Abstract
Researchers have reported sex-differentiated maturation of white matter (WM) during puberty. It is not clear, however, whether such distinctions contribute to documented sex differences in sensitivity to reward and punishment during adolescence. Given the role of the orbitofrontal cortex (OFC) and nucleus accumbens (NAcc) in reward and punishment-related behaviors, we tested in a cross-sectional study whether males and females (N=156, 89 females; ages 9–14 years) differ in the association between pubertal stage and fixel-based morphometry of WM fibers connecting the OFC and NAcc (i.e., the fronto-accumbal tract). Further, we examined whether males and females differ in associations between fronto-accumbal WM measures and self-reported sensitivity to reward and punishment. Pubertal stage was positively associated with fronto-accumbal fiber density and cross-section (FDC) in males, but not in females. Consistent with previous reports, males reported higher reward sensitivity than did females, although fronto-accumbal combined FDC was not related to reward sensitivity in either sex. Meanwhile, only males showed a negative association between fronto-accumbal tract FDC and sensitivity to punishment. Follow-up analyses revealed that fiber cross-section, but not density, was related to pubertal stage and punishment sensitivity in males, as well as to reward sensitivity in all participants. Our findings suggest there are sex differences in puberty-related maturation of the fronto-accumbal tract, and this tract is related to lower punishment sensitivity in adolescent males compared to females.
Keywords: adolescence, puberty, white matter, fronto-accumbal tract, reward and punishment sensitivity, sex differences
1. Introduction
Puberty marks the beginning of adolescence – the transition between childhood and adulthood that is associated with profound changes in brain structure and function (Goddings et al., 2019; Spear, 2000). Sex differences in aspects of social motivation also become more pronounced during puberty (Defoe et al., 2020; Harden et al., 2018), including sensitivity to reward (i.e., the influence of reward-related stimuli on motivating behaviors), and punishment (i.e., the influence of punishment-related stimuli on avoidance). Although, on average, adolescents show puberty-related increases in reward sensitivity (Harden et al., 2018) and decreases in avoidance (Colder et al., 2013; Ernst, 2014), researchers have noted sex differences in the magnitude and direction of these changes. Specifically, males exhibit greater increases in reward sensitivity across adolescence than do females (Harden et al., 2018; Shulman et al., 2015); conversely, females exhibit consistently higher punishment sensitivity than do males throughout adolescence (Pagliaccio et al., 2016), which may be attributed to a female-specific positive association between threat sensitivity and pubertal development (Urošević et al., 2014).
Data suggest that developmental changes in reward and punishment sensitivity are related to the structural and functional maturation of key neural circuits involved in reward processing, such as those connecting the nucleus accumbens (NAcc) and orbitofrontal cortex (OFC). Indeed, during adolescence, enhanced reward sensitivity is accompanied by corresponding increases in activation of the NAcc (Ernst et al., 2005; Galvan et al., 2006; Van Leijenhorst et al., 2010), which receives convergent inputs from multiple cortical regions, including the OFC (David, 2009; Haber and Knutson, 2010). Whereas the OFC in general is implicated in value representation, outcome evaluation, and salience processing (Rothkirch et al., 2012; Wallis, 2007), the lateral OFC has been implicated in inhibiting responses, when necessary, to previously rewarded stimuli (e.g., risky behaviors), and the medial OFC has been shown to play a role in monitoring and updating reward representations (e.g., learning to associate a previously non-rewarded stimulus with reward) (Elliott et al., 2000). In addition to their responsiveness to monetary reward and punishment (O’Doherty et al., 2001; Shigemune et al., 2014), the OFC and NAcc have also been implicated in human motivation for obtaining social reward (e.g., praise from others) and avoiding social punishment (e.g., disapproval from others) (Kohls et al., 2013). The roles of the OFC and NAcc in bivalent encoding of both aversive and appetitive motivation for social cues is especially important to understand during adolescence, a period of heightened sensitivity to social stimuli that is related to both adaptive changes (e.g., positive risk-taking and maturation of cognitive control via reinforcement learning) (Davidow et al., 2016; DePasque and Galván, 2017; Telzer, 2016) and the emergence of potentially maladaptive behaviors (e.g., impulsivity and negative risk-taking behaviors in the context of peers) (Chein et al., 2011; Colder et al., 2013). Developmental changes in top-down corticostriatal projections may underlie changes in NAcc activation during the processing of reward and punishment throughout adolescence (Galvan, Delevich, Wilbrecht 2020; Larsen et al., 2018). Studies examining subcortical volume have shown that longitudinal increases in sensitivity to reward are predicted by OFC and NAcc volume in adolescent boys and girls (Urošević et al., 2012). However, pubertal stage has been shown to be correlated with NAcc volume positively in boys, but negatively in girls (Urošević et al., 2014), suggesting that aspects of reward-related neurodevelopment are coupled with puberty in sex-specific ways.
Several lines of evidence support the formulation that white matter (WM) micro- and macrostructure, including within frontostriatal pathways, is influenced by puberty. In humans, studies using diffusion tensor imaging (DTI) and structural MRI have found that pubertal timing (i.e., the estimated age at which pubertal onset is achieved), pubertal stage, and gonadal and adrenal hormones are associated with characteristics of WM microstructure, volume, fiber density, and fiber cross-section (e.g., Asato et al., 2010; Chahal et al., 2018; Genc et al., 2019, 2018a; Herting et al., 2012; Ho et al., 2020; Menzies et al., 2015; Perrin et al., 2008). Broadly, these studies have documented that pubertal stage is positively associated with fractional anisotropy (FA), volume, and fiber cross-section and density, although specific regional variations have been reported. Importantly, these studies often report sex differences in the relation between indices of pubertal development and WM. While various studies have found that females exhibit peak levels of WM volume and FA in several tracts earlier than do males (Lenroot et al., 2007; Kaczkurkin et al., 2019) (see reviews by Ladouceur et al., 2012; Lebel and Deoni, 2018), it is important to note that sex differences in WM maturation are not uniform across the brain (e.g., Simmonds et al., 2014). The fronto-accumbal tract, which connects the OFC and NAcc (Haber and Knutson, 2010; Rigoard et al., 2011), appears to mature earlier in males than in females, as evidenced by an earlier age-related peak in FA (Karlsgodt et al., 2015). Additionally, higher FA of the fronto-accumbal tract in adults has been shown to be associated with greater self-reported impulsivity (Ikuta et al., 2018), a characteristic associated with higher reward sensitivity (Martin and Potts, 2004) and lower punishment sensitivity (Potts et al., 2006). Despite evidence that reward sensitivity increases during adolescence and that the NAcc and OFC are relevant for motivating or avoiding social behaviors, no study has investigated the association between puberty and WM morphometry of the fronto-accumbal tract in the context of sensitivity to reward and punishment. Given the evidence for sex differences in puberty-related volumetric development of the OFC and NAcc combined with an earlier age-related FA peak in the fronto-accumbal tract, we posited that fronto-accumbal morphometry would be related to puberty and sensitivity to reward and punishment in sex-specific ways.
No prior study has sought to simultaneously test relations among pubertal stage, fronto-accumbal WM morphology, and reward and punishment sensitivity. The main rationale for the current study is based on the formulation that puberty, perhaps through a combination of changes in circulating hormones and remodeling of the brain’s dopaminergic system (Steinberg, 2008), may relate to sex differences in risk-taking behaviors and related processes (e.g., reward and punishment sensitivity) during adolescence. The fronto-accumbal tract connects reward-processing regions that have been shown to be positively correlated (in volume) with pubertal stage in boys, but not girls (Harden et al., 2018). Further, FA of the fronto-accumbal tract has been shown to relate to impulsivity (Ikuta et al., 2018) and mature earlier (in age) in males compared to females (Karlsgodt et al., 2015), though it has not been investigated in relation to pubertal measures or reward and punishment sensitivity. We tested our formulation in the current study with a community sample of male and female adolescents who were matched on pubertal status at recruitment, allowing us to examine cross-sectionally puberty-related sex differences in fronto-accumbal tract morphometry and sensitivity to reward and punishment. Uniquely, we examined both micro- and macrostructural properties of WM using fixel-based analysis (Raffelt et al., 2017) and tractography to estimate per-person measures of combined fiber density and cross-section (FDC) between the OFC and NAcc; separate measures of fiber cross-section and fiber density were also estimated to further characterize the effects in question. FDC has been shown to be “more sensitive to certain pathologies and more directly interpretable” than are voxel-averaged quantitative measures of microstructure (e.g., fractional anisotropy) (page 58, Raffelt et al., 2017). In addition to predicting sex differences in reward and punishment sensitivity, we hypothesized that males would exhibit a stronger positive association between pubertal stage and fronto-accumbal FDC than would females, indicating that this tract is related to pubertal staging more strongly in males than in females. In addition, because males peak in fronto-accumbal microstructural properties at an earlier age than do females (Karlsgodt et al., 2015), we expected that males would have higher fronto-accumbal FDC than would females. We also tested whether the association between fronto-accumbal FDC and sensitivity to reward and punishment would differ by sex. Given our overarching hypothesis that male-biased increases in reward proclivity and decreases in avoidance of potentially negative outcomes during adolescence reflect sex-specific changes in fronto-accumbal WM, we hypothesized that the association between fronto-accumbal morphometry and sensitivity to reward (positive association) and punishment (negative association) would be stronger in males than in females. We also examined whether the association between pubertal stage and sensitivity to reward and punishment would differ by sex; we hypothesized that, compared to females, males would show a more pronounced positive association between pubertal stage and reward sensitivity, and a stronger negative association between pubertal stage and punishment sensitivity. As an exploratory analysis, we computed the ratio of sensitivity to reward relative to sensitivity to punishment (rew:pun), and then tested whether males and females differ in the associations between fronto-accumbal FDC and rew:pun, and between pubertal stage and rew:pun. This allowed us to examine whether the proclivity for reward vs. avoidance of negative outcomes is related to our variables of interest independent of overall levels of sensitivity.
2. Materials and methods
2.1. Sample
156 adolescents (89 females), ages 9–14 years (M=11.43, SD=1.08), were recruited from the San Francisco Bay Area to participate in a study assessing the effects of early life stress on psychobiological characteristics during puberty. Adolescents were recruited to be in early pubertal stages, and males and females were matched on pubertal stage. All adolescents and their parent(s)/legal guardian(s) provided informed assent and consent, respectively. Exclusion criteria included inability to participate in MRI scanning (e.g., non-removable metal in/on the body and pregnancy), intellectual delay, current or parent neurological disorders, non-fluent English speakers, and self-reported onset of menses for females (to ensure that adolescents were in early stages of puberty). Participants were not excluded based on the presence of DSM-IV Axis-I disorders (N=9 with current diagnoses; two with social phobia, 6 with specific phobias, and one with oppositional defiant disorder, according to the Schedule for Affective Disorders and Schizophrenia for School-Age Children; KSADS; Kaufman et al., 1997), body mass index (M=19, SD=3.80), or medication usage (N=27 taking allergy medications). This study was approved by the Stanford University Institutional Review Board. Informed consent was obtained from participants and all participants were compensated for their participation.
2.2. Measures
2.2.1. Cumulative Early Life Stress Severity.
A modified version of the Traumatic Events Screening Inventory for Children (TESI-C; Ribbe, 1996) was used to assess the impact of stressful life experiences. Interviewers asked adolescents to provide details about endorsed stressful life events in order to assess their severity and impact. We used a modified version of the UCLA Life Stress Interview coding system to rate the objective severity of each endorsed stressors, from which we computed a score of cumulative early life stress severity. Additional details about the scoring procedure in the larger sample have been previously been reported (King et al., 2017), and the scoring algorithm is available at https://github.com/lucysking/els_stress_interview.
2.2.3. Pubertal Stage.
Pubertal stage was estimated using the self-reported Tanner Staging Questionnaire (Morris and Udry, 1980), a measure shown to be significantly correlated with physician ratings of puberty-related physical development (Shirtcliff et al., 2009). We averaged the Tanner pubic hair and breast/testes ratings to compute an index of overall pubertal development (Dorn et al., 2006). The self-reported Petersen Pubertal Development Scale (PPDS; Petersen et al., 1988), height, weight, and salivary concentrations of testosterone and dehydroepiandrosterone (DHEA) were also collected (see Supplementary Material). While Tanner staging was used as the main pubertal measure in our study, we also used all pubertal measures to estimate per-person latent factor scores of pubertal status, excluding participants who did not provide salivary samples (N=16; 10%). These latent factor puberty scores were then used in exploratory follow-up tests of our main hypotheses.
2.2.4. Reward and Punishment Sensitivity.
Participants completed the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ; Torrubia et al., 2001), a self-report measure of the Behavioral Inhibition and Behavioral Approach motivation systems proposed by Gray’s model (Gray, 1982, 1981). This measure comprises two subscales: a Punishment Sensitivity scale (e.g., “I worry about punishments at home or in school”; Cronbach’s α=0.87) and a Reward Sensitivity scale (e.g., “I like to do things that bring immediate rewards”; Cronbach’s α=0.87). Importantly, the questions included in the SPSRQ assess proclivity for socially rewarding behaviors (e.g., “I sometimes do things to be popular, even if those things ae not nice or are not the right things to do”), as well as avoidance of behaviors that have the potential to be socially punishing (e.g., “Whenever possible, I avoid showing my skills because I am afraid of being embarrassed”). The SPSRQ has been validated in adolescents (Vandeweghe et al., 2016), in whom the Reward scale was positively correlated with impulsivity, sensation-seeking, and extraversion, and the Punishment scale was positively correlated with neuroticism and anxiety. Further, the SPSRQ has been shown to have good psychometric properties (e.g., reliability, validity, and test-retest reliability; Conner et al., 2018). In addition to examining the Reward and Punishment sensitivity scales separately, we also computed the ratio of reward to punishment sensitivity (rew:pun) using the following equation:
This ratio score was examined to elucidate sex differences in the effects of the fronto-accumbal tract and pubertal stage on the amount of sensitivity that is attributed to reward relative to punishment.
2.3. Acquisition of diffusion imaging
Diffusion-weighted MRI data were collected at the Stanford Center for Cognitive and Neurobiological Imaging (https://cni.stanford.edu) using an echo planar imaging sequence with 60 diffusion-weighted directions, with anterior-to-posterior phase encoding. Scan parameters included: echo time=93.5 ms; repetition time=8500 ms; voxel size=0.938 × 0.939 × 2.00 mm; slices=64; flip angle=12°; b=2000mm2 (6 volumes b=0) A small number of participants (N=19; 12.18%) were scanned using acquisition of 2.00 mm3 voxel sizes following a scanner upgrade. To control for any effects of this difference in acquisition parameters, we included a dichotomous covariate for scan acquisition in all statistical models.
2.4. Fixel-based analysis
We used Fixel-based Analysis (FBA) to assess the morphometry of WM tracts. Briefly, we applied higher-order diffusion models to fiber populations within each voxel (i.e., fixels) in order to estimate a combined measure of fiber density and cross-section (FDC). We estimated FDC per fixel, rather than separating the metrics of fiber density and cross-section because FDC allows for the measurement of both micro- and macro-structural properties of WM. As stated above, FDC has been shown to be more sensitive to developmental changes and more directly interpretable than are voxel-averaged quantitative measures of microstructure (e.g., fractional anisotropy) (Genc et al., 2018b; Raffelt et al., 2017). All diffusion-weighted MRI data processing was performed using MRtrix3 (Tournier et al., 2019). Following data denoising, and eddy-current induced distortion and motion correction, we estimated a brain mask for each individual and performed bias field correction to eliminate low-frequency intensity inhomogeneities in the images. We then performed 1) intensity normalization across subjects; 2) estimation of a study-specific WM mask; 3) estimation of the group-average response function; 4) up-sampling of diffusion data and brain mask images; 5) estimation of fiber orientation distribution (FOD) using Constrained Spherical Deconvolution via the group average response function; 6) study-specific FOD template generation; 7) registration of subject FOD images to the FOD template; 8) generation of WM template fixel analysis mask; 9) thresholding of peak fixel image; 10) warping of FOD images to template space; 11) segmentation of FOD images to estimate fixels and their fiber density; 12) reorienting of fixel orientations in order to ensure that the subject and template fixels had angular correspondence; 13) assignment of subject fixel to template fixels; 13) computation of fiber cross-section; and 14) computation of a combined measure of fiber density and cross-section (i.e., FDC). A full description of steps taken to compute FDC is available on the MRtrix website, along with documentation of commands (https://mrtrix.readthedocs.io/en/3.0_rc1/fixel_based_analysis/ss_fibre_density_cross-section.html).
We used the Harvard-Oxford Atlas, available through FSL (Jenkinson et al., 2012), to define binarized OFC and NAcc masks. We performed tractography between the seed (OFC) and target (NAcc) regions using Fiber Assigned Continuous Tracking (FACT), a deterministic algorithm that estimates and reconstructs streamlines between regions based on fiber orientation tracking (Mori et al., 1999). FACT tractography was performed on individuals’ template space fiber orientation distribution maps. Fixel masks were then created using the input tracks identified in the previous step. This approach yielded individualized tractography-based fixel masks of two ipsilateral and two contralateral sets of fronto-accumbal streamlines that were used to extract per-person estimates of average OFC-NAcc FDC. Although we used the average (ipsi- and contralateral fibers) FDC of OFC-NAcc WM in our main analyses, in follow-up supplemental analyses we also probed whether ipsi- or contralateral streamlines differentially contributed to our findings.
Given that fiber density approximates microstructural properties relates to axonal packing, and fiber cross-section is a macrostructural measure of fiber bundle diameter (Raffelt et al., 2017), we also separately investigated these measures to better characterize whether one of these properties of the OFC-NAcc tract is more or less related to puberty and reward and punishment sensitivity.
To test whether our findings are specific to the OFC-NAcc, rather than other reward-related regions connected to the OFC, we used the Harvard-Oxford atlas to create masks of the left and right thalamus and the Brainnetome atlas (Fan et al., 2016) to create pregenual anterior cingulate cortex (ACC) masks. FACT was used to reconstruct streamlines from the OFC to the thalamus, and from the OFC to the pregenual ACC (pgACC); then these tracks were converted to masks and used to extract per-person estimates of the average FDC in the bilateral (average of left and right) OFC-Thalamus and OFC-pgACC tracts. These two additional reward-related tracts (one OFC-Thalamus cortical-subcortical and one OFC-pgACC cortical-cortical) were used to test the specificity of the fronto-accumbal tract in relation to pubertal stage and reward and punishment sensitivity.
2.5. Testing sex differences in the association between pubertal stage and OFC-NAcc FDC
All statistical analyses were conducted in R v. 3.6.1 (R Core Team, 2019) using linear regression models with age, early life stress severity (given associations between early life stress and WM microstructural properties as well as research reporting that life stress is related to earlier pubertal development; Hanson et al., 2013; Mendle et al., 2011), race (given documented differences between White and Black youth in pubertal timing and tempo; e.g., Keenan et al., 2014), and scan acquisition as covariates. First, we tested the main and interaction effects of pubertal stage and sex on OFC-NAcc FDC. Then, we evaluated the simple slopes of pubertal stage and OFC-NAcc FDC for each sex.
2.6. Testing the effects of OFC-NAcc FDC, pubertal stage, and sex on sensitivity to reward and punishment
We conducted a multivariate multiple regression (MMR) to test the main effects and interaction of sex and pubertal status, and of sex and OFC-NAcc FDC, on two outcome variables: reward sensitivity and punishment sensitivity. Specifically, we examined the following questions: 1) does the association between OFC-NAcc FDC and reward and punishment sensitivity differ by sex (tested using an interaction term of OFC-NAcc FDC and sex)?; and 2) does the effect of pubertal status on reward and punishment sensitivity differ by sex (tested using an interaction term of sex and pubertal status)? Thus, while we expected to find sex differences in the association between pubertal stage and OFC-NAcc FDC in our first model (section 2.5 above), in this MMR model, we tested whether sex differences in reward and punishment sensitivity might be, in part, dependent on pubertal stage and OFC-NAcc FDC. We conducted univariate follow-up tests of reward and punishment sensitivity as separate response variables to probe the results of the MMR, and evaluated simple slopes to probe significant two-way interactions (Potthoff, 1964). Finally, we conducted a separate linear regression model to test these same main effects and interactions using the rew:pun ratio as the dependent variable.
Both of our main regression models (described in sections 2.5 and 2.6 above) were re-conducted using separate fiber cross-section and fiber density measures of the OFC-NAcc tract, with OFC-pgACC and OFC-thalamus tracts, and with latent scores to approximate pubertal status (i.e., including self-reported pubertal ratings, height and weight measurements, and pubertal hormones; See Supplementary Material pages 2–3).
3. Results
Descriptive statistics for the variables of interest are presented in Table 1. Correlations between all study variables are presented as separate correlation matrices for males and females in the Supplementary Material (Figures S1–2). As expected given the study design, males and females did not differ significantly in pubertal status (Figure 1), but males were, on average, 0.77 years older than females (t(154)=4.70, p<.001). As expected, age was positively correlated with pubertal status (r=.33, p<.010). Also as expected based on prior findings, males had higher levels of reward sensitivity (males: M=3.08 ± 0.58, females: M=2.71 ± 0.66; t(152)=3.53, p=.001) and a higher ratio of rew:pun (males: M=0.07 ± 0.13, females: M=0.02 ± 0.12; t(152)=2.29, p=.024) than did females (Table 1; Figure S3); there were no sex differences in punishment sensitivity (males: M=2.73 ± 0.72, females: M=2.61 ± 0.68; t(152)=1.04, p=.301). We also found that compared to White participants, racial-ethnic minority youth (N=84, of whom 18% were Black, 10% Latinx, 23% Asian American, 40% multiracial, and 7% reported ‘other race’) reported being an average of 0.20 units higher on reward sensitivity (t(152)=−1.93, p=.055) and 0.26 units higher on punishment sensitivity (t(152)=−2.33, p=.022).
Table 1.
Participant characteristics
| Mean (SD/ %) | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. ELS | 6.70 (5.30) | r=−.02 | r=.12 | r=.12 | r=−.10 | r=.08 | T=0.07 | T=−1.87 | r=−.02 |
| 2. Age (Years) | 11.44 (1.08) | r=.08 | r=.14 | r=.22* | r=.09 | r=.33* | T=4.70* (M>F) | T=0.16 | |
| 3. Race (racial-ethnic minority) | 53.85% | T=0.60 | T=−2.33* (RU>W) | T=−1.93 | T=0.05 | T=0.99 | X2=0.02 | ||
| 4. Sex (Female) | 57.05% | T=2.29* (M>F) | T=1.03 | T=3.60 (M>F) | T=2.85* (M>F) | T=−1.73 | |||
| 5. Pubertal Stage | 1.99 (0.72) | r=.04 | r=.03 | r=.09 | r=.07 | ||||
| 6. OFC-NAcc FDC | 0.28 (0.03) | r=.22* | r=−.07 | r=.14 | |||||
| 7. Reward Sensitivity | 2.87 (0.65) | r=.38* | r=.47* | ||||||
| 8. Punishment Sensitivity | 2.66 (0.70) | r=−.62* | |||||||
| 9. Rew:pun | 0.04 (0.13) |
Note:
p<.05.
ELS=early life stress cumulative severity; FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens; Rew:pun=Ratio of reward to punishment sensitivity; (N=84 of which 18% were Black, 10% Latinx, 23% Asian American, 40% multiracial, and 7% other).
Figure 1.
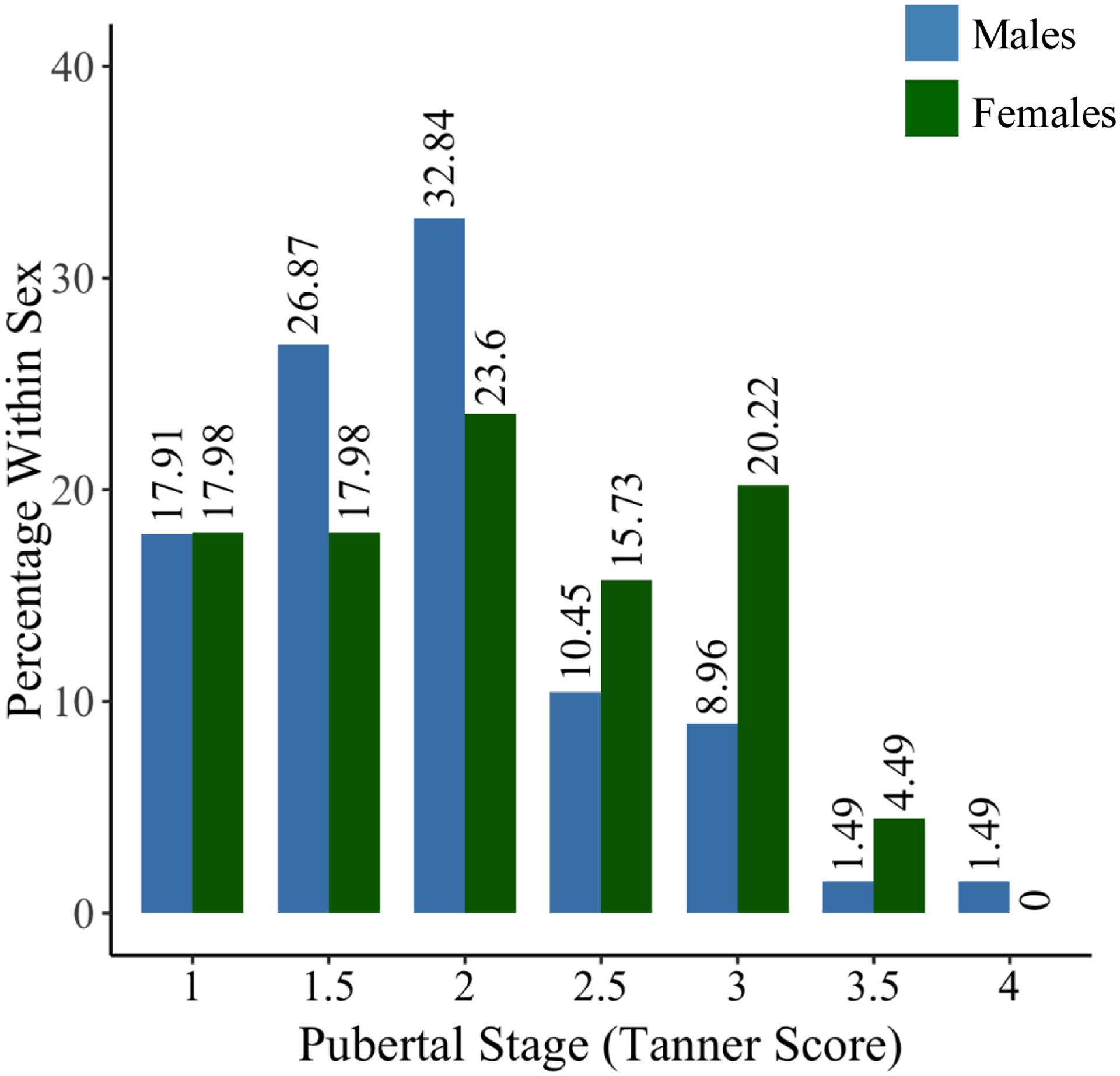
Males and females did not significantly differ in the distribution of pubertal staging.
Reconstructed fibers traversing the bilateral OFC and NAcc using FACT are presented in Figure 2. Average OFC-NAcc FDC was higher in males than in females (males: M=0.28 ± 0.03, females: M=0.27 ± 0.03; t(154)=2.85, p=.005). OFC-NAcc FDC was not correlated significantly with pubertal stage (r=.07, p=.372), reward sensitivity (r=.17, p=.075), or punishment sensitivity (r=−.07, p=.382); however, OFC-NAcc FDC was positively correlated with rew:pun (r=.22, p=.007; Table 1). OFC-NAcc FDC was highly correlated with fiber cross-section (r=.71, p<.001) and fiber density (r=.76, p<.001); however fiber cross-section and fiber density were not correlated (r=.09, p=.25). Tests for sex differences in the separate OFC-NAcc fiber density and fiber cross-section measures revealed that males showed higher cross-section compared to females (males: M=1.04 ± 0.08, females: M=0.99 ± 0.07; t(154)=4.00, p=.001), but the groups did not differ in fiber density (p=.600). Neither fiber cross-section nor density were related to pubertal stage (rs=.02 and .09, ps=.770 and .280, respectively). OFC-NAcc fiber cross-section was positively correlated with reward sensitivity (r=.18, p=.023), though fiber density was not (r=.04, p=.590). Additionally, as with FDC, fiber cross-section was positively correlated with rew:pun (r=.26, p=.001), while fiber density was not (r=.08, p=.330).
Figure 2.
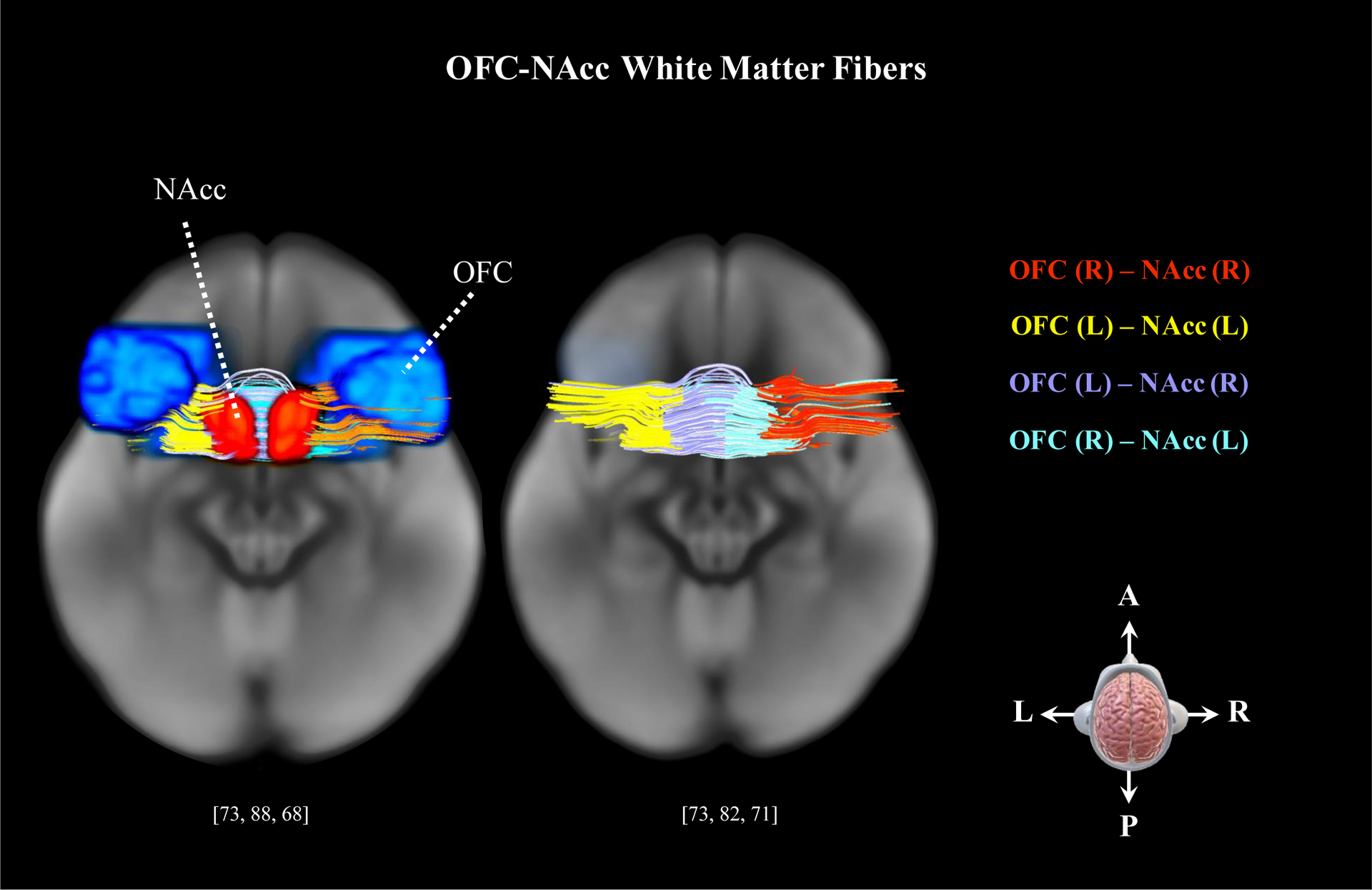
OFC-NAcc fibers reconstructed using FACT tractography.
Note: OFC=orbitofrontal cortex; NAcc=nucleus accumbens; [xyz]=MNI coordinates
Adolescents with current diagnoses on the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997) did not differ in pubertal status, reward sensitivity, punishment sensitivity, OFC-NAcc FDC, fiber cross-section, or fiber density from those without current diagnoses (all ps>.05).
3.1. Sex differences in the association between pubertal status and OFC-NAcc FDC
We found a significant interaction of sex and pubertal status on OFC-NAcc FDC, t(148)=−2.08, p=.039. Post-hoc simple slopes analyses revealed that pubertal status was positively associated with OFC-NAcc FDC in males (t(65)=2.45, p=.016), but not in females (t(87)=−0.11, p=.913) (Table 2, Figure 3). Removing early life stress as a covariate from our model did not alter the results (Table S1). Including additional covariates of body mass index (BMI) and socioeconomic status (SES; measured as highest parent education) in our model also did not affect our results (Table S2). To ensure that our findings were not driven by a small number of participants who were more advanced in pubertal status (stages 3.5 and 4; 6 of the 156 participants in the full sample), we reran our analysis only in a sub-sample of participants who were in pubertal stages 1–3. The significant interaction of sex and pubertal status on OFC-NAcc FDC was still present (Table S3). Similarly, excluding girls who were ages 13–14 (N=6 of a total of 89; i.e., older girls who had not experienced menarche and might be characterized as ‘late developing’) did not alter our findings (Table S4).
Table 2.
Association between pubertal stage and OFC-NAcc FDC in males and females
| Effect | ß Estimate | t | p |
|---|---|---|---|
| ELS | −0.06 | −0.80 | .425 |
| Age | −0.04 | −0.50 | .616 |
| Race | 0.11 | 0.70 | .485 |
| Sex | −0.54 | −3.18 | .002* |
| Pubertal Stage | 0.32 | 2.45 | .016* |
| Pubertal Stage × Sex | −0.33 | −2.08 | .039* |
| Pubertal Stage Simple Slopes: | |||
| Males | 0.32 | 2.45 | .016* |
| Females | −0.01 | −0.11 | .913 |
Note:
p<.05.
ELS=early life stress cumulative severity; FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens; Race was coded as 1=White, 2=Racial-ethnic minority; Sex was coded as 1=male, 2=female; Full model adjusted R2=.11, F(7, 148)=3.56, p<.001.
Figure 3.
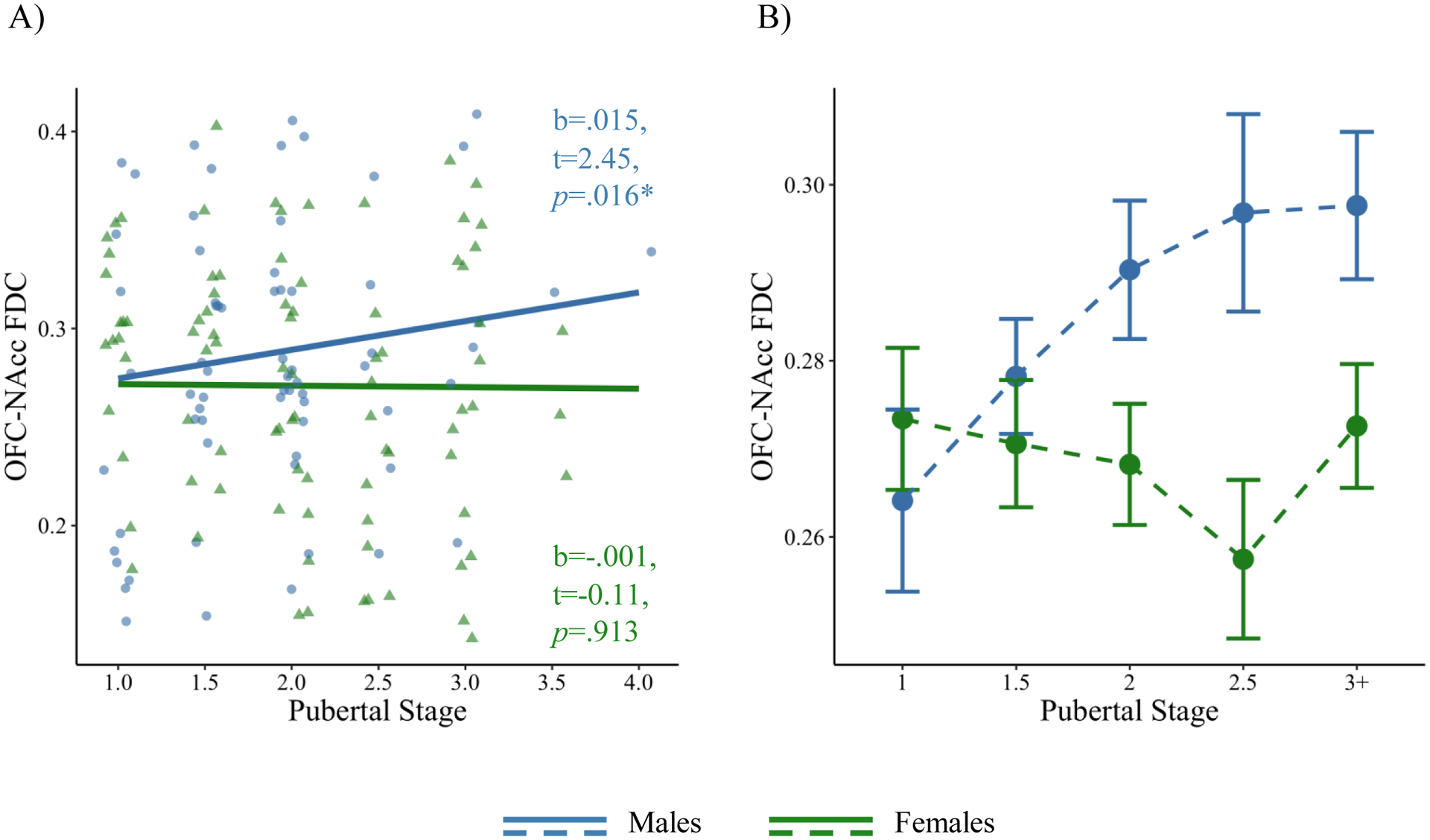
OFC-NAcc FDC is positively associated with pubertal stage in males (blue), but not females (green).
Note: *p<.05. FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens. A) Model-derived values (partial residuals) and slopes of sex by pubertal stage on OFC-NAcc FDC, controlling for age, early life stress, and race. B) Average raw (non-model-derived) OFC-NAcc FDC values per pubertal stage per sex. Error bars represent standard error of the mean per pubertal stage, per sex. Beta estimates are not standardized for visualization.
To examine whether our finding of a positive association between puberty and OFC-NAcc FDC in males was driven by ipsi- or contralateral WM fibers connecting the OFC and NAcc regions, we conducted two follow-up linear regression models in which we substituted the dependent variable for either ipsi- or contralateral fibers. Whereas the effect of pubertal stage on contralateral OFC-NAcc fibers was statistically significant (t(61)=2.20, p=.032), the effect of pubertal stage on ipsilateral fibers was not significant (t(61)=1.84, p=.071; Tables S5 and S6).
3.2. Sex differences in the association between OFC-NAcc FDC and reward and punishment sensitivity
The MMR yielded significant main effects of sex and race, as well as a significant two-way interaction of OFC-NAcc FDC and sex, on reward sensitivity and punishment sensitivity (all ps<.05). Univariate analysis with reward sensitivity as the dependent variable yielded significant main effects of sex (males > females as described above, p=.001), race (racial-ethnic minorities > White as described above, p=.045), but no interaction of OFC-NAcc FDC and sex (p=.082) or of pubertal stage and sex (p=.731) on reward sensitivity (Table 3).
Table 3.
Multivariate regression model testing associations between pubertal stage, OFC-NAcc FDC, and sex with reward and punishment sensitivity.
| Overall Model | Reward Sensitivity | Punishment Sensitivity | |||||
|---|---|---|---|---|---|---|---|
| Effect | Pillai | F | p | F | p | F | p |
| ELS | 0.02 | 1.30 | .276 | 2.02 | .158 | 1.73 | .190 |
| Age | 0.02 | 1.37 | .258 | 1.70 | .195 | 2.22 | .138 |
| Race | 0.04 | 3.14 | .046* | 4.08 | .045* | 4.95 | .028* |
| Sex | 0.08 | 6.64 | .001* | 13.07 | .001* | 1.17 | .282 |
| Pubertal Stage | 0.02 | 1.58 | .209 | 3.12 | .079 | 0.27 | .601 |
| OFC-NAcc FDC | 0.03 | 2.16 | .110 | 1.00 | .320 | 1.48 | .225 |
| Pubertal Stage × Sex | 0.01 | 0.25 | .780 | 0.12 | .731 | 0.17 | .683 |
| OFC-NAcc × Sex | 0.06 | 4.81 | .009* | 3.07 | .082 | 9.47 | .002* |
| OFC-NAcc Simple Slopes: | |||||||
| Males | t=−3.11 | .002* | |||||
| Females | t=1.06 | .289 | |||||
Note:
p<.05.
ELS=early life stress cumulative severity; FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens. Reward sensitivity model: adjusted R2=.11, F(9, 144)=3.15, p=.002. Punishment sensitivity model: adjusted R2=.08, F(9, 144)=2.41, p=.014. Age, ELS severity, race, and scanner acquisition were included as covariates; Race was coded as 1=White, 2=Racial-ethnic minority; Sex was coded as 1=male, 2=female.
Univariate analysis with punishment sensitivity as the dependent variable also yielded a significant main effect of race (racial-ethnic minorities > White as described above, p=.028), and a significant interaction of sex and OFC-NAcc FDC, F(1,144)=9.47, p=.002. Follow-up simple slopes analyses showed that in males, but not in females, there was a negative association between OFC-NAcc FDC and punishment sensitivity (t(65)=−3.11, p=.002; Table 3, Figure 4). Removing the early life stress covariate, adding BMI and SES covariates, excluding participants at pubertal stages above 3, and excluding female participants above age 13 did not alter our finding of a significant interaction between sex and OFC-NAcc FDC on punishment sensitivity; specifically, across all models, only in males was there a negative association between OFC-NAcc FDC and punishment sensitivity (all ps<.05; Tables S7–S10).
Figure 4.
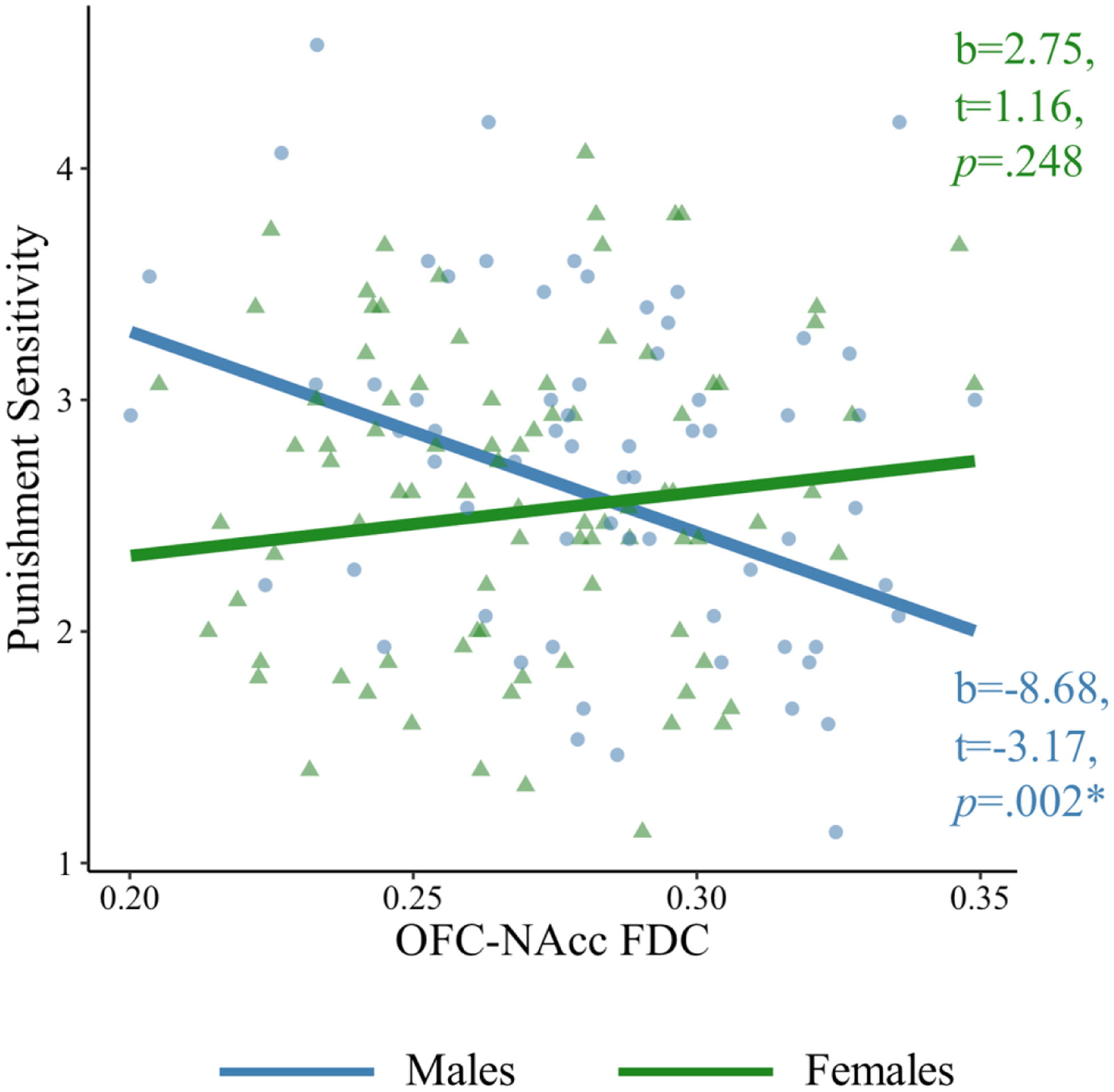
OFC-NAcc FDC is negatively associated with punishment sensitivity in males (blue), but not females (green).
Note: *p<.05. FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens. Beta estimates are not standardized for visualization.
Finally, the exploratory analysis of the rew:pun ratio yielded a significant main effect of OFC-NAcc FDC (t(0.96,144)=3.01, p=.003), and a significant interaction of OFC-NAcc FDC with sex (t(0.96, 144)=−2.15, p=.033), on rew:pun. Follow-up simple slopes analyses revealed that the positive association between OFC-NAcc FDC and rew:pun was present only in males (t(65)=3.02, p=.003), and not in females (Table 4, Figure 5).
Table 4.
Effects of OFC-NAcc FDC, pubertal stage, and sex on the ratio of reward to punishment sensitivity. Age, ELS severity, and scanner acquisition were included as covariates.
| Effect | ß Estimate | t | p |
|---|---|---|---|
| ELS | 0.01 | 0.01 | .921 |
| Age | −0.01 | −0.04 | .968 |
| Race | −0.08 | −0.51 | .612 |
| Sex | −0.20 | −1.11 | .268 |
| OFC-NAcc FDC | 0.39 | 3.01 | .003* |
| Pubertal Stage | −0.16 | −1.13 | .259 |
| Pubertal Stage × Sex | 0.27 | 1.57 | .120 |
| OFC-NAcc FDC × Sex | −0.37 | −2.15 | .033* |
| OFC-NAcc FDC Simple Slopes | |||
| Male | 0.39 | 3.02 | .003* |
| Female | 0.02 | 0.19 | .849 |
Note:
p<.05.
ELS=early life stress cumulative severity; FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus. Model adjusted R2=.07, F(9, 144)=2.20, p=.02. Age, ELS severity, race, and scanner acquisition were included as covariates; Race was coded as 1=White, 2=Racial-ethnic minority; Sex was coded as 1=male, 2=female.
Figure 5.
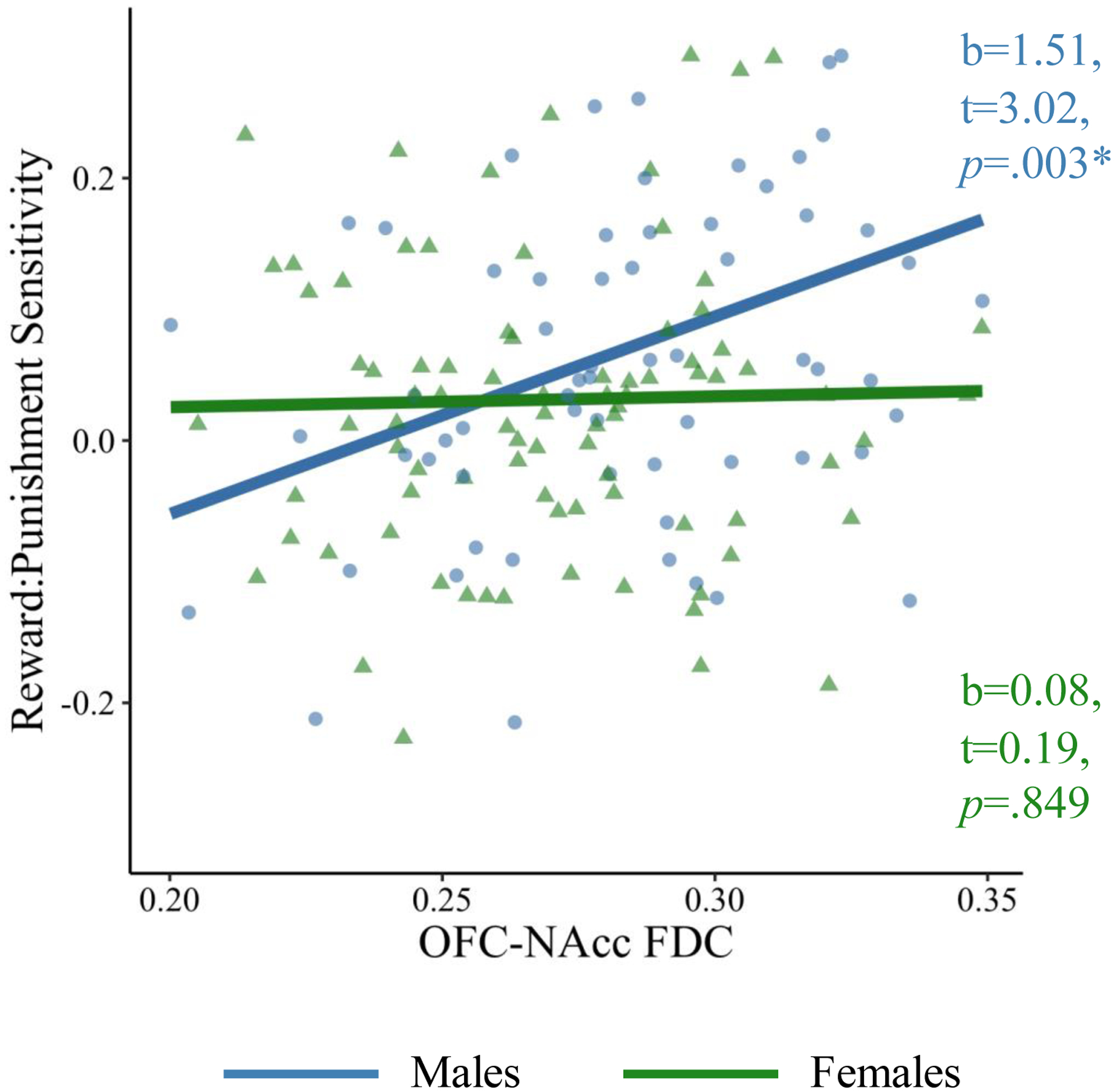
OFC-NAcc FDC is positively associated with rew:pun sensitivity in males (blue), but not females (green).
Note: *p<.05. FDC=fiber density and cross-section; OFC=orbitofrontal cortex; NAcc=nucleus accumbens. Beta estimates are not standardized for visualization.
3.3. Exploratory analyses with latent factor pubertal score
When Tanner stage was replaced by the latent factor-derived pubertal score in our main models (described in sections 3.1–2 above), we again found a significant interaction of sex and pubertal score on OFC-NAcc FDC (t(148)=−2.57, p=.011), such that only males exhibited a positive association between pubertal score and OFC-NAcc FDC (t(65)=2.65, p=.009), but females did not (t(87)=−0.31, p=.755) (Table S11 and Figure S4). We found a significant interaction between OFC-NAcc FDC and sex on reward and punishment sensitivity in the exploratory MMR model (ps<.05). Univariate follow-up tests yielded an interaction of sex and OFC-NAcc FDC on punishment sensitivity (F(1, 144)=9.80, p=.002). As expected, and shown using the raw pubertal stage measure, males, but not females, exhibited a negative association between OFC-NAcc FDC and punishment sensitivity (t(65)=−3.21, p=.002; Table S12).
3.4. Exploratory follow-up analyses with separate fiber cross-section and fiber density measures.
A set of follow-up analyses revealed that fiber cross-section, but not density, drove the above-reported associations. First, the significant interaction of pubertal stage and sex (in which only males showed a positive association with OFC-NAcc; section 3.1 above) was only observed with fiber cross-section (p=.014), but not with density (p=.510; Table S13, Figure S5).
Second, we found a significant interaction between OFC-NAcc fiber cross-section, but not density, and sex on reward and punishment sensitivity in the MMR model (p=.002 for cross-section; p=.373 for density). As with the combined FDC measure, univariate follow-up tests yielded an interaction of sex and OFC-NAcc cross-section (F(1, 144)=12.70, p<.001) on punishment sensitivity. In males, but not in females, there was a negative association between OFC-NAcc fiber cross-section and punishment sensitivity (t(65)=−3.80, p<.001; Table S14, Figure S6).
Third, a separate linear model also yielded a significant main effect of OFC-NAcc cross-section (t(0.95, 144)=3.88, p<.001) and an interaction of OFC-NAcc cross-section and sex on the rew:pun ratio (t(0.95, 144)=−2.47, p=.015); the positive association between OFC-NAcc fiber cross-section and rew:pun was present only in males (t(65)=3.88, p<.001), and not in females (Table S15; Figure S7).
3.5. Exploratory follow-up analyses with additional reward-related tracts
We re-ran our main models (described in sections 3.1 and 3.2 above) using the OFC-pgACC and OFC-Thalamus tracts to examine whether our findings generalize to other reward-related WM tracts, or specific to the fronto-accumbal OFC-NAcc tract. While we did not find a main effect of pubertal stage or an interaction of pubertal stage and sex on OFC-pgACC or OFC-Thalamus FDC (Table S16), we did find that the OFC-pgACC was negatively correlated with punishment sensitivity (r=−.17, p=.032) and positively correlated with rew:pun (r=.20, p=.015); additionally, OFC-Thalamus FDC was positively associated with reward sensitivity (r=.16, p=.045) and rew:pun (r=.22, p=.005).
4. Discussion
In a community sample of adolescents, we examined sex differences in puberty-related fronto-accumbal WM morphometry (specifically fiber density and cross-section; FDC) assessed via fixel-based analysis. Further, we assessed sex differences in sensitivity to reward and punishment as a function of fronto-accumbal FDC and pubertal status. We found that pubertal stage is positively associated with fronto-accumbal morphometry in males, but not in females; further, males with higher FDC in this tract reported lower punishment sensitivity than did males with lower FDC. We did not find an association between pubertal stage and reward or punishment sensitivity. Further, we did not find an association between fronto-accumbal FDC and reward sensitivity; however, across the full sample, we did find that fiber cross-section of the fronto-accumbal tract was positively associated with reward sensitivity. In addition, we found that cross-section, but not fiber density, drove our findings in males of an association between pubertal stage and fronto-accumbal morphometry, and between fronto-accumbal morphometry and punishment sensitivity. Taken together, these findings provide evidence for sex-differentiated fronto-accumbal maturation during puberty that may be related to sex differences in how potential rewards and punishments influence behavior.
Both males and females have been shown to exhibit increasing responsiveness to social stimuli during adolescence (Nelson et al., 2016); in this context, the reward system, including the NAcc and OFC, is thought to play a key role in both the adaptive and potentially maladaptive sequelae of this heightened social responsiveness. For example, relative to adults, adolescents have been shown to make more risky decisions in the presence of peers, compared to alone, during a simulated driving task. Further, greater activation in the NAcc and OFC predicted risk-taking in the context of peers (Chein et al., 2011). Moreover, a recent study showed that the presence of peers influenced risk-taking behaviors during this simulated driving task in male, but not female, adolescents, suggesting that male adolescents may be particularly sensitive to socially-rewarding environments (Defoe et al., 2020). However, it is important to note that the development of the NAcc has also been implicated in positive changes during adolescence. The recalibration of ventral striatum (including the NAcc) activation has been posited to improve cognitive persistence, heighten the effect of positive peer influences (Telzer, 2016), and support reinforcement learning (Davidow et al., 2016) during adolescent development. The current results suggest that morphological changes in the OFC-NAcc tract during puberty are more pronounced in males than in females, and this sex-selective developmental pattern may contribute to both adaptive increases in cognitive abilities (Gur and Gur, 2016) and, potentially, to maladaptive increases in reward-seeking behaviors (e.g., impulsivity; Ikuta et al., 2018). It is unclear why the fronto-accumbal tract is related to puberty only in males. It is possible that in males, this tract is coupled with puberty through a combination of direct hormonal action and more complex, indirect effects via changes in social salience and motivation (Dahl and Forbes, 2010; Delevich and Wilbrecht, 2020). Indeed, testosterone levels have been shown to be positively associated with ventral striatum activity in response to rewards in males and females (Braams et al., 2015; Op de Macks et al., 2011), however other research reports that pubertal testosterone is related to dorsal striatal activation, but not ventral (Laube et al., 2019). In addition, data from animal studies show that adrenal hormones, such as dehydroepiandrosterone (i.e., DHEA), are involved in neurite growth and neuroprotection (see review by Maninger et al., 2009), and that androgen signaling contributes to greater myelination and oligodendrocyte density in males, compared to females (Cerghet et al., 2009; Ghanem et al., 2017). Future work should focus on elucidating the role of hormonal changes in fronto-accumbal tract development and corresponding social behaviors.
The fronto-accumbal tract is part of the cortico-striato-thalamo-cortical loop (Rigoard et al., 2011), a reward- and salience-related circuit that supports self-regulation and cognitive control (Peters et al., 2016). We found that fronto-accumbal fiber cross-section was positively associated with reward sensitivity (and the ratio of reward relative to punishment sensitivity within individuals) in both males and females, likely reflecting the fact that individual differences in reward-seeking behaviors are attributable, in part, to developing fronto-accumbal WM morphometry. We did not find any sex-specific effect of fronto-accumbal FDC or cross-section on reward sensitivity, suggesting that this WM pathway facilitates reward-seeking behaviors in both males and females. Longitudinal research spanning adolescence is needed to understand how developmental increases in fronto-accumbal morphometry track with changes in reward-seeking behavior in both males and females.
We did observe a sex-specific association between the fronto-accumbal tract (both FDC and fiber cross-section) and punishment sensitivity: specifically, males with higher FDC (and cross-section) in this tract reported lower sensitivity to punishment. Prior research has found that males show lower punishment-guided behavioral avoidance, in both monetary and social conditions (Ding et al., 2017), and reduced neurophysiological response to social punishment (Greimel et al., 2018), than do females. Given that risk-taking behavior during a computerized gambling task has been shown to be negatively correlated with self-reported avoidance (Studer et al., 2013), it is possible that higher fronto-accumbal FDC observed in males in our study contributes to the increases in sensation-seeking, impulsivity, and risk-taking behavior documented in adolescent males (Cross et al., 2011; Shulman et al., 2015). Supporting this formulation, we found that fronto-accumbal FDC was positively linked with higher rew:pun in males, but not females; this observation may contribute to male-biased increases in risk-taking behavior during adolescence. The rew:pun findings also support recent formulations that threat and reward circuit development might interact during puberty to influence sex differences in risk-taking and anxiety during adolescence (Baker and Galván, 2020). Follow-up longitudinal studies might help us to understand the role of fronto-accumbal WM development in the emergence of internalizing and externalizing behaviors.
Fiber cross-section, but not fiber density, was positively associated with pubertal stage and negatively associated with punishment sensitivity. These findings suggest that in adolescent boys, the expansion of fronto-accumbal fiber bundles during puberty coincides with lower behavioral avoidance of potentially negative outcomes. Fiber bundle expansion has previously been reported in a longitudinal study of adolescents in early puberty (Brouwer et al., 2012). In addition, an electron microscopy study of young rats revealed that males had larger axonal diameter than did females, due in part to testosterone (Pesaresi et al., 2015). Human longitudinal studies have also reported that males show faster expansion of white matter than do females in adolescence (Lenroot et al., 2007). Although researchers have not explored fiber density and cross-section of the fronto-accumbal tract specifically, particularly in relation to puberty and sex, one study found that age-related changes in fractional anisotropy of this tract are more pronounced in males than in females (Karlsgodt et al., 2015). While increases in anisotropy have been attributed to axonal packing and density (Takahashi et al., 2002), it is not clear whether other micro- or macro-structural properties of WM contribute to anisotropic diffusion (Winston, 2012). Nevertheless, fiber density is conceptually understood to modulate fiber cross-section, and vice versa (Raffelt et al., 2017); thus, these ‘separate’ measures should be interpreted with caution. In sum, while the FDC measure should be investigated to ensure correct interpretation of findings as both micro- and macro-structural variations are combined, separate examinations of fiber density and cross-section might provide biologically useful information that should be interpreted with care (Raffelt et al., 2017).
Future research is required examining puberty-related development of medial vs. lateral aspects of the OFC with respect to their structural connectivity with the NAcc. As we described above, studies suggest that there are functional distinctions between the medial and lateral OFC, particularly in the contexts of reward processing and reward-based learning (Elliott et al., 2000; Noonan et al., 2017). Further, anatomical and functional connections between these OFC sub-regions and other regions of the brain (e.g., striatum, thalamus, anterior cingulate cortex) have previously been reported (Fettes et al., 2017; Zald et al., 2014). Specifically, the medial OFC projects to the NAcc (Bonelli and Cummings, 2007), whereas both the lateral and medial OFC project to the caudate (Jarbo and Verstynen, 2015). Functionally, the medial OFC has been shown to co-activate with limbic and default-mode regions, whereas the lateral OFC co-activates with prefrontal regions involved in cognitive processing; however, both medial and lateral OFC sub-regions are functionally connected with the striatum, amygdala, hippocampus, and thalamus (Zald et al., 2014). Future multi-modal investigations of the development of structural and functional connectivity within distinct OFC sub-regions will advance our understanding of specific cognitive processes that underlie reward and punishment sensitivity and how they change over puberty in males and females.
It is possible that other WM connections with the OFC might also be related to puberty and the processing of rewards and punishment. Therefore, we examined whether our findings generalize to other reward-related WM tracts, or are specific to the fronto-accumbal OFC-NAcc tract. We found that while neither males nor females showed pubertal associations with the OFC-pgACC and OFC-Thalamus tracts, both tracts were positively associated with rew:pun but through different mechanisms: whereas OFC-pgACC FDC was negatively related to punishment sensitivity, OFC-Thalamus FDC was positively associated with reward sensitivity. The pgACC receives information about reward outcomes from the medial OFC and punishment from the lateral OFC (Rolls, 2019) through structural and functional connections (Rolls et al., 2020). The pgACC has been implicated in social cognition (Amodio and Frith, 2006; Mao et al., 2017; Mitchell et al., 2006), including in regulating negative emotional valence in decision-making (Amemori and Graybiel, 2012) and processing pleasant social touch (Lindgren et al., 2012). Thus, WM connecting the OFC and pgACC might be important in social reward processing, although the pubertal process may not be coupled as strongly with this tract as it is with the OFC-NAcc tract in males. In addition, the OFC and thalamus are reciprocally connected structurally (Burks et al., 2017) and functionally (Zald et al., 2014); thalamic projections to the OFC have been shown to support reward-related learning in mice (Fresno et al., 2019; Namboodiri et al., 2020). Consistent with this evidence, we found that OFC-Thalamus tract FDC was positively associated with reward sensitivity in both males and females, but was not related to pubertal staging. Although changes in reinforcement learning have been noted as an important aspect of adolescent development (Davidow et al., 2016; Master et al., 2020; Moin Afshar et al., 2020), our measures of reward and punishment sensitivity were relatively coarse and more strongly related to social processes. Future studies are needed to elucidate how different aspects of reward processing and decision-making (e.g., learning, shaping social interactions, positive and negative risk-taking) and their WM correlates develop during puberty.
We also found that racial-ethnic minority participants had higher sensitivity to reward and punishment than did White participants. To our knowledge, race differences in reward and punishment sensitivity specifically have not previously been reported, though trajectories of sensation-seeking and impulsivity have been explored in Black and White children through adolescence (Pedersen et al., 2012). While Pederson et al. (2012) reported greater increases in sensation-seeking in White, relative to Black, youth, they also reported that Black children had higher initial levels of impulsivity. Our findings warrant research that assesses potential cultural, societal, and economic sources of such differences, as well as the role of the processing of reward and punishment in contributing to mental health disparities. For example, higher behavioral avoidance, which is correlated with the SPSRQ punishment sub-scale, has been shown to be associated with elevated risk for internalizing and posttraumatic stress symptoms; further, behavioral approach, which is related to the SPSRQ reward sub-scale, has been shown to be associated with higher risk for externalizing problems in youth (Gudiño et al., 2012).
This study is the first to examine the role of puberty in fronto-accumbal WM morphometry and sex differences in the contribution of puberty-related frontostriatal WM to sensitivity to reward and punishment; nevertheless, we should note four limitations of this work. First, because of the cross-sectional design of our investigation, we cannot draw strong conclusions about the directionality of our findings. Studying these questions longitudinally will allow investigators to determine the extent to which pubertal advancement is coupled with fronto-accumbal morphometric development. Second, many of our participants were in early or middle stages of puberty; future work is needed with adolescents across a wider span of pubertal development to determine whether our findings are specific to adolescents in the early and middle stages of puberty. Also, given our recruitment procedure, only pre-menarcheal females were included in our sample and, because a small proportion of the girls (6.70%; N=6 of 89) were ages 13–14, they might be considered to be late-maturing. It is important to note, however, that the majority of girls in our sample (93%; N=83) were ages 9–12 and 80% (N=71) were ages 9–11. Epidemiological research suggests that fewer than 10% of girls in the United States start to menstruate before age 11 (Chumlea et al., 2003) and the median age of menarche is 12.25 years (Biro et al., 2018). Thus, pubertal stage in our overall sample was largely representative of national trends. Further, our follow-up analyses excluding female participants who were ages 13–14 years yielded the same results as we obtained when they were included. Third, we used self-report measures to assess adolescents’ sensitivity to reward and punishment. To understand the real-world sequelae of sex-differentiated fronto-accumbal maturation, future studies should examine behavioral differences using reward- and punishment-related paradigms or other-reports of sensation-seeking behaviors (e.g., drug use and risky sexual behaviors). Finally, while fixel-based analysis is fiber specific and has been shown to be more interpretable than voxel-averaged approaches that contain multiple fiber populations (Raffelt et al., 2017), FDC is nevertheless a relative measure that is dependent on the sample being studied. Future work with larger sample sizes is needed to determine whether the current findings are reproducible.
5. Conclusion
Puberty-related changes in WM pathways relevant to outcome valuation may differ for males and females, thereby contributing to sex differences in sensitivity to reward and punishment in adolescence. The fronto-accumbal tract connecting the OFC and NAcc (both implicated in reward-motivated and punishment-aversive behaviors) is one such pathway that has previously been shown to peak higher and earlier (in age) in WM in males than in females; microstructural properties of this fiber bundle have also been shown to be positively associated with impulsivity in adults. We examined whether males and females differ in the association between pubertal stage and fronto-accumbal morphometry in a community sample of adolescents in which males and females were matched in distribution of pubertal staging. Males showed higher reward sensitivity than did females; in addition, males, but not females, exhibited a positive association between pubertal stage and fronto-accumbal FDC. Further, males showed a negative association between fronto-accumbal FDC and punishment sensitivity, whereas females did not. Differences in fiber cross-section of this tract specifically drove our findings; further, fronto-accumbal cross-section was positively correlated with reward sensitivity in both males and females. Morphometric characteristics of the fronto-accumbal WM tract may be more strongly associated with pubertal development in males than females. Further, sex differences in the fronto-accumbal tract may contribute to adolescent-typical biases in reward seeking behaviors, even in the face of potentially negative outcomes.
Supplementary Material
Acknowledgements:
We thank all the families who participated in this study, as well as the staff at the Stanford Neurodevelopment, Affect, and Psychopathology Lab.
Funding: This research was supported by the National Institutes of Health (NIH; R37MH101495 to IHG, F32MH120975 to RC, K01MH117442 to TCH) and the Stanford University Precision Health and Integrated Diagnostics Center (PHIND to IHG, JSK, TCH).
Footnotes
Disclosures: All authors reported no biomedical financial interests or potential conflicts of interest.
References
- Amemori K, Graybiel AM, 2012. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci 15, 776–785. 10.1038/nn.3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD, 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7, 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B, 2010. White matter development in adolescence: a DTI study. Cereb. Cortex 20, 2122–2131. 10.1093/cercor/bhp282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AE, Galván A, 2020. Threat or thrill? the neural mechanisms underlying the development of anxiety and risk taking in adolescence. Developmental Cognitive Neuroscience 45, 100841 10.1016/j.dcn.2020.100841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, Greenspan LC, Kushi LH, Teitelbaum SL, 2018. Age of Menarche in a Longitudinal US Cohort. J Pediatr Adolesc Gynecol 31, 339–345. 10.1016/j.jpag.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL, 2007. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 9, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Duijvenvoorde A.C.K. van, Peper JS, Crone EA, 2015. Longitudinal Changes in Adolescent Risk-Taking: A Comprehensive Study of Neural Responses to Rewards, Pubertal Development, and Risk-Taking Behavior. J. Neurosci 35, 7226–7238. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Mandl RCW, Schnack HG, Soelen I.L.C. van, Baal GC, Peper JS, Kahn RS, Boomsma DI, Pol HEH, 2012. White Matter Development in Early Puberty: A Longitudinal Volumetric and Diffusion Tensor Imaging Twin Study. PLOS ONE 7, e32316 10.1371/journal.pone.0032316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks JD, Conner AK, Bonney PA, Glenn CA, Baker CM, Boettcher LB, Briggs RG, O’Donoghue DL, Wu DH, Sughrue ME, 2017. Anatomy and white matter connections of the orbitofrontal gyrus. Journal of Neurosurgery 128, 1865–1872. 10.3171/2017.3.JNS162070 [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Swamydas M, Bessert D, 2009. Sexual dimorphism in the white matter of rodents. J. Neurol. Sci 286, 76–80. 10.1016/j.jns.2009.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R, Vilgis V, Grimm KJ, Hipwell AE, Forbes EE, Keenan K, Guyer AE, 2018. Girls’ pubertal development is associated with white matter microstructure in late adolescence. Neuroimage 181, 659–669. 10.1016/j.neuroimage.2018.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L, 2011. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci 14, F1–F10. 10.1111/j.1467-7687.2010.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, Sun SS, 2003. Age at menarche and racial comparisons in US girls. Pediatrics 111, 110–113. 10.1542/peds.111.1.110 [DOI] [PubMed] [Google Scholar]
- Colder CR, Hawk LW, Lengua LJ, Wiezcorek W, Eiden RD, Read JP, 2013. Trajectories of Reinforcement Sensitivity During Adolescence and Risk for Substance Use. J Res Adolesc 23, 345–356. 10.1111/jora.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner BT, Rahm-Knigge RL, Jenkins AL, 2018. Revision and clarification of the sensitivity to punishment sensitivity to reward questionnaire. Personality and Individual Differences 121, 31–40. 10.1016/j.paid.2017.09.016 [DOI] [Google Scholar]
- Cross CP, Copping LT, Campbell A, 2011. Sex differences in impulsivity: a meta-analysis. Psychol Bull 137, 97–130. 10.1037/a0021591 [DOI] [PubMed] [Google Scholar]
- Dahl RE, Forbes EE, 2010. Pubertal Development and Behavior: Hormonal Activation of Social and Motivational Tendencies. Brain Cogn 72, 66–72. 10.1016/j.bandc.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, 2009. Towards a Reconceptualization of Striatal Interactions Between Glutamatergic and Dopaminergic Neurotransmission and Their Contribution to the Production of Movements. Curr Neuropharmacol 7, 132–141. 10.2174/157015909788848893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidow JY, Foerde K, Galván A, Shohamy D, 2016. An Upside to Reward Sensitivity: The Hippocampus Supports Enhanced Reinforcement Learning in Adolescence. Neuron 92, 93–99. 10.1016/j.neuron.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Defoe IN, Dubas JS, Dalmaijer ES, van Aken MAG, 2020. Is the Peer Presence Effect on Heightened Adolescent Risky Decision-Making only Present in Males? J Youth Adolesc 49, 693–705. 10.1007/s10964-019-01179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Wilbrecht L, 2020. Role of Puberty on Adult Behaviors [WWW Document]. Oxford Research Encyclopedia of Neuroscience; 10.1093/acrefore/9780190264086.013.248 [DOI] [Google Scholar]
- DePasque S, Galván A, 2017. Frontostriatal development and probabilistic reinforcement learning during adolescence. Neurobiol Learn Mem 143, 1–7. 10.1016/j.nlm.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Ding Y, Wang E, Zou Y, Song Y, Xiao X, Huang W, Li Y, 2017. Gender differences in reward and punishment for monetary and social feedback in children: An ERP study. PLoS One 12 10.1371/journal.pone.0174100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl R, Biro F, 2006. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science 10, 30–56. [Google Scholar]
- Elliott R, Dolan RJ, Frith CD, 2000. Dissociable Functions in the Medial and Lateral Orbitofrontal Cortex: Evidence from Human Neuroimaging Studies. Cereb Cortex 10, 308–317. 10.1093/cercor/10.3.308 [DOI] [PubMed] [Google Scholar]
- Ernst M, 2014. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn 89, 104–111. 10.1016/j.bandc.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS, 2005. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage 25, 1279–1291. 10.1016/j.neuroimage.2004.12.038 [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T, 2016. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 26, 3508–3526. 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettes P, Schulze L, Downar J, 2017. Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Front. Syst. Neurosci 11 10.3389/fnsys.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno V, Parkes SL, Faugère A, Coutureau E, Wolff M, 2019. A thalamocortical circuit for updating action-outcome associations. eLife 8, e46187 10.7554/eLife.46187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ, 2006. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. J. Neurosci 26, 6885–6892. 10.1523/JNEUROSCI.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Malpas CB, Ball G, Silk TJ, Seal ML, 2018a. Age, sex, and puberty related development of the corpus callosum: a multi-technique diffusion MRI study. Brain Struct Funct 223, 2753–2765. 10.1007/s00429-018-1658-5 [DOI] [PubMed] [Google Scholar]
- Genc S, Malpas CB, Gulenc A, Sciberras E, Efron D, Silk TJ, Seal ML, 2019. Longitudinal white matter development in children is associated with puberty, attentional difficulties, and mental health. bioRxiv. [Google Scholar]
- Genc S, Smith RE, Malpas CB, Anderson V, Nicholson JM, Efron D, Sciberras E, Seal ML, Silk TJ, 2018b. Development of white matter fibre density and morphology over childhood: A longitudinal fixel-based analysis. Neuroimage 183, 666–676. 10.1016/j.neuroimage.2018.08.043 [DOI] [PubMed] [Google Scholar]
- Ghanem CA, Degerny C, Hussain R, Liere P, Pianos A, Tourpin S, Habert R, Macklin WB, Schumacher M, Ghoumari AM, 2017. Long-lasting masculinizing effects of postnatal androgens on myelin governed by the brain androgen receptor. PLOS Genetics 13, e1007049 10.1371/journal.pgen.1007049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A-L, Beltz A, Peper JS, Crone EA, Braams BR, 2019. Understanding the Role of Puberty in Structural and Functional Development of the Adolescent Brain. Journal of Research on Adolescence 29, 32–53. 10.1111/jora.12408 [DOI] [PubMed] [Google Scholar]
- Gray JA, 1982. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system, The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Clarendon Press/Oxford University Press, New York, NY, US. [Google Scholar]
- Gray JA, 1981. A Critique of Eysenck’s Theory of Personality, in: Eysenck HJ (Ed.), A Model for Personality. Springer, Berlin, Heidelberg, pp. 246–276. 10.1007/978-3-642-67783-0_8 [DOI] [Google Scholar]
- Greimel E, Bakos S, Landes I, Töllner T, Bartling J, Kohls G, Schulte-Körne G, 2018. Sex differences in the neural underpinnings of social and monetary incentive processing during adolescence. Cogn Affect Behav Neurosci 18, 296–312. 10.3758/s13415-018-0570-z [DOI] [PubMed] [Google Scholar]
- Gudiño OG, Nadeem E, Kataoka SH, Lau AS, 2012. Reinforcement Sensitivity and Risk for Psychopathology Following Exposure to Violence: A Vulnerability-Specificity Model in Latino Youth. Child Psychiatry Hum Dev 43, 306–321. 10.1007/s10578-011-0266-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC, 2016. Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neurosci Biobehav Rev 70, 159–170. 10.1016/j.neubiorev.2016.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 35, 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD, 2013. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev 84, 1566–1578. 10.1111/cdev.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Mann FD, Grotzinger AD, Patterson MW, Steinberg L, Tackett JL, Tucker-Drob EM, 2018. Developmental differences in reward sensitivity and sensation seeking in adolescence: Testing sex-specific associations with gonadal hormones and pubertal development. J Pers Soc Psychol 115, 161–178. 10.1037/pspp0000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ, 2012. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex 22, 1979–1992. 10.1093/cercor/bhr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Colich NL, Sisk LM, Oskirko K, Jo B, Gotlib IH, 2020. Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Developmental Cognitive Neuroscience 100773 10.1016/j.dcn.2020.100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Del Arco A, Karlsgodt KH, 2018. White matter integrity in the fronto-striatal accumbofrontal tract predicts impulsivity. Brain Imaging Behav 12, 1524–1528. 10.1007/s11682-017-9820-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K, Verstynen TD, 2015. Converging Structural and Functional Connectivity of Orbitofrontal, Dorsolateral Prefrontal, and Posterior Parietal Cortex in the Human Striatum. J. Neurosci 35, 3865–3878. 10.1523/JNEUROSCI.2636-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM, 2012. FSL. Neuroimage 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD, 2019. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 44, 71–85. 10.1038/s41386-018-0111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, John M, Ikuta T, Rigoard P, Peters BD, DeRosse P, Malhotra AK, Szeszko PR, 2015. The Accumbofrontal Tract: Diffusion Tensor Imaging Characterization and Developmental Change from Childhood to Adulthood. Hum Brain Mapp 36, 4954–4963. 10.1002/hbm.22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry 36, 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Keenan K, Culbert K, Grimm KJ, Hipwell A, Stepp S, 2014. Timing and tempo: Exploring the complex association between pubertal development and depression in African American and European American girls. J Abnorm Psychol 123, 725–736. 10.1037/a0038003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, Gotlib IH, 2017. The Impact of the Severity of Early Life Stress on Diurnal Cortisol: The Role of Puberty. Psychoneuroendocrinology 77, 68–74. 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, Price E, Faja S, Herrington JD, Schultz RT, 2013. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia 51, 2062–2069. 10.1016/j.neuropsychologia.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Peper JS, Crone EA, Dahl RE, 2012. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev Cogn Neurosci 2, 36–54. 10.1016/j.dcn.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Verstynen TD, Yeh F-C, Luna B, 2018. Developmental Changes in the Integration of Affective and Cognitive Corticostriatal Pathways are Associated with Reward-Driven Behavior. Cereb Cortex 28, 2834–2845. 10.1093/cercor/bhx162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube C, Lorenz R, van den Bos W, 2019. Pubertal testosterone correlates with adolescent impatience and dorsal striatal activity. Dev Cogn Neurosci 42 10.1016/j.dcn.2019.100749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Deoni S, 2018. The development of brain white matter microstructure. NeuroImage, Microstructural Imaging 182, 207–218. 10.1016/j.neuroimage.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN, 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073. 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L, 2012. Pleasant human touch is represented in pregenual anterior cingulate cortex. NeuroImage 59, 3427–3432. 10.1016/j.neuroimage.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30, 65–91. 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CV, Araujo MFP, Nishimaru H, Matsumoto J, Tran AH, Hori E, Ono T, Nishijo H, 2017. Pregenual Anterior Cingulate Gyrus Involvement in Spontaneous Social Interactions in Primates—Evidence from Behavioral, Pharmacological, Neuropsychiatric, and Neurophysiological Findings. Front Neurosci 11 10.3389/fnins.2017.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Potts GF, 2004. Reward sensitivity in impulsivity. Neuroreport 15, 1519–1522. 10.1097/01.wnr.0000132920.12990.b9 [DOI] [PubMed] [Google Scholar]
- Master SL, Eckstein MK, Gotlieb N, Dahl R, Wilbrecht L, Collins AGE, 2020. Disentangling the systems contributing to changes in learning during adolescence. Developmental Cognitive Neuroscience 41, 100732 10.1016/j.dcn.2019.100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle, Leve LD, Van Ryzin M, Natsuaki MN, Ge X, 2011. Associations Between Early Life Stress, Child Maltreatment, and Pubertal Development Among Girls in Foster Care. Journal of Research on Adolescence 21, 871–880. 10.1111/j.1532-7795.2011.00746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Goddings A-L, Whitaker KJ, Blakemore S-J, Viner RM, 2015. The effects of puberty on white matter development in boys. Dev Cogn Neurosci 11, 116–128. 10.1016/j.dcn.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR, 2006. Dissociable Medial Prefrontal Contributions to Judgments of Similar and Dissimilar Others. Neuron 50, 655–663. 10.1016/j.neuron.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Moin Afshar N, Keip AJ, Taylor JR, Lee D, Groman SM, 2020. Reinforcement Learning during Adolescence in Rats. J Neurosci 40, 5857–5870. 10.1523/JNEUROSCI.0910-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC, 1999. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol 45, 265–269. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolescence 9, 271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- Namboodiri VMK, Hobbs T, Pisanty IT, Simon RC, Stuber GD, 2020. Meta-reinforcement learning in a thalamo-orbitofrontal circuit. bioRxiv 2020.04.28.066878. 10.1101/2020.04.28.066878 [DOI] [Google Scholar]
- Nelson EE, Jarcho JM, Guyer AE, 2016. Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, Special Section: The Developmental Neuroscience of Adolescence: Revisiting, Refining, and Extending Seminal Models 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Chau BKH, Rushworth MFS, Fellows LK, 2017. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans. J. Neurosci 37, 7023–7035. 10.1523/JNEUROSCI.0692-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C, 2001. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience 4, 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Gunther Moor B, Overgaauw S, Güroğlu B, Dahl RE, Crone EA, 2011. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev Cogn Neurosci 1, 506–516. 10.1016/j.dcn.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luking KR, Anokhin AP, Gotlib IH, Hayden EP, Olino TM, Peng C-Z, Hajcak G, Barch DM, 2016. Revising the BIS/BAS Scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychol Assess 28, 429–442. 10.1037/pas0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SL, Molina BSG, Belendiuk KA, Donovan JE, 2012. Racial Differences in the Development of Impulsivity and Sensation Seeking from Childhood into Adolescence and Their Relation to Alcohol Use. Alcoholism: Clinical and Experimental Research 36, 1794–1802. 10.1111/j.1530-0277.2012.01797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Hervé P-Y, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T, 2008. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J. Neurosci 28, 9519–9524. 10.1523/JNEUROSCI.1212-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T, 2015. Axon diameter and axonal transport: In vivo and in vitro effects of androgens. Neuroimage 115, 191–201. 10.1016/j.neuroimage.2015.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, Downar J, 2016. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci 10 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Potthoff RF, 1964. On the Johnson-Neyman technique and some extensions thereof. Psychometrika 29, 241–256. 10.1007/BF02289721 [DOI] [Google Scholar]
- Potts GF, George MRM, Martin LE, Barratt ES, 2006. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters 397, 130–134. 10.1016/j.neulet.2005.12.003 [DOI] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Raffelt DA, Tournier J-D, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A, 2017. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144, 58–73. 10.1016/j.neuroimage.2016.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbe D, 1996. Psychometric review of Traumatic Events Screening Inventory for Children (TESI-C), in: Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran. [Google Scholar]
- Rigoard P, Buffenoir K, Jaafari N, Giot JP, Houeto JL, Mertens P, Velut S, Bataille B, 2011. The Accumbofrontal Fasciculus in the Human Brain: A Microsurgical Anatomical Study. Neurosurgery 68, 1102–1111. 10.1227/NEU.0b013e3182098e48 [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2019. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct 224, 3001–3018. 10.1007/s00429-019-01945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Du J, Wei D, Qiu J, Dai D, Zhou Q, Xie P, Feng J, 2020. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cogn Affect Neurosci 15, 75–86. 10.1093/scan/nsaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M, Schmack K, Schlagenhauf F, Sterzer P, 2012. Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage 62, 1717–1725. 10.1016/j.neuroimage.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Shigemune Y, Tsukiura T, Kambara T, Kawashima R, 2014. Remembering with gains and losses: effects of monetary reward and punishment on successful encoding activation of source memories. Cereb. Cortex 24, 1319–1331. 10.1093/cercor/bhs415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: correspondence between hormonal and physical development. Child Dev 80, 327–337. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Harden KP, Chein JM, Steinberg L, 2015. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. J Youth Adolesc 44, 1–17. 10.1007/s10964-014-0116-9 [DOI] [PubMed] [Google Scholar]
- Simmonds D, Hallquist MN, Asato M, Luna B, 2014. Developmental Stages and Sex Differences of White Matter and Behavioral Development through Adolescence: A Longitudinal Diffusion Tensor Imaging (DTI) Study. Neuroimage 92, 356–368. 10.1016/j.neuroimage.2013.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24, 417–463. 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2008. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev 28, 78–106. 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer B, Pedroni A, Rieskamp J, 2013. Predicting Risk-Taking Behavior from Prefrontal Resting-State Activity and Personality. PLOS ONE 8, e76861 10.1371/journal.pone.0076861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Hackney DB, Zhang G, Wehrli SL, Wright AC, O’Brien WT, Uematsu H, Wehrli FW, Selzer ME, 2002. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc. Natl. Acad. Sci. U.S.A 99, 16192–16196. 10.1073/pnas.252249999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, 2016. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, Special Section: The Developmental Neuroscience of Adolescence: Revisiting, Refining, and Extending Seminal Models 17, 57–67. 10.1016/j.dcn.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X, 2001. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences 31, 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Tournier J-D, Smith RE, Raffelt DA, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh C-H, Connelly A, 2019. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation (preprint). Neuroscience. 10.1101/551739 [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, Luciana M, 2012. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology 48, 1488–1500. 10.1037/a0027502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim KO, Luciana M, 2014. Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain and Cognition, Special Issue on Reward and Regulatory Processes in Adolescence 89, 15–26. 10.1016/j.bandc.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA, 2010. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage 51, 345–355. 10.1016/j.neuroimage.2010.02.038 [DOI] [PubMed] [Google Scholar]
- Vandeweghe L, Matton A, Beyers W, Vervaet M, Braet C, Goossens L, 2016. Psychometric Properties of the BIS/BAS Scales and the SPSRQ in Flemish Adolescents. Psychol Belg 56, 406–420. 10.5334/pb.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, 2007. Orbitofrontal Cortex and Its Contribution to Decision-Making. Annual Review of Neuroscience 30, 31–56. 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- Winston GP, 2012. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quantitative Imaging in Medicine and Surgery 2, 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR, 2014. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb. Cortex 24, 232–248. 10.1093/cercor/bhs308 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


