Abstract
Previous studies have shown that miR-222 targets the p53 upregulated modulator of apoptosis (PUMA) to regulate cell biological behavior in some human malignancies. We hypothesized that there was a negative regulation, which might induce apoptosis, between miR-222 and PUMA in adenoid cystic carcinoma (ACC). In this study, the expression levels of miR-222 and the PUMA gene after transfection with antisense miR-222 (As-miR-222) were evaluated by RT-PCR and Western blot assays. Cell proliferation and migratory abilities were detected by CCK-8 and Transwell assays. Cell cycle and apoptosis were analyzed by flow cytometry. Our results showed that, when compared with the control and scramble-transfected groups, the expression of miR-222 in the As-miR-222 group was downregulated, while the expression of PUMA at both mRNA and protein levels was upregulated, cell proliferation and migratory abilities were inhibited, and apoptosis was increased. Our results suggested that As-miR-222 transfection could upregulate the expression of PUMA to induce apoptosis in ACC, providing a new concept for the treatment of ACC.
Key words: Adenoid cystic carcinoma (ACC), miR-222, p53 upregulated modulator of apoptosis (PUMA), Apoptosis, Migration
INTRODUCTION
Adenoid cystic carcinoma (ACC) is a common malignant salivary gland tumor occurring mainly in the major maxillofacial salivary glands but seldom in other glands, such as, for example, the minor salivary, esophageal, bronchial, and mammary glands (1). The clinical and pathological characteristics of ACC include slow growth, neural invasion, distant metastasis, and the potential for local recurrence. ACC has a high incidence rate of metastasis, most commonly in the lung. Although complete tumor resection and radiotherapy have been shown to improve long-term survival, the prognosis of ACC remains unsatisfactory (2).
MicroRNAs (miRNAs) are small noncoding RNA molecules containing about 22 nucleotides. By perfectly or partially complementary sequences with the 3′ end of target mRNAs, they regulate the expression of target genes at the posttranscription level. This regulation aims to affect cell proliferation, differentiation, and apoptosis and has intimate contact with eukaryotes throughout the life movement process (3). As an important member of the family of miRNAs, miR-222 is one of the common “oncomirs” that have been found to link with various types of cancers. Its expression level relates to cell proliferation, migration, invasion, and apoptosis in various tumors (4,5). However, the role of miR-222 in ACC has not yet been reported.
p53 upregulated modulator of apoptosis (PUMA), a BH3-only protein, is a member of the Bcl-2 protein family. Induced by a variety of signals, PUMA plays an important role in p53-dependent and -independent apoptosis via the mitochondrial pathway. Numerous studies have shown that PUMA expression is closely related to the development of malignant tumors, including those in colorectal, breast, liver, and oral cancers (6–8).
Studies have suggested that miR-222 can regulate PUMA expression and cell apoptosis by targeting binding sites in the 3′-UTR (9). In research on breast cancer, lung cancer, and glioblastoma, Zhang and colleagues discovered that antisense miR-222 (As-miR-222) transfection could induce tumor cell apoptosis by upregulating PUMA expression (10). Our previous studies found that miR-222 expression was positive in oral squamous cell carcinoma. Experiments confirmed that As-miR-222 transfection could induce apoptosis in oral squamous cell carcinoma by upregulating PUMA expression. However, the roles of miR-222 and PUMA in human ACC have not been reported.
In this study, by utilizing As-miR-222 transfection into ACC cells, we observed its effect on PUMA expression and detected cell proliferation, migration, and apoptosis. We then identified the relationship between miR-222 and PUMA in ACC, and the effect on ACC cell apoptosis after As-miR-222 transfection, to provide a new method for ACC gene therapy.
MATERIALS AND METHODS
Cell Culture and Transfection
The ACC cell lines SACC-83 and SACC-LM were provided by Prof. Shenglin Li (Beijing University School of Stomatology, P.R. China). They were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM/high glucose; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone), penicillin (100 U/ml), and streptomycin (100 μg/ml), and incubated in a humidified 5% CO2 environment at 37°C. The 2′-O-methyl (OMe) oligonucleotides were synthesized and purified by high-performance liquid chromatography (GenePharma Co. Ltd., Shanghai, P.R. China). All the bases were 2′-OMe modified to obtain the following sequences: hsa-miR-222 inhibitor (As-miR-222), 5′-ACCCAGUAGCCAGAUGUAGCU-3′; miRNA, which mimics the negative control (NC) inhibitor, 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′. As-miR-222 and NC inhibitor were transfected by the use of Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA). After transfection for 24 h, cells were used for subsequent analysis and were divided into three groups: the control group, the scramble group, and the As-miR-222 group.
RNA Preparation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
After transfection for 24 h, total RNA was extracted with TRIzol reagent (Invitrogen) and purified with Dnase I (Promega, Madison, WI, USA) for removal of genomic DNA. The purity and integrity of total RNA were detected, after which RT-PCR was carried out. The U6 snRNA gene was used as the internal control against the hsa-miR-222 gene, while the 18s rRNA gene was used against the PUMA gene. The hsa-miR-222 gene primers were 5′-ACACTCCAGCTGGGAGCTACATCTGGCTACTG-3′ (forward) and 5′-CTCAACTGGTGTCGTGGA-3′ (reverse). The U6 snRNA gene primers were 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse). The PUMA gene primers were 5′-GGGCCCAGACTGTGAATCC-3′ (forward) and 5′-TCACACGTGCTCTCTCTAAACC-3′ (reverse). The 18s rRNA gene primers were 5′-CCTGGATACCGCAGCTAGGA-3′ (forward) and 5′-GCGGCGCAATACGAATGCCCC-3′ (reverse). Relative expression levels were calculated by the 2−ΔΔCt method (11).
Western Blot Analysis
After transfection for 48 h, cells were washed three times with cold phosphate-buffered saline (PBS; Hyclone) and maintained in lysis buffer for 30 min, followed by centrifugation at 15,000 × g for 15 min at 4°C. The protein concentration was detected by means of the BCA protein assay kit (Nanjing KeyGen Biotech Co. Ltd., P.R. China) according to the manufacturer’s instructions. Equal amounts of protein were separated by 12% SDS–polyacrylamide gel and transferred to a PVDF membrane, which was blocked in 5% skim milk for 1 h at room temperature and incubated with the primary antibodies against PUMA and GAPDH at 4°C overnight. The membrane was then incubated with the secondary antibody for 1 h at room temperature and developed by enhanced chemiluminescence. The density of PUMA protein bands was quantified after normalization with the density of GAPDH.
Cell Viability Assay
After transfection as described previously, the SACC-83 and SACC-LM cell lines were seeded into 96-well plates with 5,000 cells/well. A 10-μl quantity of Cell Counting Kit-8 solution (CCK-8; Beyotime Institute of Biotechnology, P.R. China) was added to each well at 0, 24, 48, and 72 h after transfection. The cells were subsequently incubated for 2 h at 37°C. Optical density (OD) was measured at a wavelength of 450 nm.
Cell Migration Assay
After transfection for 48 h, 1 × 105 cells were seeded into the upper chamber in 100 μl of serum-free DMEM, while a 600-μl quantity of DMEM supplemented with 10% FBS was placed in the lower chamber. The two chambers were separated by Transwell inserts with 8-μm pores. After 12 h of incubation in a 5% CO2 environment at 37°C, the nonmigrating cells on the upper surfaces of the inserts were removed, while cells on the lower surfaces were fixed, stained, and counted under a microscope.
Cell Cycle Assay
After transfection for 48 h, 1 × 106 cells of each group were washed twice with cold PBS and fixed with 70% ethanol. The cells were stained with propidium iodide (PI) and examined by flow cytometry.
Apoptosis Assay
Forty-eight hours after transfection, cells were washed twice with cold PBS and collected in 0.5 ml of buffer at a density of 1 × 106 cells/ml. The buffer was mixed with 1.25 μl of annexin V-FITC and 10 μl of PI and then incubated for 15 min at room temperature in the dark. Finally, the cells were examined by flow cytometry.
Statistical Analysis
The experimental data were expressed as means ± standard deviation (SD) from a minimum of three analyses. Statistical analyses were performed by independent sample t-test or one-way analysis of variance (ANOVA) with SPSS software 20.0. Statistical significance was set at p < 0.05.
RESULTS
Expression of miR-222 and the PUMA Gene in ACC Cells
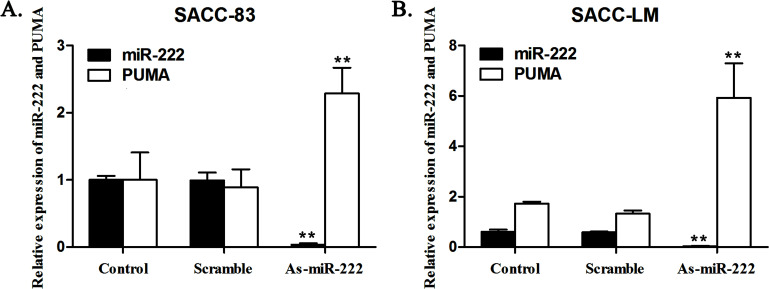
As-miR-222 was chemically synthesized and transfected into SACC-83 and SACC-LM cells to knock down endogenous miR-222. In the As-miR-222 group of SACC-83 and SACC-LM cells, As-miR-222 inhibited the expression of miR-222. RT-PCR analysis indicated that endogenous miR-222 was silenced by As-miR-222 specifically and efficiently. In contrast, the expression of the PUMA gene in the As-miR-222 group was obviously upregulated compared with that in the control and scramble groups. However, no significant differences were noted between the control and scramble groups. The results showed that miR-222 was negatively correlated with the expression of the PUMA gene in ACC cells (Fig. 1).
Figure 1.
In (A) SACC-83 and (B) SACC-LM cells, As-miR-222 transfection inhibited the expression of miR-222 and upregulated the expression of the PUMA gene. The RT-PCR analysis indicated the relative expression of miR-222 and PUMA in SACC-83 and SACC-LM cells after transfection. RT-PCR was performed as described in materials and Methods.
As-miR-222 Upregulates the Expression of PUMA Proteins
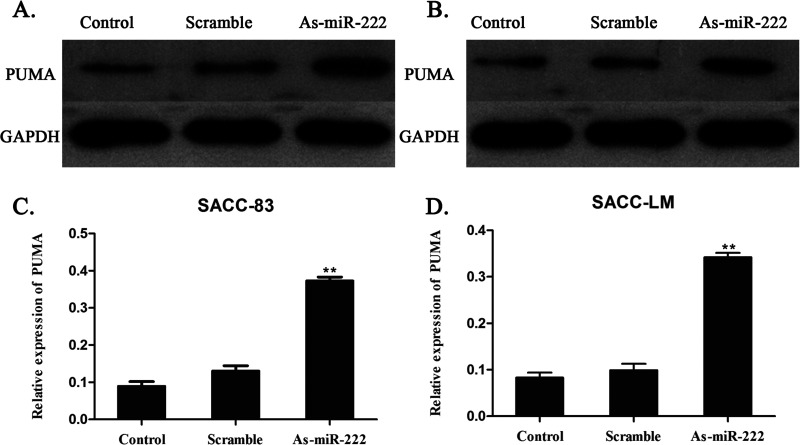
The expression of the PUMA proteins was measured by Western blot analysis for the identification of the molecular mechanism of the relationship between miR-222 and PUMA proteins in ACC cells. A significant increase in the expression of PUMA proteins was observed in SACC-83 and SACC-LM cells in the As-miR-222 group, when compared with that in the control and scramble groups. The results showed that the transfection of As-miR-222 upregulated the expression of PUMA proteins (Fig. 2).
Figure 2.
Expression of PUMA in (A, C) SACC-83 and (B, D) SACC-LM cells after transfection. The Western blot assay determined that PUMA was observed to be overexpressed in the SACC-83 and SACC-LM cells in the As-miR-222 group, compared with the other two groups.
As-miR-222 Influences Proliferation of ACC Cells
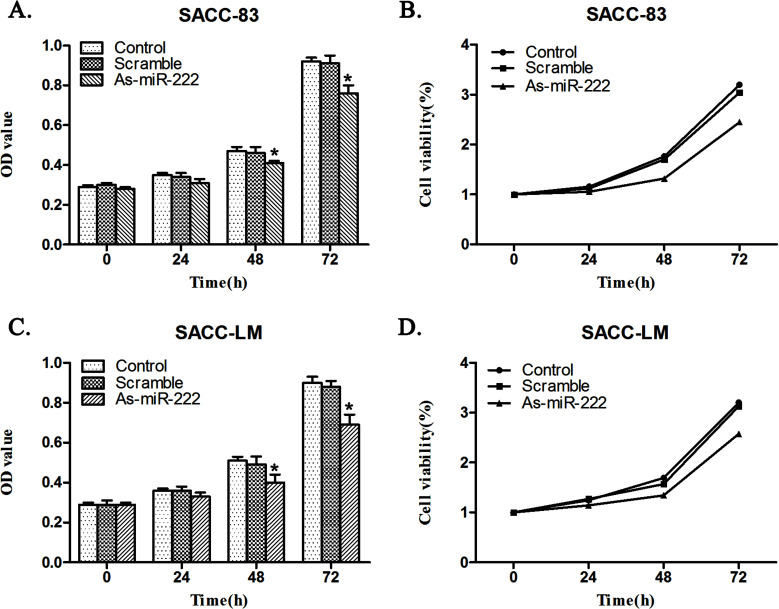
The CCK-8 assay indicated that the proliferation of SACC-83 and SACC-LM cells in the As-miR-222 group, when transfected with As-miR-222, was significantly inhibited when compared with that in the control and scramble groups (Fig. 3).
Figure 3.
As-miR-222 transfection influenced ACC cell growth. The CCK-8 assay showed that (A, B) SACC-83 and (C, D) SACC-LM cells in the As-miR-222 group proliferated at a significantly lower rate than in the other two groups. No significant differences between the control and scramble groups were noted.
As-miR-222 Affects the Migratory Ability of ACC Cells
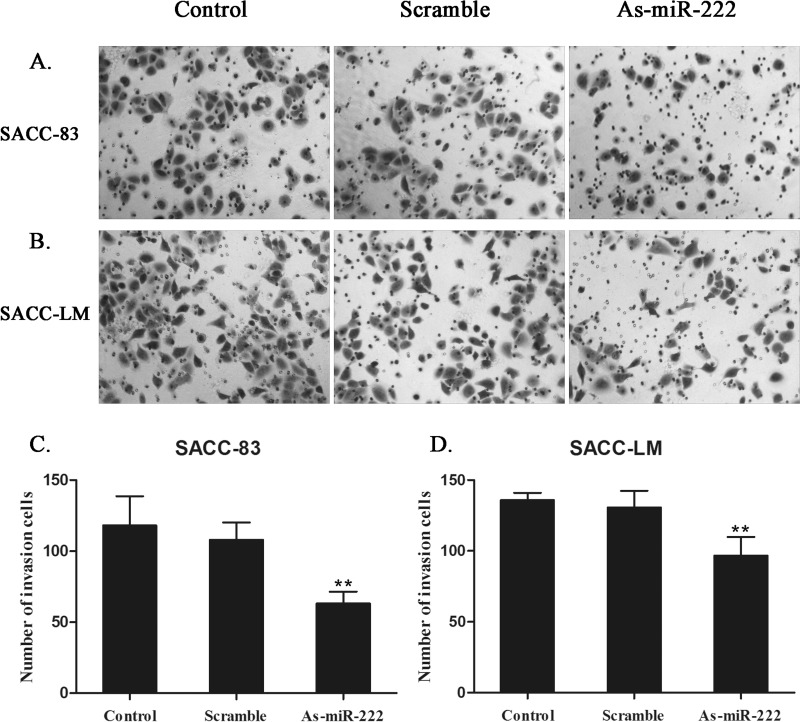
Since cell motility plays an important role in metastasis, the motility of ACC cells was examined by the Transwell assay. The migration of SACC-83 and SACC-LM cells transfected with As-miR-222 was significantly reduced compared with that in the control groups. The results indicated that transfection with As-miR-222 impaired the migratory ability of ACC cells (Fig. 4).
Figure 4.
Effect of As-miR-222 transfection on the migratory ability of ACC cells. Images of (A) SACC-83 and (B) SACC-LM cells, which were transferred to inserts after transfection for Transwell assay. ACC cells in the As-miR-222 group showed inhibited migratory ability. No significant differences between the control and scramble groups were observed. (C, D) The numbers of migrated cells were expressed as the means ± SD of three different experiments.
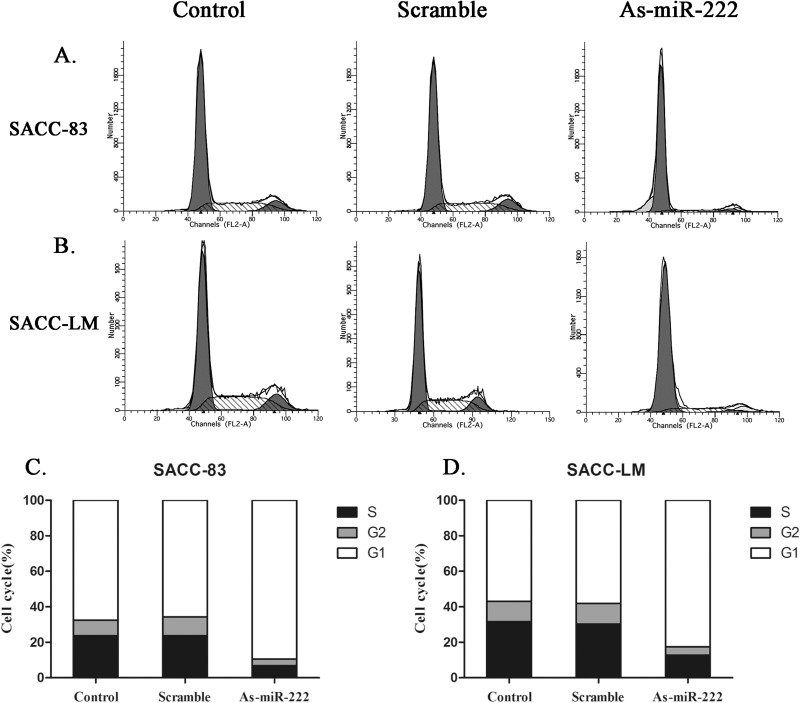
As-miR-222 Influences Cell Cycle and Apoptosis
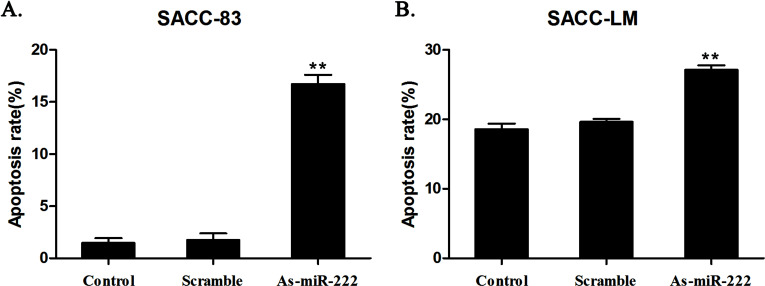
The cell cycle assay showed that cells in the G0/G1 phase increased significantly in the As-miR-222 group, while cells in the G2/M and S phases decreased noticeably when compared with the other two groups (Fig. 5). The apoptosis assay showed that the apoptosis rate of ACC cells in the As-miR-222 group was obviously increased compared with that in the other two groups (Fig. 6). The results suggested that apoptosis was significantly induced in ACC cells transfected with As-miR-222.
Figure 5.
The changes of cell cycle in (A, C) SACC-83 and (B, D) SACC-LM cells were examined by flow cytometry after transfection. The cells in the G0/G1 phase increased significantly in the As-miR-222 group, while the cells in the G2/M and S phases did not. No significant differences between the control and scramble groups were noted.
Figure 6.
The distribution of apoptosis in (A) SACC-83 and (B) SACC-LM cells was detected by flow cytometry after transfection. The apoptosis rate of ACC cells in the As-miR-222 group was significantly increased. No significant differences between the control and scramble groups were observed.
DISCUSSION
In recent years, more studies have shown that miRNAs function in the posttranscriptional regulation of gene expression. miRNAs can promote or suppress cancer in tumor cells (12,13). miR-222 is one of the most common “oncomirs,” and its increased expression is related to proliferation, invasion, and apoptosis in a variety of tumor cells. Researchers have reported that miR-221/222 was associated with the development of various tumors, such as glioblastoma, prostatic carcinoma, and thyroid papillary carcinoma (14–16). Others also confirmed that miR-221/222 promoted the growth of tumor cells by downregulating the expression of p27kip1 and p57kip2 proteins (4,17).
As a member of the Bcl-2 protein family, PUMA can be rapidly induced by p53, playing a key role in apoptosis pathways (18). Investigators have suggested that PUMA is involved in p53-dependent and -independent apoptosis induced by a variety of signals, such as DNA damage, oncogene activation, low oxygen levels, growth factors, tissue ischemia, and glucocorticoid (19–25). Mutation or absence of the PUMA gene could lead to reduced rates of apoptosis (23,26), indicating that PUMA is essential in the process of apoptosis.
The bioinformatics analysis showed that miR-222 is closely related to the PUMA gene. There have been no reports on the relationship between miR-222 and PUMA in ACC, but there has been related research on other tumors. Zhang and coworkers found that knocking down miR-221/222 could upregulate the expression of PUMA and induce cell apoptosis in glioblastoma (10). They suggested that there is a negative regulation between miR-221/222 and PUMA and that miR-221/222 targeted PUMA to induce cell apoptosis in glioblastoma. Jiang and colleagues also confirmed that miR-222 could control the biological behavior of oral squamous cell carcinoma by negatively regulating the expression of PUMA (27).
With the continuous development of related research, PUMA has received increased attention for its role in the development of malignant tumors, but the research on PUMA in ACC is negligible. In this study, by transfecting As-miR-222 into SACC-83 and SACC-LM cells, we knocked down the expression of endogenous miR-222. The results of the RT-PCR showed that the expression of PUMA, one of the apoptosis-promoting genes, could be upregulated by knocking down miR-222. There was a negative regulation between miR-222 and PUMA. This point of view was confirmed by Western blot assay at the protein level. The results of the CCK-8 assay indicated that the cell proliferation rate was significantly suppressed when the expression of miR-222 decreased. miR-222 could be regarded as an “oncomir” in ACC cells. In cell cycle and apoptosis assays, cells in the G0/G1 phase and cell apoptosis rate increased significantly in the As-miR-222 group compared with the other two groups. The results confirmed that downregulation of miR-222 could induce cell apoptosis and influence cell cycle distribution to suppress cancer. In our experiments, cell apoptosis rates of the As-miR-222 group in SACC-83 and SACC-LM cells were 16.73% and 27.13%, respectively, which was significantly increased compared with rates in the other two groups. The differences were statistically significant. However, the ideal therapeutic effect has not yet been achieved and may be related to specific mechanisms or other targets of miR-222, requiring further research. With the continuous development of such research, As-miR-222 transfection by targeting PUMA to induce cell apoptosis is expected to provide an experimental basis for the clinical treatment of ACC.
ACKNOWLEDGMENTS
This study was supported by the National Natural Sciences Foundation of China (Nos. 81272554 and 81472526), the Guangdong Natural Sciences Foundation (No. S2011020003247), and the Guangdong Sciences and Technology Project (Nos. 2011B050400030 and 2012B031800387).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Terada T. Adenoid cystic carcinoma of the oral cavity: Immunohistochemical study of four cases. Int. J. Clin. Exp. Pathol. 6:932–938; 2013. [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang L. C.; Huang S. Y.; Zhang D. S.; Zhang S. H.; Li W. G.; Zheng P. H.; Chen Z. W. Expression of beclin 1 in primary salivary adenoid cystic carcinoma and its relation to Bcl-2 and p53 and prognosis. Braz. J. Med. Biol. Res. 47:252–258; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srinivasan S.; Selvan S. T.; Archunan G.; Gulyas B.; Padmanabhan P. MicroRNAs—The next generation therapeutic targets in human diseases. Theranostics 3:930–942; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garofalo M.; Quintavalle C.; Di Leva G.; Zanca C.; Romano G.; Taccioli C.; Liu C. G.; Croce C. M.; Condorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene 27:3845–3855; 2008. [DOI] [PubMed] [Google Scholar]
- 5. le Sage C.; Nagel R.; Egan D. A.; Schrier M.; Mesman E.; Mangiola A.; Anile C.; Maira G.; Mercatelli N.; Ciafre S. A.; Farace M. G.; Agami R. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim M. R.; Jeong E. G.; Chae B.; Lee J. W.; Soung Y. H.; Nam S. W.; Lee J. Y.; Yoo N. J.; Lee S. H. Pro-apoptotic PUMA and anti-apoptotic phospho-BAD are highly expressed in colorectal carcinomas. Dig. Dis. Sci. 52:2751–2756; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Qiu W.; Wang X.; Leibowitz B.; Yang W.; Zhang L.; Yu J. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology 54:1249–1258; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang R.; Wang X.; Li B.; Lin F.; Dong K.; Gao P.; Zhang H. Z. Tumor-specific adenovirus-mediated PUMA gene transfer using the survivin promoter enhances radiosensitivity of breast cancer cells in vitro and in vivo. Breast Cancer Res. Treat. 117:45–54; 2009. [DOI] [PubMed] [Google Scholar]
- 9. Zhang C.; Zhang J.; Zhang A.; Wang Y.; Han L.; You Y.; Pu P.; Kang C. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int. J. Oncol. 37:1621–1626; 2010. [DOI] [PubMed] [Google Scholar]
- 10. Zhang C. Z.; Zhang J. X.; Zhang A. L.; Shi Z. D.; Han L.; Jia Z. F.; Yang W. D.; Wang G. X.; Jiang T.; You Y. P.; Pu P. Y.; Cheng J. Q.; Kang C. S. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 9:229; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408; 2001. [DOI] [PubMed] [Google Scholar]
- 12. Hede K. Studies define role of microRNA in cancer. J. Natl. Cancer Inst. 97:1114–1115; 2005. [DOI] [PubMed] [Google Scholar]
- 13. Gregory R. I.; Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 65:3509–3512; 2005. [DOI] [PubMed] [Google Scholar]
- 14. Gillies J. K.; Lorimer I. A. Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 6:2005–2009; 2007. [DOI] [PubMed] [Google Scholar]
- 15. Galardi S.; Mercatelli N.; Giorda E.; Massalini S.; Frajese G. V.; Ciafre S. A.; Farace M. G. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 282:23716–23724; 2007. [DOI] [PubMed] [Google Scholar]
- 16. Pallante P.; Visone R.; Ferracin M.; Ferraro A.; Berlingieri M. T.; Troncone G.; Chiappetta G.; Liu C. G.; Santoro M.; Negrini M.; Croce C. M.; Fusco A. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr. Relat. Cancer 13:497–508; 2006. [DOI] [PubMed] [Google Scholar]
- 17. Fornari F.; Gramantieri L.; Ferracin M.; Veronese A.; Sabbioni S.; Calin G. A.; Grazi G. L.; Giovannini C.; Croce C. M.; Bolondi L.; Negrini M. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27:5651–5661; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Yu J.; Zhang L.; Hwang P. M.; Kinzler K. W.; Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673–682; 2001. [DOI] [PubMed] [Google Scholar]
- 19. Yu J.; Wang Z.; Kinzler K. W.; Vogelstein B.; Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 100:1931–1936; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chipuk J. E.; Bouchier-Hayes L.; Kuwana T.; Newmeyer D. D.; Green D. R. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309:1732–1735; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Ming L.; Sakaida T.; Yue W.; Jha A.; Zhang L.; Yu J. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis 29:1878–1884; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You H.; Pellegrini M.; Tsuchihara K.; Yamamoto K.; Hacker G.; Erlacher M.; Villunger A.; Mak T. W. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 203:1657–1663; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeffers J. R.; Parganas E.; Lee Y.; Yang C.; Wang J.; Brennan J.; MacLean K. H.; Han J.; Chittenden T.; Ihle J. N.; McKinnon P. J.; Cleveland J. L.; Zambetti G. P. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4:321–328; 2003. [DOI] [PubMed] [Google Scholar]
- 24. Villunger A.; Michalak E. M.; Coultas L.; Mullauer F.; Bock G.; Ausserlechner M. J.; Adams J. M.; Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302:1036–1038; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Wu B.; Qiu W.; Wang P.; Yu H.; Cheng T.; Zambetti G. P.; Zhang L.; Yu J. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut 56:645–654; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu F. T.; Newland A. C.; Jia L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem. Biophys. Res. Commun. 310:956–962; 2003. [DOI] [PubMed] [Google Scholar]
- 27. Jiang F.; Zhao W.; Zhou L.; Zhang L.; Liu Z.; Yu D. miR-222 regulates the cell biological behavior of oral squamous cell carcinoma by targeting PUMA. Oncol. Rep. 31:1255–1262; 2014. [DOI] [PubMed] [Google Scholar]