Abstract
Hepatocellular carcinoma (HCC) is highly resistant to traditional chemotherapeutic approaches, which causes difficulty in the development of effective drugs for the treatment of HCC. Berberine, a major ingredient of Rhizoma coptidis, is a natural alkaloid used in traditional Chinese medicine. Berberine exhibits potent antitumor activity against HCC due to its high efficiency and low toxicity. In the present study, we found that berberine sensitized HepG cells to NF-κB-mediated apoptosis. Berberine exhibited a significant antiproliferation effect on the HepG2 cells and promoted apoptosis. Both qRT-PCR and immunofluorescence staining revealed that berberine reduced the NF-κB p65 levels in HepG2 cells. Moreover, p65 overexpression rescued berberine-induced cell proliferation and prevented HepG2 cells from undergoing apoptosis. These results suggest that berberine inhibits the growth of HepG2 cells by promoting apoptosis through the NF-κB p65 pathway.
Key words: Hepatocellular carcinoma (HCC), Berberine, NF-κB, p65, Apoptosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers in the world, with more than 600,000 people being diagnosed every year1. For HCC patients with a well-preserved hepatic function and long-term survival, hepatic resection or transplantation is typically the first-line option. However, surgical therapies have limitations, and advanced tumors might recur even after complete surgical resection. Once the tumor size is more than 5 cm or has advanced, patient prognosis is poor, yielding a 5-year survival rate of 30%–40%1. Currently, chemotherapy remains an effective way to treat HCC2. Therefore, there is a need for potential new agents, with minimal off-target effects and tolerance, to be developed for patients with unresectable HCC.
Berberine (Fig. 1), a natural alkaloid isolated from the Chinese herb Rhizoma coptidis, has been widely used in traditional Chinese medicine for centuries. Multiple pharmaceutical effects of berberine on several diseases have been reported. For example, berberine acts as an immunoregulator by suppressing the expression of the proinflammatory genes, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), in macrophages3. Berberine also induces a significant reduction in triglycerides and plasma cholesterol in diabetic patients, improves lipid and glucose profiles, and decreases body mass index in subjects with metabolic syndrome4. Increasing evidence supports the inhibitory effect of berberine in various human cancer cells such as breast5,6, colorectal7,8, lymphoma9, and hepatoma10,11. Berberine suppresses cancer cell growth and proliferation by inducing cell cycle arrest, stimulating caspase-dependent apoptosis, and inhibiting metastasis by suppressing matrix metalloproteinases12. These findings suggest that berberine acts on different tumor cell types through several potential mechanisms. However, the precise molecular mechanisms of berberine-induced apoptosis are not clear.
Figure 1.
Chemical structure of berberine.
Apoptosis is the major modality of programmed cell death, and dysregulation of apoptosis results in pathological conditions including cancer, autoimmune, and neurodegenerative diseases13. Recent studies showed that nuclear factor κB (NF-κB) plays an important role in the regulation of cell inflammation14, oncogenesis15, and apoptosis16. Classical chemotherapeutic agents in combination with NF-κB inhibitors are considered a new treatment strategy for cancer patients17,18.
Moreover, NF-κB levels were found to be higher in HCC tumor tissue compared with normal tissue. It is possible that constitutive activation of NF-κB accompanied by dysregulation of IκBα may play a role in hepatocarcinogenesis19. However, whether physiological function can be adjusted by berberine treatment through NF-κB or its downstream pathway is not clear yet. To explore the mechanism of the inhibition of berberine in the proliferation of the HepG2 cells, we need to assess the expression levels of NF-κB in berberine-treated HepG2 cells and investigate the molecular mechanism of berberine-induced apoptosis in these cells. Findings from our study suggest that berberine induces apoptosis in hepatocellular carcinoma cells primarily in an NF-κB-dependent manner, providing a new mechanism for berberine-induced apoptosis.
MATERIALS AND METHODS
Materials
Berberine and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit anti-NF-κB p65 and rabbit anti-caspase 8 antibodies were purchased from Abcam (Cambridge, MA, USA). Rabbit anti-GAPDH antibody and Alexa Fluor® 488-labeled goat anti-rabbit IgG were purchased from Cell Signaling Technology (Danvers, MA, USA). All other chemicals were of analytical grade unless indicated otherwise. The NF-κB p65 expression vector pCMV4-p65 (plasmid #21966; Addgene) and empty vector (pCMV4-3HA; plasmid #24165; Addgene) were a gift from Dr. Warner Greene.
Cell Culture
The HepG2 cell line was obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. HepG2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (Gibco, USA), 2 mM glutamine, 1% nonessential amino acids (NEAAs), and 1% antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin) in a humidified atmosphere containing 5% CO2 at 37°C.
Cell Transfection
HepG2 cells were seeded in a six-well plate at a density of 2 × 105 cells/well in DMEM containing 10% FBS and 1% penicillin–streptomycin. After a 24-h incubation period, cells were transfected with expression constructs encoding p65 (pCMV4-p65) or empty control vector (pCMV4-3HA) using Lipofectamine LTX (Invitrogen) according to the manufacturer’s instructions, respectively. Cells were incubated for 4 h in transfection solution and were then cultivated with complete culture medium. After a further 48 h of incubation, the cells were harvested for assay analysis.
Cell Viability Assay
Cell viability was determined with cell counting kit-8 (CCK-8; Dojindo Laboratories, Japan) as previously reported20. Briefly, 3 × 103 HepG2 cells were plated on 96-well plates in 100 μl of DMEM and incubated with berberine (0, 10, 50, and 100 μM) for 72 h. In certain groups, they were also treated with NF-κB activator simultaneously. CCK-8 solution (10 μl) was then added to each well, and the cells were incubated for another 1 h at 37°C. Optical density at 450 nm was read as cell viability.
RNA Extraction, Assay for mRNA Levels
The HepG2 cells were treated with 50 μM of berberine for 0, 24, 48, or 72 h, and total RNA was extracted with TRIzol reagent (Invitrogen) and purified using the Qiagen RNeasy Mini Kit (Qiagen) as described previously21. Total RNA samples were reverse transcribed with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher). qRT-PCR was performed on a thermocycler (Applied Biosystems® 7500 Real-Time PCR Systems; Thermo Fisher) for 40 cycles. The GAPDH gene was used as a reference control. Relative mRNA level data were analyzed using the 2−ΔΔCt method12. The primer sequences used for qRT-PCR were as follows: p65, 5′-CCCCACGAGCTTGTAGGAAAG-3′ (forward) and 5′-CCAGGTTCTGGAAACTGTGGAT-3′ (reverse); GAPDH, 5′-CAGCCTCAAGATCATCAGCA-3′ (forward) and 5′-ACAGTCTTCTGGGTGGCAGT-3′ (reverse).
Western Blotting
Cells were washed twice with precold PBS and then lysed with RIPA buffer with protease inhibitor cocktail. After 30 min of bath sonication at 4°C, the mixtures were centrifuged (12,000 × g) for 20 min, and the supernatants were collected for total protein concentration determination using the BCA Assay Kit (Pierce, USA). Whole-cell protein (20 μg) samples were separated by SDS-PAGE (8%–15%) and transferred onto PVDF membranes using a wet transfer system (Bio-Rad). Membranes were blocked with 5% nonfat milk and then incubated with the respective primary antibodies. After washing membranes with TBST, they were incubated with the respective secondary antibodies labeled with HRP. GAPDH antibody was used as a protein-loading control. Membranes were detected by Pierce ECL Western Blotting Kit (Pierce) and autoradiography using X-ray film.
Flow Cytometric Assay
Berberine-induced HepG2 cell apoptosis was quantified by flow cytometry, as described previously22. Briefly, HepG2 cells were treated with berberine (50 μM) for 48 h. Cells were then harvested by trypsinization, washed twice with PBS, and resuspended in binding buffer, followed by incubation with annexin V-conjugated Alexa Fluor 488 and PI for 15 min in the dark at room temperature. The stained cells were analyzed by a FACSCalibur instrument (BD Biosciences, USA). Data were analyzed by FlowJo software.
Hoechst Staining Assay
HepG2 cells were treated with berberine (50 μM) for 48 and then harvested by trypsinization, washed twice with PBS, and resuspended in binding buffer, and then the cells were cultured at 37°C for 24 h and stained with 0.1 μg/ml Hoechst 33342 (Sigma-Aldrich). Fluorescence microscopy (Olympus IX71; Olympus Corporation, Tokyo, Japan) with a filter for Hoechst 33342 (365 nm) was used to detect any change in nuclear morphology.
Immunofluorescence Analysis
HepG2 cells treated with or without berberine were fixed in 4.0% polyformaldehyde for 15 min, washed with PBS, and then treated with permeabilization solution (PBS, 0.25% Triton X-100) for 10 min. After the cells were washed with PBS, they were blocked with 3% bovine serum albumin in PBS for 1 h at room temperature. Cells were stained with anti-caspase 8 antibody or anti-NF-κB antibody overnight at 4°C, followed by incubation with an Alexa Fluor® 488-labeled goat anti-rabbit IgG (Cell Signaling Technology) antibody at room temperature for 1 h. Cells were then incubated with 2 μg/ml of DAPI (Invitrogen) in PBS for 5 min. Coverslips were mounted, and images were acquired with fluorescence microscopy (Olympus IX71). Alexa Fluor1 pt 488 and DAPI images were taken from the same field.
Statistical Analysis
Data used for statistical analysis are shown as mean ± SD of three independent experiments. The results from treated and untreated control cells were analyzed using Student’s t-test or one-way ANOVA by GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA) program, and a value of p < 0.05 was considered as statistically significant.
RESULTS
Effects of Berberine on HepG2 Proliferation and Apoptosis
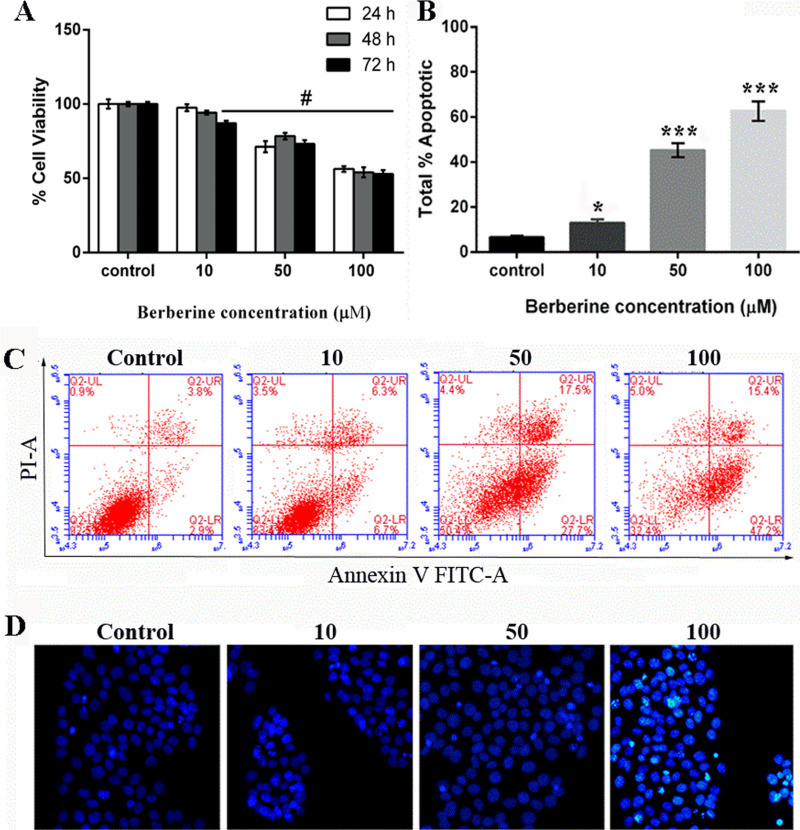
The growth of HepG2 cells incubated with berberine at concentrations of 10, 50, and 100 μg/ml was monitored within 24, 48, and 72 h of cultivation. The survival columns show that berberine had a significant reduction effect on the viability of HepG2 cells. The inhibition rates of 50 and 100 μM of berberine were 21.0% to 26.9% (p < 0.01) after 24 h, 21.5% to 45.9% after 48 h, and 26.8% to 47.0% after 72 h of treatment, whereas HepG2 cells treated with 10 μM of berberine induced significant inhibition of cell survival only after 48 and 72 h (Fig. 2A). After cells were treated for 48 h with 10, 50, or 100 μM of berberine, the percentage of cell apoptosis increased in a dose-dependent manner (Fig. 2B and C). Furthermore, the Hoechst staining assay also indicated that berberine significantly induced HepG2 apoptosis (Fig. 2D).
Figure 2.
Berberine induced the inhibition of human hepatocellular carcinoma HepG2 cells and promoted apoptosis. Cell viability (A) and apoptosis (B, C) were analyzed in the HepG2 cell line by treatment with varying concentrations of berberine (0, 10, 50, and 100 μM). (D) Hoechst staining assay. Data are presented as means ± SD of the percentage of untreated controls (0.1% DMSO). #p < 0.01 versus control; *p < 0.05 versus control; ***p < 0.001 versus control.
Downregulation of p65 by Berberine In Vitro
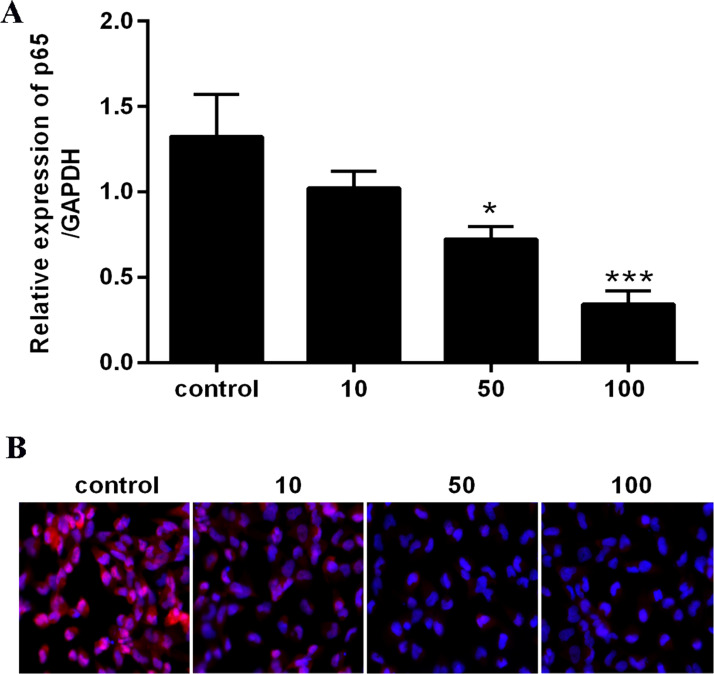
To determine the relationship between NF-κB signaling and the apoptotic effect of berberine in HepG2 cells, we investigated the concentration of cellular NF-κB p65 in cells treated with 50 μM of berberine. Data from qRT-PCR showed that the NF-κB p65 level was significantly lower than that in the control group in a dose-dependent manner (Fig. 3A). Immunofluorescence staining demonstrated that NF-κB p65 was suppressed by berberine in HepG2 cells (Fig. 3B). Subsequently, a 50-μM concentration of berberine was selected for all further assays to explore the mechanism in cell viability of HepG2 cells.
Figure 3.
Effects of berberine on expression of NF-κB p65 proteins and mRNA levels in HepG2 cells. (A) NF-κB p65 mRNA expression levels. (B) NF-κB p65 was examined by immunofluorescence staining. *p < 0.05 versus control; ***p < 0.001 versus control.
Overexpression of NF-κB p65 Reduced the Function of Berberine on HepG2 Cells
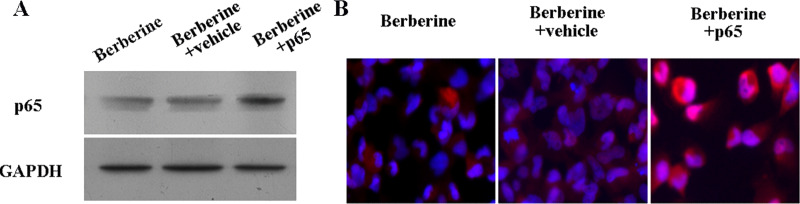
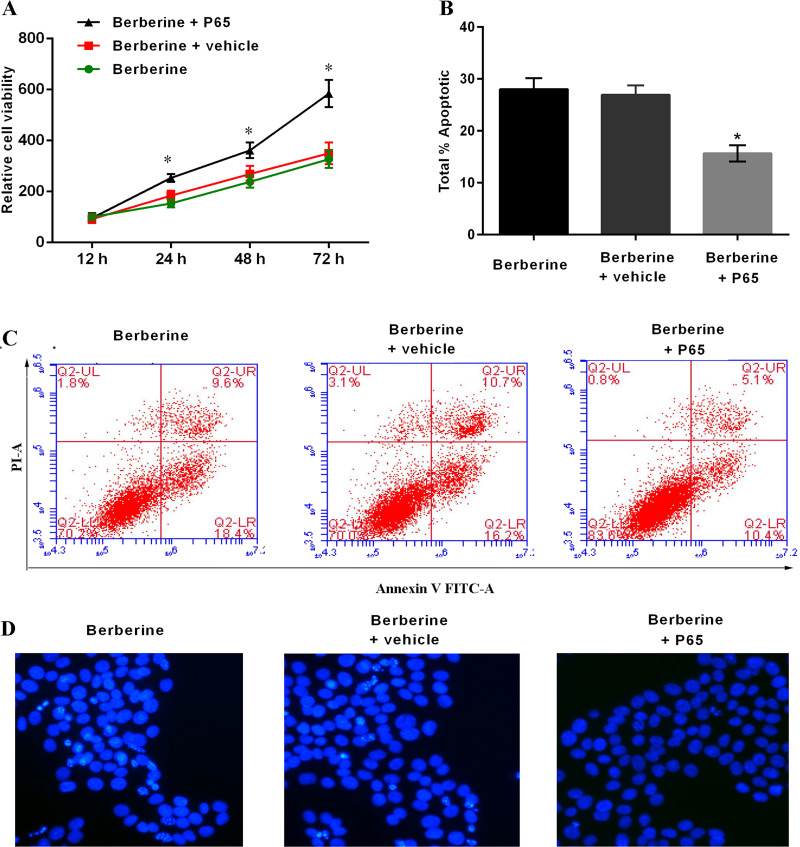
Berberine has been shown to inhibit NF-κB levels in HepG2 cells. To determine whether the effects of berberine on cell proliferation were reversible with activated NF-κB, HepG2 cells were transfected with the pCMV4-p65 plasmid for 48 h before treatment with berberine. When cells were treated with 50 μM of berberine for 48 h, the expression of NF-κB p65 was increased in p65-overexpressed cells (Fig. 4), and the proliferation was significantly rescued in p65-overexpressed cells (Fig. 5A). Furthermore, in these cells, the berberine-induced apoptosis was reduced significantly: 28.0% (berberine), 26.9% (berberine + vehicle), and 15.6% (berberine + pCMV4-p65) at 48 h (Fig. 5B and C). The Hoechst staining assay also indicated that the berberine-induced apoptosis was reduced (Fig. 5D).
Figure 4.
HepG2 cells were transfected with p65, and the protein levels of p65 were determined. (A) Western blotting assay. (B) Immunofluorescence staining.
Figure 5.
Moderation of berberine-induced HepG2 cell proliferation and apoptosis. (A) CCK-8 assay. (B, C) Apoptosis of HepG2 cells. (D) Hoechst staining assay. Data are presented as means ± SD of three independent experiments. *p < 0.05 versus control.
Taken together, these results indicate that berberine-induced apoptosis in HepG2 cells is mediated through the NF-κB p65 Pathway.
DISCUSSION
Effective and well-tolerated compounds are desired in order to treat HCC. Natural products are excellent sources of novel compounds for the treatment of various diseases, with nonsynthetic antitumor agents comprising approximately 60% of the approved anticancer drug candidates developed in the last 30 years23. The low cytotoxicity of natural products has an advantage over chemotherapeutic drugs. Berberine, a natural alkaloid, is an attractive candidate for its antitumor effect on different cancer cell lines12. In this study, we examined the effect of berberine on HepG2 cancer cell viability and apoptosis.
Apoptosis is an essential process for the purging of useless cells in various biological systems and is a key mechanism of chemotherapy24. Recently, inhibition of NF-κB has been shown to enhance the anticancer effect of HepG2 cells25. This suggested that the NF-κB signaling cascade may be involved in the anticancer activity of berberine.
The exact molecular mechanisms of the action of apoptosis, and understanding the subsequent interaction of berberine on apoptosis signaling pathways, are still not known. A variety of factors may account for berberine-induced apoptosis, including receptors, cytokines, and their downstream signaling cascades. Apoptosis induced by different stimulation, such as death ligands, drugs, or ionizing irradiation, leads to the activation of caspase 11. It is well established that inhibition of NF-κB induces apoptosis in hepatocellular carcinoma cells2,26. This indicates that NF-κB may be an important molecular target for chemopreventive compounds. To delineate the effect of NF-κB on HepG2 cells, further studies were designed to determine the effect of berberine on NF-κB and caspase expression in HepG2 cells. We treated HepG2 cells with berberine and a p65 overexpression plasmid (pCMV4-p65) and observed that the expression of NF-κB was increased. This present study demonstrated that p65 overexpression rescued HepG2 cell proliferation from the effects of berberine (50 μM) treatment and prevented HepG2 cells from undergoing apoptosis.
In conclusion, we showed that cytotoxicity of berberine is due to the induction of apoptosis through the suppression of NF-κB expression. Furthermore, our data support the hypothesis that NF-κB may be involved in the antitumor effect of berberine. These results suggest that berberine appears to have anticancer potential in hepatocellular carcinoma.
ACKNOWLEDGMENT
The authors alone are responsible for the content and writing of this article.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Yim HJ, Suh SJ, Um SH. Current management of hepatocellular carcinoma: An Eastern perspective. World J Gastroenterol. 2015;21(13):3826–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim SC, Choi JE, Kang HS, Han SI. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int J Cancer 2010;126(7):1582–95. [DOI] [PubMed] [Google Scholar]
- 3. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296(4):E955–64. [DOI] [PubMed] [Google Scholar]
- 4. Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015;243(2):449–61. [DOI] [PubMed] [Google Scholar]
- 5. Patil JB, Kim J, Jayaprakasha GK. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur J Pharmacol. 2010;645(1–3):70–8. [DOI] [PubMed] [Google Scholar]
- 6. Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park SY, Shin I, Han W, Noh DY. The alkaloid berberine inhibits the growth of anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine 2010;17(17):436–40. [DOI] [PubMed] [Google Scholar]
- 7. Guaman Ortiz LM, Tillhon M. Multiple effects of berberine derivatives on colon cancer cells. Biomed Res Int. 2014;2014(2):924585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piyanuch R, Sukhthankar M, Wandee G, Baek SJ. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007;258(2):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jantova S, Cipak L, Letasiova S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol In Vitro 2007;21(1):25–31. [DOI] [PubMed] [Google Scholar]
- 10. Shukla S, Sharma A, Pandey VK, Raisuddin S, Kakkar P. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharm. 2016;291:70–83. [DOI] [PubMed] [Google Scholar]
- 11. Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80(2):62–73. [DOI] [PubMed] [Google Scholar]
- 12. Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules 2014;19(8):12349–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1–2):41–58. [DOI] [PubMed] [Google Scholar]
- 14. Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–48. [DOI] [PubMed] [Google Scholar]
- 15. Arsura M, Cavin LG. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005;229(2):157–69. [DOI] [PubMed] [Google Scholar]
- 16. Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006;441(7092):431–6. [DOI] [PubMed] [Google Scholar]
- 17. Braun T, Carvalho G, Fabre C, Grosjean J, Fenaux P, Kroemer G. Targeting NF-kappaB in hematologic malignancies. Cell Death Differ. 2006;13(5):748–758. [DOI] [PubMed] [Google Scholar]
- 18. Orlowski RZ, Baldwin AS. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8(8):385–9. [DOI] [PubMed] [Google Scholar]
- 19. Tai DI, Tai SL, Chang YH, Huang SN, Chen TC, Chang KS, Liaw YF. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer 2000;89(11):2274–81. [PubMed] [Google Scholar]
- 20. Wu Y, Ma Y, Xu Z, Wang D, Zhao B, Pan H, Wang J, Xu D, Zhao X, Pan S, Liu L, Dai W, Jiang H. Sodium orthovanadate inhibits growth of human hepatocellular carcinoma cells in vitro and in an orthotopic model in vivo. Cancer Lett. 2014;351(1):108–16. [DOI] [PubMed] [Google Scholar]
- 21. Kollipara PS, Jeong HS, Han SB, Hong JT. (E)-2,4-bis(p-hydroxyphenyl)-2-butenal has an antiproliferative effect on NSCLC cells induced by p38MAPK-mediated suppression of NF-kappaB and up-regulation of TNFRSF10B (DR5). Br J Pharmacol. 2013;168(6):1471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan K, Zhang C, Feng J, Hou L, Yan L, Zhou Z, Liu Z, Liu C, Fan Y, Zheng B, Xu Z. Induction of G1 cell cycle arrest and apoptosis by berberine in bladder cancer cells. Eur J Pharmacol. 2011;661(1–3):1–7. [DOI] [PubMed] [Google Scholar]
- 23. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kobayashi Y, Yamashita Y, Fujii N, Takaboshi K, Kawakami T, Kawamura M, Mizukami T, Nakano H. Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med. 1995;61(5):414–8. [DOI] [PubMed] [Google Scholar]
- 25. Tiwari P, Kumar A, Balakrishnan S, Kushwaha HS, Mishra KP. Silibinin-induced apoptosis in MCF7 and T47D human breast carcinoma cells involve caspase-8 activation and mitochondrial pathway. Cancer Invest. 2011;29(1):12–20. [DOI] [PubMed] [Google Scholar]
- 26. Yin Y, Chen W, Tang C, Ding H, Jang J, Weng M, Cai Y, Zou G. NF-kappaB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G(2)/M cell cycle arrest. Food Chem Toxicol. 2011;49(12):3046–54. [DOI] [PubMed] [Google Scholar]