Abstract
Sex-determining region Y (SRY)-box 9 (SOX9) is a member of the SOX transcription factor family. Increasing evidence has reported that SOX9 plays different roles in various types of malignancies. However, the role of SOX9 in papillary thyroid cancer (PTC) is still unclear. The aim of this study was to investigate the role of SOX9 in PTC. Our results showed that SOX9 was upregulated in PTC tissues and cell lines. In addition, knockdown of SOX9 significantly inhibited PTC proliferation, colony formation, migration, and invasion, as well as epithelial–mesenchymal transition (EMT) phenotype in TPC-1 and BCPAP cells. Moreover, knockdown of SOX9 significantly inhibited the expression levels of β-catenin, cyclin D1, and c-Myc in PTC cells. In conclusion, this is the first report demonstrating that knockdown of SOX9 inhibited PTC cell proliferation, invasion, and the EMT process via suppressing Wnt/β-catenin signaling pathway. Thus, SOX9 may act as a novel molecular target for the prevention and treatment of PTC.
Key words: SOX9, Papillary thyroid cancer (PTC), Invasion, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Thyroid cancer is one of the most common endocrine malignancies worldwide. Papillary thyroid cancer (PTC), referred to as a differentiated neoplasia, is the most common histological type, accounting for 80%–90% of all thyroid cancers (1). PTC is usually removed by thyroidectomy and treated with iodine-131 radiation therapy. However, a few PTC carriers develop recurrences and distant metastasis (2,3). The pathogenesis of PTC is believed to be a multistep process that involves multiple genetic changes, including loss of tumor suppressor genes and activation of oncogenes. Although this cancer has been widely studied, the precise genetic changes underlying the development and progression of PTC are not completely understood. Therefore, there is an urgent need to explore the molecular mechanisms responsible for PTC.
The sex-determining region Y (SRY)-box (SOX) genes encode a family of high-mobility groups that are a family of transcriptional factors and have emerged as potent modulators involved in orchestrating embryonic development and cell fate, organogenesis, and stem cell maintenance (4). The role of the SOX gene family in carcinogenesis has been attributed to their properties involved in the regulation of cell differentiation, proliferation, and survival in multiple processes (5–8). SOX9 is a member of the SOX transcription factor family. Increasing evidence has reported that SOX9 plays different roles in various types of malignancies. Some reports demonstrated that SOX9 was overexpressed in gastric carcinoma, breast cancer, pancreatic ductal adenocarcinoma, and glioma (9–12), whereas other studies showed that SOX9 may function as a tumor suppressor, such as in bladder cancer and melanoma (13,14). However, the role of SOX9 in PTC is still unclear. The aim of this study was to investigate the role of SOX9 in PTC. Our data indicated that knockdown of SOX9 inhibited PTC cell proliferation, invasion, and epithelial–mesenchymal transition (EMT) via suppressing the Wnt/β-catenin signaling pathway.
MATERIALS AND METHODS
Tissue Samples
PTC specimens (n = 12) and normal thyroid specimens (n = 12) were collected between 2011 and 2014 at the Department of Normal Surgery, Weifang People’s Hospital (P.R. China). The mean age of the patients (ranging from 13 to 69 years) was 47 years. None of these patients received chemotherapy or radiotherapy before surgery, and informed consent was obtained from each patient before surgery. Experimental procedures were approved by the Weifang People’s Hospital.
Cell Culture
Human PTC cell lines TPC-1 and BCPAP, and the normal thyroid epithelial cell-derived cell line HTori-3 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum (FBS), 1% glutamine, and 1% penicillin–streptomycin.
RT-PCR
Total RNA was extracted from PTC specimens and cell lines using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. Up to 5 μg of the total RNA was reverse transcribed into cDNA using M-MLV reverse transcriptase (Clontech, Palo Alto, CA, USA). RT-qPCR was performed in a final volume of 10 μl, which contained 5 μl of SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories), 1 μl of cDNA (1:50 dilution), and 2 μl each of the forward and reverse primers (1 mM). The specific primers used were as follows: SOX9, 5′-TGCTCAAGGGCTACGACTG-3′ (sense) and 5′-ACGCTTCTCGCTCTCATTCA-3′ (antisense); β-actin, 5′-CTCCATCCTGGCCTCGCTGT-3′ (sense) and 5′-GCTGTCACCTTCACCGTTCC-3′ (antisense). The PCR procedure was as follows: polymerase activation for 30 s at 95°C, 35 cycles of amplification each consisting of 95°C for 5 s, 60°C for 20 s, and 1 cycle of dissociation consisting of 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s. Results were normalized to β-actin expression. Relative quantification of the gene of interest was determined using the comparative ΔCt method.
Western Blot Analysis
The proteins were extracted from the PTC cells using RIPA lysis buffer (Beyotime, Nantong, P.R. China). The protein concentration in the lysates was determined using a BCA protein assay kit (Beyotime). For Western blot analysis, proteins were fractionated by 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes (Amersham, Little Chalfont, UK). The membranes were shaken and blocked at room temperature with 5% nonfat dry milk in Tris-buffered saline (TBS) for 1 h followed by incubation in the primary antibodies (mouse polyclonal SOX9, N-cadherin, E-cadherin, β-catenin, cyclin D1, c-Myc, and β-actin; from Santa Cruz Biotechnology) diluted in blocking buffer (1:10,000) at 4°C overnight. After washing with TBST buffer (0.05 mol/L Tris, 0.15 mol/L NaCl, and 0.05% Tween 20), the membranes were incubated in peroxidase-conjugated secondary antibody goat anti-rabbit IgG (diluted 1:3,000 in blocking buffer; Boster Corp., Wuhan, Hubei, P.R. China) for 1 h. Protein–antibody complexes were visualized using the enhanced chemiluminescence Western blotting detection system according to the manufacturer’s protocol (GE, Orsay, France).
RNA Silencing and Cell Transfection
siRNA-SOX9 oligoribonucleotide and control siRNA were obtained from Shanghai Sangon Co., Ltd. (Shanghai, P.R. China). The sequences corresponding to the siRNA of SOX9 were 5′-GCAGCGACGUCAUCUCCAAdTdT-3′ (sense) and 5′-dTdTCGUCGCUGCAGUAGAGGUU-3′ (antisense). Cells were transfected with siRNA-SOX9 or siRNA-scramble using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Knockdown efficiency of SOX9 gene expression was then assessed using Western blot analysis 48 h after transfection.
MTT Assay
For the MTT assay, cells were seeded into a 96-well plate in a density of 5 × 104 cells/well and cultured under regular conditions until they reached 80% confluence. siRNA-SOX9 was transfected according to standard protocols and was continually incubated with cells at 37°C with 5% CO2 for 24, 48, or 72 h. The culture medium was replaced with fresh medium containing MTT (5 mg/ml in PBS, 200 μl/well) and incubated with the cells for an additional 4 h. Then formazan was dissolved in DMSO (150 μl/well; Sigma-Aldrich) for 10 min. The results were analyzed by an automated plate reader at 490 nm. The experiments were repeated at least three times.
Soft Agar Assay
TPC-1 and BCPAP cells transfected with siRNA-SOX9 were seeded into 100-mm petri dishes with soft agar. The cells were allowed to grow for 12 days until colonies formed. Colonies were stained with crystal violet and counted under a microscope using OpenCFU 3.8-BETA software.
Nucleosome ELISA for Detection of Apoptosis
Cell apoptosis was carried out using Nucleosome ELISA Kit. TPC-1 and BCPAP cells transfected with siRNA-SOX9 were harvested. Nucleosome ELISAs were performed according to the manufacturer’s instructions (Oncogene Research Products, Cambridge, MA, USA).
Migration and Invasion Assays
Cell migration was measured by Transwell assay. In brief, TPC-1 and BCPAP cells transfected with siRNA-SOX9 were added to the upper compartment with an 8-μm microporous filter. Then 500 μl of DMEM containing 10% FBS was added to the bottom chamber. After 24 h, cells on the upper surface of the filters were removed using cotton swabs. Cells that migrated to the lower surface of the filters were washed, fixed, stained with crystal violet, and counted under a microscope.
Cell invasion was assessed by Matrigel-precoated Transwell inserts (8.0-mm pore size with polyethylene tetraphthalate membrane) according to the manufacturer’s protocol. To assess invasion, filters were precoated with 10 mg of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Cells transfected with siRNA-SOX9 were seeded into the upper Transwell chambers (BD Biosciences) in medium, and 10% FBS was added into the lower wells as the chemoattractant. After 24 h, the filters were stained with crystal violet. The number of cells that invaded the lower side of the membrane was determined by counting cells in a minimum of four randomly selected areas.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical differences were analyzed by one-way ANOVA followed by multiple comparisons performed with post hoc Bonferroni test (SPSS version 16.0; SPSS, Chicago, IL, USA). A value of p < 0.05 was considered to indicate a statistically significant result.
RESULTS
SOX9 Is Upregulated in Human PTC Samples and Cell Lines
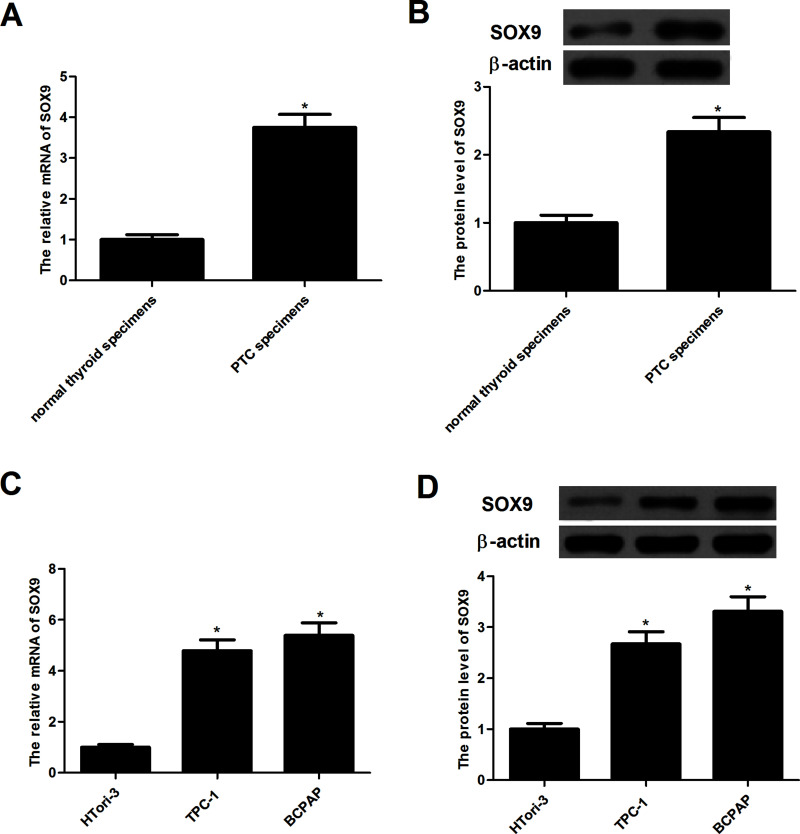
To investigate the role of SOX9 in the tumorigenesis of PTC, we detected the expression of SOX9 in PTC samples. As indicated in Figure 1A, the expression of SOX9 mRNA was significantly increased in PTC samples when compared with those in normal thyroid specimens. Western blot analysis demonstrated that the expression of SOX9 protein was obviously increased in PTC samples (Fig. 1B). In addition, we evaluated the expression of SOX9 in PTC cell lines; the expression levels of SOX9 mRNA and protein were also increased in TPC-1 and BCPAP cells (Fig. 1C and D). These results suggest that SOX9 is upregulated in PTC.
Figure 1.
Expression of SOX9 in human papillary thyroid cancer tissue specimens and cell lines. (A) SOX9 mRNA levels in papillary thyroid cancer tissue specimens were significantly higher than in normal thyroid tissue specimens. *p < 0.05 compared to normal thyroid tissue specimens. (B) SOX9 protein levels in papillary thyroid cancer tissue specimens were obviously higher than in normal thyroid tissue specimens. *p < 0.05 compared to normal thyroid tissue specimens. (C) Representative mRNA expression of SOX9 in papillary thyroid cancer cell lines. (D) Representative Western blot image of SOX9 protein in PTC cell lines. Data represent mean ± SD from three independent experiments. *p < 0.05.
Knockdown of SOX9 Inhibits Cell Growth in PTC Cells
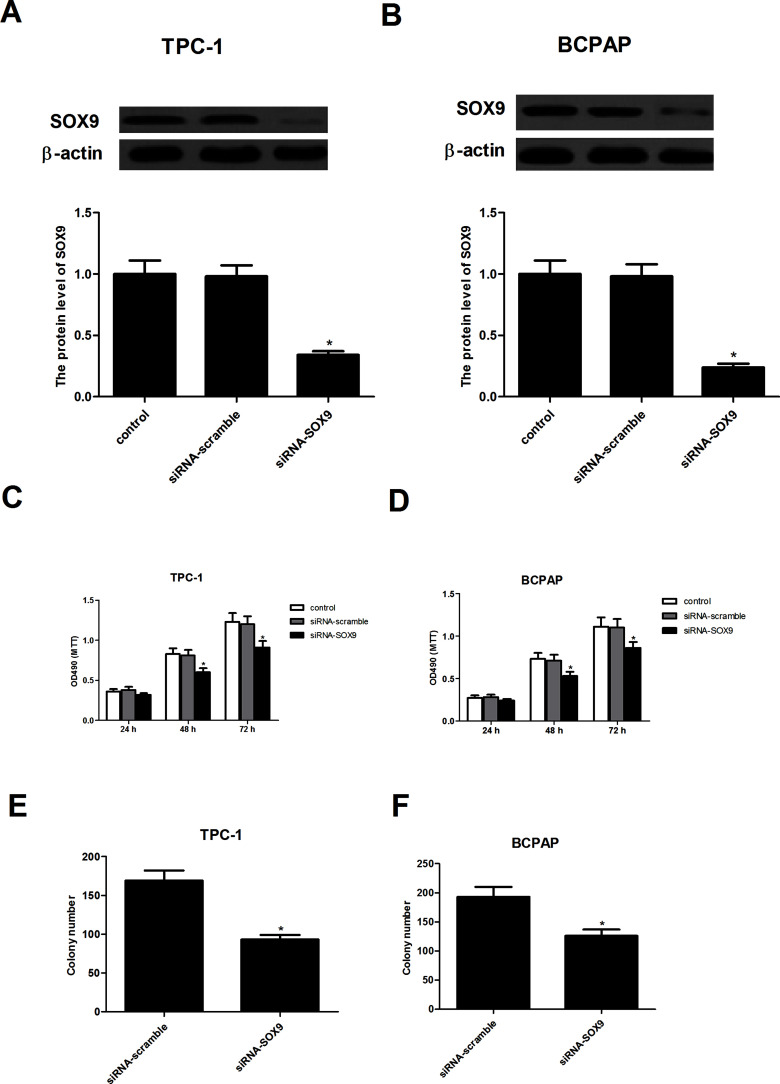
In order to investigate the role of SOX9 in PTC, SOX9 was silenced by siRNA in the human TPC-1 and BCPAP cells, respectively. The results showed that SOX9 was significantly downregulated in TPC-1 and BCPAP cells transfected with siRNA-SOX9, respectively (Fig. 2A and B). Then cell proliferation was determined using an MTT assay; knockdown of SOX9 significantly inhibited proliferation of TPC-1 and BCPAP cells 48 and 72 h after transfection (Fig. 2C and D). Additionally, colony formation was detected by the soft agar assay. We found that knockdown of SOX9 significantly inhibited colony formation in TPC-1 and BCPAP cells, respectively (Fig. 2E and F).
Figure 2.
Knockdown of SOX9 inhibited the proliferation of PTC cells. (A) The mRNA and protein levels of SOX9 in siRNA-SOX9-transfected TPC-1 cells. (B) The mRNA and protein levels of SOX9 in siRNA-SOX9-transfected BCPAP cells. (C) Knockdown of SOX9 inhibited the proliferation of TPC-1 cells in a time-dependent manner. (D) Knockdown of SOX9 inhibited the proliferation of BCPAP cells in a time-dependent manner. (E) Knockdown of SOX9 inhibited colony formation in TPC-1 cells. (F) Knockdown of SOX9 inhibited colony formation in BCPAP cells. Data represent mean ± SD from three independent experiments. *p < 0.05.
Knockdown of SOX9 Promotes Cell Apoptosis in PTC Cells
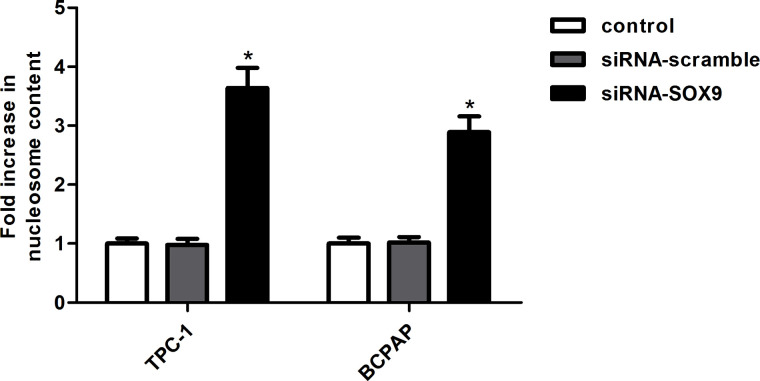
We performed Nucleosome ELISA to measure cell apoptosis. The results showed that the siRNA-SOX9-transfected group has more apoptotic cells than the control group, and there was no significant difference between the siRNA-scramble group and the control group (Fig. 3).
Figure 3.
Knockdown of SOX9 promoted cell apoptosis in PTC cells. TPC-1 and BCPAP cells were transfected with siRNA-SOX9 or siRNA-scramble, respectively. Cell apoptosis was carried out using the Nucleosome ELISA kit. Data represent mean ± SD from three independent experiments. *p < 0.05.
Knockdown of SOX9 Reduces Migration and Invasion of PTC Cells
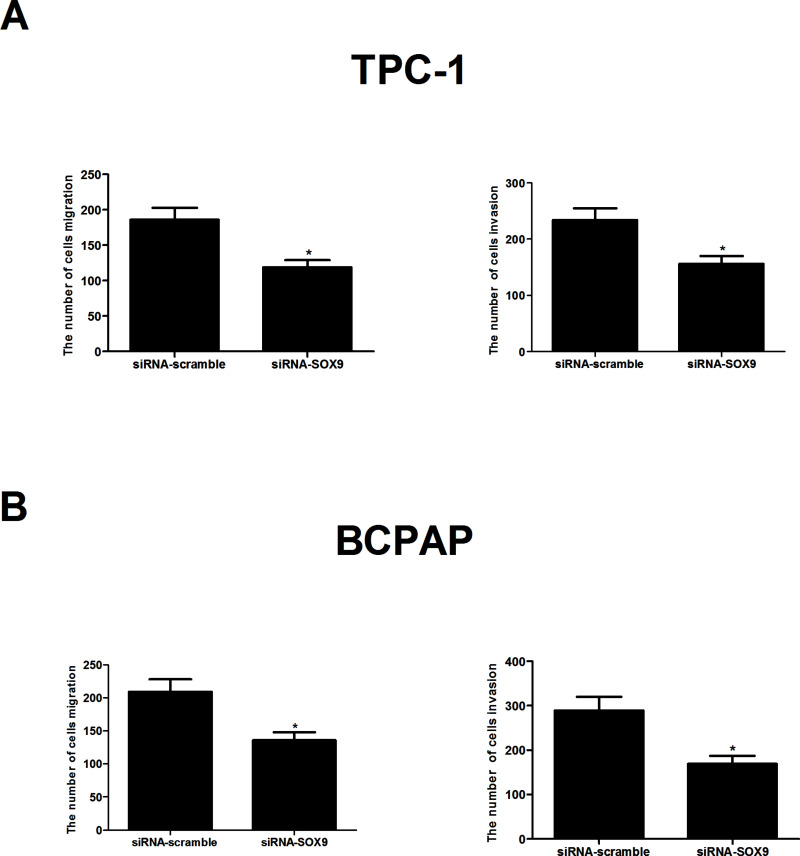
We next determined the potential impact of SOX9 on PTC cell migration and invasion. As shown in Figure 4A, the number of migrated TPC-1 cells was reduced to 119 after transfection with siRNA-SOX9 when compared with the siRNA-scramble group. In addition, knockdown of SOX9 drastically inhibited TPC-1 cell invasion. Similar results were obtained in BCPAP cells in which SOX9 expression was depleted (Fig. 4B).
Figure 4.
Knockdown of SOX9 inhibited the migration and invasion of PTC cells. (A) The number of migrated/invaded cells in siRNA-SOX9-transfected TPC-1 cells was obviously decreased, compared with the mock group. (B) Knockdown of SOX9 significantly inhibited the migration and invasion of BCPAP cells. Data represent mean ± SD from three independent experiments. *p < 0.05.
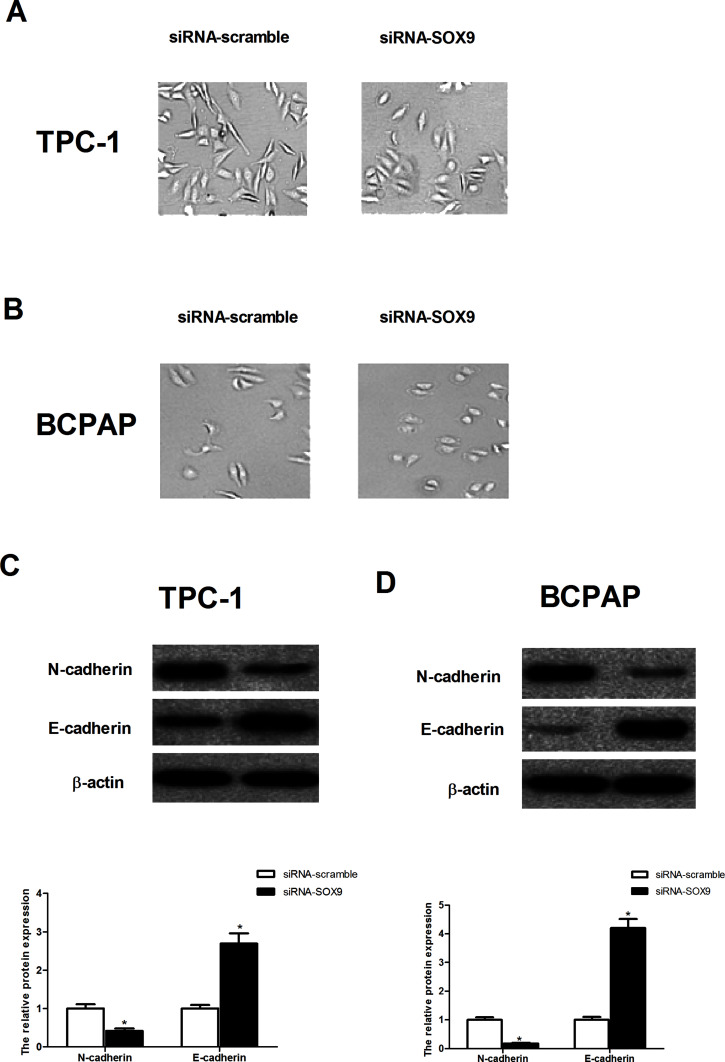
Knockdown of SOX9 Reverses Epithelial–Mesenchymal Transition (EMT) Phenotype in PTC Cells
We then investigated the effect of SOX9 on EMT phenotype in PTC cells. As shown in Figure 5A and B, TPC-1 and BCPAP cells transfected with siRNA-SOX9 displayed more cobblestone-like cell morphology and cluster formation when compared with siRNA-scramble-transfected cells, which exhibited a more mesenchymal phenotype with elongated shape. Furthermore, knockdown of SOX9 inhibited the expression of N-cadherin and upregulated the expression of E-cadherin in TPC-1 cells (Fig. 5C). Similar results were observed in BCPAP cells transfected with siRNA-SOX9 (Fig. 5D).
Figure 5.
Knockdown of SOX9 reversed EMT phenotype in PTC cells. PTC cells were transfected with siRNA-SOX9 or siRNA-scramble for 24 h. Morphological changes of TPC-1 (A) and BCPAP cells (B) after knockdown of SOX9 expression for indicated groups. (C) The protein expression levels of N-cadherin and E-cadherin were detected by Western blot in TPC-1 cells and quantification of protein levels from three independent experiments. (D) The protein expression levels of N-cadherin and E-cadherin were detected by Western blot in BCPAP cells and quantification of protein levels from three independent experiments. Data represent mean ± SD. *p < 0.05.
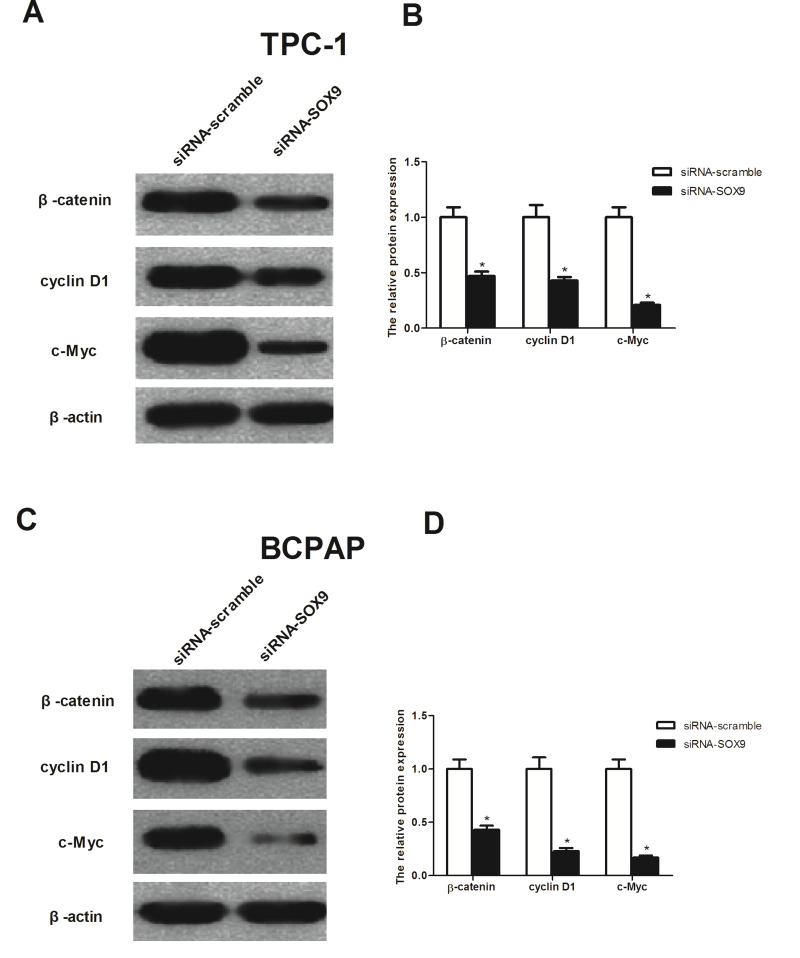
SOX9 Promotes the Metastatic Process of PTC by Suppressing Wnt/β-Catenin Signaling Pathway
It has been reported that SOX9 regulated cell proliferation and cell invasion by the Wnt/β-catenin signaling pathway, so we investigated the effects of SOX9 on the downstream target genes of the Wnt pathway, β-catenin, cyclin D1, and c-Myc. The results showed that the expression levels of β-catenin, cyclin D1, and c-Myc were significantly inhibited by siRNA-SOX9 in TPC-1 and BCPAP cells, respectively (Fig. 6A and C).
Figure 6.
Knockdown of SOX9 inhibited cell growth and invasion by suppressing the Wnt/β-catenin signaling in PTC cells. Western blot measurement of β-catenin, cyclin D1, and c-Myc expression in siRNA-SOX9-transfected TPC-1 (A) and BCPAP cells (C); relative expression of β-catenin, cyclin D1, and c-Myc in TPC-1 (B) and BCPAP cells (D) was quantified using Image-Pro Plus 6.0 software after normalization with β-actin. Data represent mean ± SD from three independent experiments. *p < 0.05.
DISCUSSION
To the best of our knowledge, the current study represents the first demonstration that SOX9 was upregulated in PTC. We also showed that knockdown of SOX9 significantly inhibited PTC proliferation, migration, and invasion, as well as EMT phenotype in TPC-1 and BCPAP cells. The data indicate that upregulation of SOX9 favors PTC progression and metastasis.
SOX9 has different functions in various cancer cells. Our study showed that SOX9 was overexpressed in PTC tissues and cell lines. This finding is in agreement with previous reports that SOX9 was overexpressed in colorectal and prostate cancers (15,16). These results suggest that SOX9 was an oncogene, which was required for the progression of PTC.
SOX9 is a member of the high-mobility group-box class DNA-binding protein family of transcription factors and plays an important role in the control of cellular proliferation (17). Our study showed that siRNA-mediated attenuation of SOX9 in human PTC cells led to a decrease in cell proliferation and colony formation. In addition, knockdown of SOX9 promoted cell apoptosis in PTC cells. These results suggested that SOX9 is an essential factor involved in the growth of human PTC cells. These observations are in agreement with the findings of similar studies on a variety of tumor cells, which further confirms the critical role of SOX9 in the survival and proliferation of PTC cells.
Previous studies reported that EMT occurs during the progression of epithelial tumors to increase the motility and invasiveness of cancer cells. A particular characteristic of EMT is downregulation of the epithelial marker E-cadherin and increase in mesenchymal markers such as vimentin, fibronectin, and N-cadherin (18). In the present study, we observed that knockdown of SOX9 significantly inhibited the number of PTC cell migration and invasion, as well as the expression of N-cadherin and vimentin and upregulated the expression of E-cadherin in PTC cells. These results suggest that SOX9 promotes PTC cell invasion and induces EMT phenotype in TPC-1 and BCPAP cells.
Mounting evidence has shown that aberrant activation of the Wnt/β-catenin signaling plays an important role in the progression of human cancers including PTC (19–21). This signaling pathway regulates a variety of cellular process such as cell fate, cell proliferation, cell survival, cell migration, EMT, and apoptosis (22,23). Wnt proteins bind to the frizzled receptor, subsequently activate disheveled, and then result in stabilization and translocation of β-catenin into the nucleus. Finally, β-catenin interacts with T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors and activates Wnt/β-catenin target genes such as c-Myc and cyclin D1 (24). Therefore, targeting the Wnt/β-catenin signaling cascade is a potential good therapeutic approach to PTC. Numerous studies have demonstrated that the SOX family regulates the Wnt/β-catenin signaling in a variety of tumors (24–28). Chan et al. demonstrated that SOX7 could significantly suppress the expressions of Wnt targets cyclin D1 and c-Myc in endometrial cells (29). Another study showed that SOX1 could regulate TCF-responsive transcriptional activity and inhibit the expression of Wnt downstream genes in hepatocellular carcinoma (30). In the present study, we studied the expression of β-catenin downstream genes, cyclin D1 and c-Myc, to clarify the effects of SOX9 gene expression on the Wnt/β-catenin pathway. We observed that the expression levels of β-catenin, cyclin D1, and c-Myc were significantly downregulated in TPC-1 and BCPAP cells with siRNA-SOX9. These results support the notion that siNRA-SOX9 inhibits cell proliferation and invasion by suppressing the Wnt/β-catenin signaling pathway.
In conclusion, this is the first report demonstrating that knockdown of SOX9 inhibited PTC cell proliferation, invasion, and EMT via suppressing Wnt signaling pathway. Thus, SOX9 may act as a novel molecular target for the prevention and treatment of PTC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R.; Ma J.; Zou Z.; Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 64(1):9–29; 2014. [DOI] [PubMed] [Google Scholar]
- 2. Xing M.; Haugen B. R.; Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 381(9871):1058–1069; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 13(3):184–199; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu Y.; Li Y.; Jun Wei J. W.; Liu X. The role of sox genes in lung morphogenesis and cancer. Int. J. Mol. Sci. 13(12):15767–15783; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiang R.; Liao D.; Cheng T.; Zhou H.; Shi Q.; Chuang T.; Markowitz D.; Reisfeld R.; Luo Y. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br. J. Cancer 104(9):1410–1417; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chou Y. T.; Lee C. C.; Hsiao S. H.; Lin S. E.; Lin S. C.; Chung C. H.; Chung C. H.; Kao Y. R.; Wang Y. H.; Chen C. T. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells 31(12):2607–2619; 2013. [DOI] [PubMed] [Google Scholar]
- 7. Vervoort S.; van Boxtel R.; Coffer P. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene 32(29):3397–3409; 2013. [DOI] [PubMed] [Google Scholar]
- 8. Castillo S. D.; Sanchez-Cespedes M. The SOX family of genes in cancer development: Biological relevance and opportunities for therapy. Expert Opin. Ther. Targets 16(9):903–919; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C. J.; Guo J. Q.; Zhu K. X.; Zhang Q. H.; Pan C. R.; Xu W. H.; Wang H. J.; Liu B. Elevated expression of SOX9 is related with the progression of gastric carcinoma. Diagn. Cytopathol. 39(2):105–109; 2011. [DOI] [PubMed] [Google Scholar]
- 10. Chakravarty G.; Moroz K.; Makridakis N. M.; Lloyd S. A.; Galvez S. E.; Canavello P. R.; Lacey M. R.; Agrawal K.; Mondal D. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp. Biol. Med. 236(2):145–155; 2011. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka T.; Kuroki T.; Adachi T.; Ono S.; Hirabaru M.; Soyama A.; Kitasato A.; Takatsuki M.; Hayashi T.; Eguchi S. Evaluation of SOX9 expression in pancreatic ductal adenocarcinoma and intraductal papillary mucinous neoplasm. Pancreas 42(3):488–493; 2013. [DOI] [PubMed] [Google Scholar]
- 12. Wang L.; He S.; Yuan J.; Mao X.; Cao Y.; Zong J.; Tu Y.; Zhang Y. Oncogenic role of SOX9 expression in human malignant glioma. Med. Oncol. 29(5):3484–3490; 2012. [DOI] [PubMed] [Google Scholar]
- 13. Passeron T.; Valencia J. C.; Namiki T.; Vieira W. D.; Passeron H.; Miyamura Y.; Hearing V. J. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J. Clin. Invest. 119(4):954–963; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aleman A.; Adrien L.; Lopez-Serra L.; Cordon-Cardo C.; Esteller M.; Belbin T.; Sanchez-Carbayo M. Identification of DNA hypermethylation of SOX9 in association with bladder cancer progression using CpG microarrays. Br. J. Cancer 98(2):466–473; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang G.; Lunardi A.; Zhang J.; Chen Z.; Ala U.; Webster K. A.; Tay Y.; Gonzalez-Billalabeitia E.; Egia A.; Shaffer D. R. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nat. Genet. 45(7):739–746; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lü B.; Fang Y.; Xu J.; Wang L.; Xu F.; Xu E.; Huang Q.; Lai M. Analysis of SOX9 expression in colorectal cancer. Am. J. Clin. Pathol. 130(6):897–904; 2008. [DOI] [PubMed] [Google Scholar]
- 17. Lee Y. H.; Saint-Jeannet J. P. Sox9 function in craniofacial development and disease. Genesis 49(4):200–208; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L.-K.; Jiang X.-Y.; Zhou X.-X.; Wang D.-M.; Song X.-L.; Jiang H.-B. Upregulation of vimentin and aberrant expression of E-cadherin/β-catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod. Pathol. 23(2):213–224; 2010. [DOI] [PubMed] [Google Scholar]
- 19. Dellinger T. H.; Planutis K.; Tewari K. S.; Holcombe R. F. Role of canonical Wnt signaling in endometrial carcinogenesis. Expert Rev. Anticancer Ther. 12(1):51–62; 2012. [DOI] [PubMed] [Google Scholar]
- 20. Dahmani R.; Just P.-A.; Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 35(11):709–713; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Sastre-Perona A.; Santisteban P. Role of the wnt pathway in thyroid cancer. Front. Endocrinol. 3:31; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logan C. Y.; Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20: 781–810; 2004. [DOI] [PubMed] [Google Scholar]
- 23. Ko H.; Kim H. S.; Kim N. H.; Lee S. H.; Kim K. H.; Hong S. H.; Yook J. I. Nuclear localization signals of the E-cadherin transcriptional repressor Snail. Cells Tissues Organs. 185(1–3):66–72; 2007. [DOI] [PubMed] [Google Scholar]
- 24. Giles R. H.; van Es J. H.; Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653(1):1–24; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Lin Y.-W.; Tsao C.-M.; Yu P.-N.; Shih Y.-L.; Lin C.-H.; Yan M.-D. SOX1 suppresses cell growth and invasion in cervical cancer. Gynecol. Oncol. 131(1):174–181; 2013. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y.; Huang S.; Dong W.; Li L.; Feng Y.; Pan L.; Han Z.; Wang X.; Ren G.; Su D. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 277(1):29–37; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Sinner D.; Kordich J. J.; Spence J. R.; Opoka R.; Rankin S.; Lin S.-C. J.; Jonatan D.; Zorn A. M.; Wells J. M. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol. Cell. Biol. 27(22):7802–7815; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W.; Glöckner S. C.; Guo M.; Machida E. O.; Wang D. H.; Easwaran H.; Van Neste L.; Herman J. G.; Schuebel K. E.; Watkins D. N. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 68(8):2764–2772; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan D. W.; Mak C. S.; Leung T. H.; Chan K. K.; Ngan H. Y. Down-regulation of Sox7 is associated with aberrant activation of Wnt/β-catenin signaling in endometrial cancer. Oncotarget 3(12):1546–1556; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsao C. M.; Yan M. D.; Shih Y. L.; Yu P. N.; Kuo C. C.; Lin W. C.; Li H. J.; Lin Y. W. SOX1 functions as a tumor suppressor by antagonizing the WNT/β-catenin signaling pathway in hepatocellular carcinoma. Hepatology 56(6):2277–2287; 2012. [DOI] [PubMed] [Google Scholar]