanding tumor in remnant cystic duct(arrow). b solid lines: tumor spreading along to common hepatic duct wall. spotted line: tumor in remnant cystic duct.
Fig. 5.
Papillary adenocarcinoma is filling in the cystic duct(arrow). Low papillary neoplasm is spreading laterally on common hepatic duct(arrow head).
3. Discussion
We herein describe a case of T1b RCD cancer following cholecystectomy. On 1 year prior CT image, we can detect a nodule in CBD retrospectively, but its findings was overlooked because the main purpose was detection of HCC recurrence and there was no bile duct dilation. His symptoms such as loss of appetite and liver dysfunction led to diagnosis of RCD cancer. Cystic duct cancer is a rare entity. Tu-Nan Yu et al. reviewed extrahepatic cholangiocarcinoma diagnosed between 2006 and 2015, cystic duct cancer was only accounted 0.44 % [2]. In 1951, Farrar proposed diagnostic criteria for cystic duct carcinoma. Cystic duce cancer was defined based on the following three criteria: “a) The growth must be restricted to the cystic duct; b) There must be no neoplastic process in the gallbladder, hepatic or common bile ducts; and c) A histological examination of the growth must confirm the presence of carcinoma cells” [3]. But Farrar’s criteria is only applicable to limited carcinoma and excludes advanced one originating from the cystic duct. In most cases, the tumor extends to gallbladder and CBD and the origin is difficult to determine. Ozden et al. proposed new working definition which describes a cystic duct adenocarcinoma as a gallbladder cancer whose center is located in the cystic duct [4].
Cystic duct cancer is rare but might be suspected in patients presenting with distended gallbladder or cholecystitis. However, RCD cancer doesn’t cause symptoms related to gallbladder swelling. If it advanced to CBD, obstructive jaundice will occur.
There are limited reports about RCD cancer following cholecystectomy. To the best of our knowledge, only 9 cases of subsequent remnant cystic duct cancer following cholecystectomy including one case of T1b cancer have been reported (Table 1; found by searching the PubMed databases by using the keywords “remnant cystic duct cancer” or “cystic duct cancer after cholecystectomy” all articles were written in English) [[5], [6], [7], [8], [9], [10], [11], [12]].
Table 1.
Summary of the previous reported cases of remnant cancer [[5], [6], [7], [8], [9], [10], [11], [12]].
| Author | Year | Country | age(y.o.) | Gender | Symptom | T(yrs) | Histological type | Surgery | Stage |
|---|---|---|---|---|---|---|---|---|---|
| Kuwayti et al. [5] | 1957 | USA | 73 | female | ND | 3 | ND | ND | ND |
| Phillips et al. [6] | 1969 | USA | 57 | male | abdominal pain, jaundice | 6 | AC | biopsy and T-tube drainage | liver metastases |
| Dixon et al. [7] | 1971 | USA | 77 | female | abdominal pain, loss of weight | 5 | muc | biopsy and T-tube drainage | involvement of RHA,PV |
| Gabata et al. [8] | 2003 | Japan | 70 | male | abdominal pain, fever, jaundice | 0.5 | pap | PD | T3NxMx |
| Noji et al. [9] | 2003 | Japan | 62 | male | abdominal discomfort | 15 | tub2-por | ERH, BD, PC | T3N2M0 |
| Eum et al. [10] | 2008 | Korea | 45 | male | none | 20 | por | BD | T3N0M0 |
| Do et al. [11] | 2014 | Korea | 74 | male | abdominal pain | 10 | AC | WR, BD | T2N0M0 |
| Prasoon et al. [12] | 2019 | Japan | 81 | female | abdominal pain | 2 | tub1 | BD | T1bN0M0 |
| Present case | 2021 | Japan | 81 | male | loss of appetite | 5 | pap > tub2 | BD | T1bN0M0 |
ND: not documented.
T: Time interval after prior cholecystectomy.
AC: Adenocarcinoma(degree of differenriation isn't available).
PD: Pancreatoduodenectomy.
ERH: Extended right hepatecyomy.
BD: Bile duct resection.
PC: Partial colectomy.
All their cause of prior cholecystectomy were cholelithiasis or cholecystitis. Cases since 2003 were reported from Korea or Japan. This would be affected by worldwide prevalence of biliary tract cancer [13]. Although histological type of the RCD cancer case series were various, early stage cancer cases were tub1 or pap [12]. Our case is not only resected in early stage, but also 1 year has passed since it had detected firstly with CECT. This suggests that the tumor progressed slowly. The Japanese Society of Hepato‐Biliary‐Pancreatic Surgery proposed that extrahepatic cholangiocarcinoma could be classified as one of three types based on gross morphology: papillary type, nodular type or flat type types [14]. Papillary type is reported to significant tendency of noninvasiveness to deeper layers and better prognosis. Frequent occurrence of papillary type with early extrahepatic bile duct cancer may be explained by the fact that these tumor types grow and spread superficially along the bile duct mucosa and do not invade deeply into the bile duct wall [[15], [16], [17], [18]].
To confirm RCD cancer is challenging if there isn’t jaundice or bile duct dilation. Although this kind of cancer is extremely, careful radiographic image interpretation, EUS and IDUS may be helpful to diagnose early stage RCD cancer. In our case, RCD cancer was spreading to CBD. This enabled us to diagnose as malignant by ERCP preoperatively.
4. Conclusion
This is a rare case of primary carcinoma of RCD which is detected during CT follow up for HCC recurrence. We confirmed RCD cancer and its superficial extension to CBD with EUS and IDUS. Proper curative surgery was performed.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This study is exempt from ethnical approval in Kishiwada city hospital ethics review committee.
Consent
Informed consent was obtained from this patient to be included in the study.
Author contribution
All authors in this manuscript contributed to the interpretation of data, and drafting and writing of this manuscript. Muneji Yasuda is first author and corresponding author of this paper. Yuichi Tanaka, Shinji Miyajima, Toyokazu Fukunaga and Haruo Takaya were engaged in patient’s care in our hospital. Kozo Kajimura reviewed the final manuscript. All the authors read and approved the final manuscript.
Registration of research studies
Not Applicable.
Guarantor
Kozo Kajimura.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgments
None.
References
- 1.Zhu A.X., Pawlik T.M., Kooby D.A., Schefter T.E., Vauthey J.N. Gallbladder. In: Amin M.B., Edge S.B., Greene F.L., Byrd D.R., Brookland R.K., Washington M.K., editors. American Joint Committee on Cancer Staging Manual. 8. Springer; New York: 2017. pp. 303–309. [Google Scholar]
- 2.Yu T.N. Cystic duct cancer: should it be deemed as a type of gallbladder cancer? World J. Gastroenterol. 2019;25(44):6541–6550. doi: 10.3748/wjg.v25.i44.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar D.A. Carcinoma of the cystic duct. Br. J. Surg. 1951;39(154):183–185. doi: 10.1002/bjs.18003915414. [DOI] [PubMed] [Google Scholar]
- 4.Ozden I. Cystic duct carcinoma: a proposal for a new “working definition”. Langenbecks Arch. Surg. 2003;387(9–10):337–342. doi: 10.1007/s00423-002-0333-7. [DOI] [PubMed] [Google Scholar]
- 5.Kuwayti K. Carcinoma of the major intrahepatic and the extrahepatic bile ducts exclusive of the papilla of Vater. Surg. Gynecol. Obstet. 1957;104(3):357–366. [PubMed] [Google Scholar]
- 6.Phillips S.J., Estrin J. Primary adenocarcinoma in a cystic duct stump. Report of a case and review of the literature. Arch. Surg. 1969;98(2):225–227. doi: 10.1001/archsurg.1969.01340080117026. [DOI] [PubMed] [Google Scholar]
- 7.Dixon J.A., Christensen C. Primary adenocarcinoma in cystic duct remnants. Am. J. Surg. 1971;121(5):610–611. doi: 10.1016/0002-9610(71)90151-6. [DOI] [PubMed] [Google Scholar]
- 8.Gabata T. Cystic duct remnant carcinoma with widespread invasion along the extrahepatic bile duct wall: dynamic CT findings. Abdom. Imaging. 2003;28(1):79–82. doi: 10.1007/s00261-001-0159-8. [DOI] [PubMed] [Google Scholar]
- 9.Noji T. Carcinoma of the cystic duct remnant with direct colonic invasion. Int. J. Gastrointest. Cancer. 2003;34(2–3):117–120. doi: 10.1385/IJGC:34:2-3:117. [DOI] [PubMed] [Google Scholar]
- 10.Eum J.S. A remnant cystic duct cancer presenting as a duodenal submucosal tumor. Gastrointest. Endosc. 2008;67(6):975–976. doi: 10.1016/j.gie.2007.11.010. discussion 976. [DOI] [PubMed] [Google Scholar]
- 11.Do J.H., Choi Y.S., Ze E.Y. Adenocarcinoma developed from remnant cystic duct after cholecystectomy. BMC Gastroenterol. 2014;14:175. doi: 10.1186/1471-230X-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasoon P. Early-stage T1b adenocarcinoma arising in the remnant cystic duct after laparoscopic cholecystectomy: a case report and literature review. BMC Surg. 2019;19(1):183. doi: 10.1186/s12893-019-0647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridgewater J.A. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am. Soc. Clin. Oncol. Educ. Book. 2016;35:e194–203. doi: 10.1200/EDBK_160831. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki M. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J. Hepatobiliary Pancreat Sci. 2015;22(3):181–196. doi: 10.1002/jhbp.211. [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda T. Early carcinoma of the extrahepatic bile duct. Jpn. J. Surg. 1989;19(6):691–698. doi: 10.1007/BF02471720. [DOI] [PubMed] [Google Scholar]
- 16.Mizumoto R., Ogura Y., Kusuda T. Definition and diagnosis of early cancer of the biliary tract. Hepatogastroenterology. 1993;40(1):69–77. [PubMed] [Google Scholar]
- 17.Kozuka S., Tsubone M., Hachisuka K. Evolution of carcinoma in the extrahepatic bile ducts. Cancer. 1984;54(1):65–72. doi: 10.1002/1097-0142(19840701)54:1<65::aid-cncr2820540115>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Tamada K. Cholangiographic findings of early-stage extrahepatic bile duct carcinoma. J. Gastroenterol. 2001;36(12):837–841. doi: 10.1007/s005350170006. [DOI] [PubMed] [Google Scholar]