Abstract
Purpose
Data on non-steroidal anti-inflammatory drug (NSAID) hypersensitivity in Southeast Asia are scarce. Increased urinary leukotriene E4 (uLTE4) levels have been suggested as a biomarker of NSAID-exacerbated respiratory disease (NERD). This study investigated clinical patterns of NSAID sensitivity in Thailand and the diagnostic roles of uLTE4 measurement in various phenotypes.
Methods
The clinical phenotypes in 92 Thai adults with cross-reactive NSAID hypersensitivity were characterized based on the clinical history and drug provocation. The uLTE4 levels were measured at baseline, after aspirin provocation and after desensitization.
Results
More than half of the patients (56.5%) presented with cutaneous symptoms (NSAID-exacerbated cutaneous disease), while one-third (33.7%) developed symptoms in at least 2 systems (NSAID-induced blended reactions; NIBR). Fifty-two patients underwent drug provocation and 59.6% of them yielded positive results. After drug provocation, a significant number of patients with confirmed NSAID cross-reactivity experienced clinical symptoms in more than one organ system. The uLTE4 levels at baseline were comparable between the NSAID-tolerant and NSAID-sensitive groups, but were substantially increased after aspirin provocation predominantly in NERD (983.4 pg/mg creatinine) and NIBR (501.0 pg/mg creatinine) compared to NSAID-tolerant subjects (122.1 pg/mg creatinine, P < 0.01 and 0.05, respectively). The uLTE4 levels were elevated after aspirin desensitization, although nasal polyposis and asthma were under control in 3 NERD and 3 NIBR subjects.
Conclusions
NIBR is not uncommon among NSAID-sensitive patients in Thailand. The diagnostic value of basal uLTE4 levels was limited, but increased uLTE4 levels upon aspirin provocation suggest NSAID cross-reactivity with respiratory components. This study indicates that aspirin desensitization, if necessary, might be effective in both NERD and NIBR.
Trial Registration
ClinicalTrials.gov Identifier: NCT03849625
Keywords: Leukotriene E4, aspirin, desensitization, anti-inflammatory agents, non-steroidal, biomarkers, phenotype, drug hypersensitivity
INTRODUCTION
Hypersensitivity reactions to aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most common drug allergic reactions in clinical practice. They are generally categorized into 5 major phenotypes: NSAID-exacerbated respiratory disease (NERD), NSAID-exacerbated cutaneous (urticaria/angioedema) disease (NECD) in patients with underlying chronic urticaria, NSAID-induced urticaria/angioedema (NIUA) in patients without underlying chronic urticaria, single NSAID-induced urticaria/angioedema or anaphylaxis, and single NSAID-induced delayed hypersensitivity reactions.1 The first 3 phenotypes are non-immunologically mediated, and patients can develop cross-reactive hypersensitivity to chemically unrelated NSAIDs, while the latter 2 are selective NSAID sensitivities associated with drug-specific immunoglobulin E (IgE) and T-cell response, respectively.
Genetic and epigenetic variations play significant roles in the development of NERD.2 The proposed underlying mechanism is that the disturbance in arachidonic acid synthesis pathways leads to overproduction of cysteinyl leukotrienes (cysLT) and persistent airway inflammation.3 Mast cells are believed to be the major source of cysLT, but platelet-adherent leukocytes may be responsible for cysLT overproduction in NERD as well.4 The consumption of drugs inhibiting the cyclooxygenase-1 (COX-1) enzyme, such as aspirin and conventional NSAIDs, aggravates respiratory symptoms by enhancing cysLT production in NSAID-sensitive subjects, while the administration of specific COX-2 inhibitors, such as celecoxib, is generally safe.5,6 Besides aspirin/NSAID avoidance, aspirin desensitization has also been proven to be helpful for long term management in NERD subjects. Beneficial effects of aspirin desensitization in preventing nasal polyp recurrence and control symptoms of chronic rhinosinusitis and asthma have been well documented.7 Rapid aspirin desensitization has shown to be effective for the treatment of coronary artery disease in patients with a history of aspirin/NSAID-induced urticaria/angioedema, but it is not indicated for the treatment of chronic urticaria.8
Worldwide epidemiological data on clinical characteristics of NSAID hypersensitivity are limited. Most studies on NERD were performed in the United States and northern European countries.9,10,11,12 However, the predominant manifestation in Latin Americans and southern Europeans is the cutaneous phenotype (NIUA, NECD, and NSAID-induced isolated periorbital angioedema).13,14 Studies in Asian populations have suggested that the prevalence of the NERD phenotype is not common in this region of the world. A study performed in China indicated that the prevalence of NSAID hypersensitivity in Chinese patients with chronic rhinosinusitis is very low (0.57%) compared to their European counterparts.15 It was also noted that NSAID-induced blended reactions (NIBR) are common in young Asian, atopic children in Singapore.16 Reports from Singapore and Thailand indicated that aspirin/NSAID-induced angioedema/urticaria is probably the prominent phenotype in Southeast Asia, with acetaminophen hypersensitivity being frequent.17,18
In clinical practice, the current classification of cross-reactive NSAID hypersensitivity into 3 distinct phenotypes has some limitations. Clinical characteristics in NSAID-sensitive patients may not be well defined, and a combination of respiratory and cutaneous or other symptoms, such as gastrointestinal symptoms (mixed or blended reactions), is frequently observed.19 A previous report stated that aspirin nasal provocation is potentially useful for diagnosing patients with the blended reaction as well.20 However, the role of aspirin desensitization in other phenotypes of NSAID hypersensitivity, besides NERD, for long-term management of inflammatory airway diseases has not yet been established. Whether aspirin desensitization would be effective in controlling asthma and the recurrence of nasal polyposis after sinus surgery in NIBR is currently unknown.
At present, cross-reactive types of NSAID hypersensitivity can only be diagnosed by careful history taking and controlled-provocation testing, since there is no reliable in vitro method to confirm the diagnosis.21 There is evidence that basal urinary levels of leukotriene E4 (uLTE4), a stable product of the cysLT synthesis pathway, are elevated in NERD patients and could be a potential biomarker for differentiating between NSAID-sensitive and NSAID-tolerant asthmatics. However, these data are still controversial.22,23,24 There have also been preliminary studies showing that uLTE4 levels might be increased in NECD.25,26 Whether the measurement of basal uLTE4 levels could be a diagnostic marker for any phenotype of NSAID hypersensitivity is yet to be explored.
The purpose of this study was to analyze the clinical characteristics of patients diagnosed with NSAID-induced immediate reactions in Thailand and to explore whether the measurement of uLTE4 levels would be helpful in identifying NSAID-sensitive subjects or in differentiating among different phenotypes of cross-reactive NSAID hypersensitivities.
MATERIALS AND METHODS
Patient recruitment
Adult patients (18 years of age and older) with a suggestive history of NSAID-induced hypersensitivity reaction visiting the allergy clinic at King Chulalongkorn University Hospital between June 2014 and July 2018 were recruited into this study. Those with a history compatible with drug-induced non-immediate reactions (maculopapular rash, fixed drug eruption, acute generalized exanthematous pustulosis, drug rash with eosinophilia and systemic symptoms, Stevens-Johnson syndrome/toxic epidermal necrolysis) or NSAID-related side effects were excluded. The status of NSAID-induced immediate hypersensitivity was diagnosed in patients with a well-documented drug allergy history or in patients with a suggestive history of NSAID sensitivity who yielded a positive drug provocation test as follows.
Clinical diagnosis of NSAID hypersensitivity
The diagnosis of cross-reactive NSAID hypersensitivity was clinically made in patients with a well-documented history of multiple episodes of respiratory, cutaneous, and/or gastrointestinal reactions within 2 hours after exposure to at least 2 different NSAID classes.1,21,27,28 Patient demographic data were collected, including the underlying diseases, atopic family history, and details on drug allergy history as well as the presenting symptoms and types of the culprit NSAIDs.
Aspirin provocation test
Oral aspirin provocation was performed in patients with a suggestive medical history or in those who had a history of an immediate reaction to a single NSAID to confirm the diagnosis of cross-reactive NSAID hypersensitivity according to the EAACI/GA2LEN guidelines, with some modifications.29,30,31 Four increasing doses of aspirin (40.5, 81, 150, and 300 mg) were administered at 90-minute intervals until a positive reaction occurred. Clinical symptoms, fractional exhaled nitric oxide (FeNO), and the forced expiratory volume in 1 second (FEV1) were monitored to evaluate the reaction. A positive provocation test was defined if any of the following reactions developed: lower respiratory/bronchial reaction (a 15% decrease in FEV1 plus naso-ocular reaction or a 20% decrease in FEV1 alone), upper respiratory/naso-ocular reaction (nasal congestion and rhinorrhea; conjunctival injection), cutaneous reactions (urticaria/angioedema, periorbital edema), and/or other reactions (gastrointestinal symptoms such as nausea/vomiting, stomach cramps, and diarrhea). The test was considered negative if a patient tolerated the final dose (300 mg of aspirin) without any significant symptoms mentioned above.
Oral provocation test (OPT) with other NSAIDs, acetaminophen, or celecoxib
Patients with a history of an immediate reaction to only aspirin were challenged with escalating doses of ibuprofen to confirm the diagnosis of cross-reactive NSAID sensitivity. Patients with a history of an immediate reaction to a single NSAID were provoked with the suspected drug to verify the status of single NSAID hypersensitivity after a negative aspirin provocation test. Escalating doses of ibuprofen, diclofenac, and acetaminophen were administered, if applicable, as follows: ibuprofen (50, 100, and 200 mg), diclofenac (6.25, 12.5, and 25 mg), and acetaminophen (125, 250, and 500 mg). Celecoxib was also provoked in 3 doses (50, 100, and 200 mg) in patients with confirmed hypersensitivity to multiple NSAIDs who were willing to identify a safe alternative drug. The provocation test for each drug was performed on separate days at least 7 days apart. Each dose of drug provocation was provided at 90-minute intervals until the final dose was reached.
Classification of NSAID hypersensitivity according to drug provocation test results
Patients with cross-reactive NSAID phenotypes were classified according to the results of the drug provocation test as 1) NECD/NIUA if they developed cutaneous symptoms alone; 2) NERD if they developed respiratory symptoms alone; and 3) NIBR if they developed a combination of symptoms involving more than one organ system (respiratory, cutaneous, and/or gastrointestinal symptoms). Single NSAID hypersensitivity was diagnosed in patients with a positive provocation test to the suspected NSAID, but a negative aspirin provocation test. Patients who had negative provocations to both aspirin and the suspected culprit drug were labeled as NSAID-tolerant subjects. NECD/NIUA were purposely categorized together in our study to compare the differences and similarities between patients diagnosed with NSAID-induced cutaneous reactions and -respiratory reactions. Moreover, NECD and NIUA share similar background characteristics from a clinical point of view and some patients diagnosed with NIUA eventually evolve to NECD over time.32
Skin prick tests with common aeroallergens in Thailand (mixed dust mites, mixed cockroaches, mixed mold, cat dander, dog hair, and southern grass mix; ALK-Abello, Hørsholm, Denmark) were also examined in tested patients to identify atopic status. Wheal size ≥ 3 mm was considered a positive test.
Measurement of FeNO and the FEV1 in patients who underwent drug provocation test
FeNO was measured in all participants at baseline and after drug provocation by using a portable electrochemical analyzer (NObreath®; Bedfont Scientific Ltd, Maidstone, UK) according to the ATS/ERS recommendations.33 Measurements of FEV1 were also performed using a Vitalograph spirometer (Vitalograph®, Buckingham, UK). The best of 3 repeated attempts was recorded.
The uLTE4 measurement
The uLTE4 levels were measured by a leukotriene E4 ELISA kit (Cayman Chemical, Ann Arbor, MI, USA) at baseline and 90 minutes after the final dose of drug provocation, as well as at the follow-up visits after aspirin desensitization and reported after adjustment to urine creatinine levels.
Aspirin desensitization
Outpatient aspirin desensitization was performed in NSAID-sensitive patients who were referred from otolaryngologists to prevent the recurrence of nasal polyposis. The procedure was carried out according to the intranasal ketorolac and modified aspirin challenge 2-day protocol.34 Aspirin desensitization was completed after patients were able to tolerate a 325-mg dose of aspirin by the end of day 2 and experience no symptoms, changes in nasal flow rates, or decrease in FEV1 values. The prescribed maintenance dose of aspirin was 325 mg twice a day.
Statistical analysis
Patient characteristics are reported as means ± standard deviation for quantitative analysis, and median and interquartile range (IQR) are used to describe non-parametric data. The Mann-Whitney U test and Kruskal-Wallis test with Dunn's multiple comparisons were used to analyze the differences between 2 groups and more than 2 groups, respectively. Statistical analyses were performed using GraphPad Prism 8.3 software (GraphPad Software Inc., San Diego, CA, USA). P values < 0.05 were considered statistically significant.
Ethical considerations
Patients enrolled in this study were those who recruited in the study entitled “Characteristics of Patients Diagnosed With NSAID Sensitivity in Thailand” registered at ClinicalTrials.gov (NCT03849625). The study was approved by the Ethics and Research Committee of the Faculty of Medicine, Chulalongkorn University, Approval Number: COA No. 659/2012 and informed consent was obtained from all participants.
RESULTS
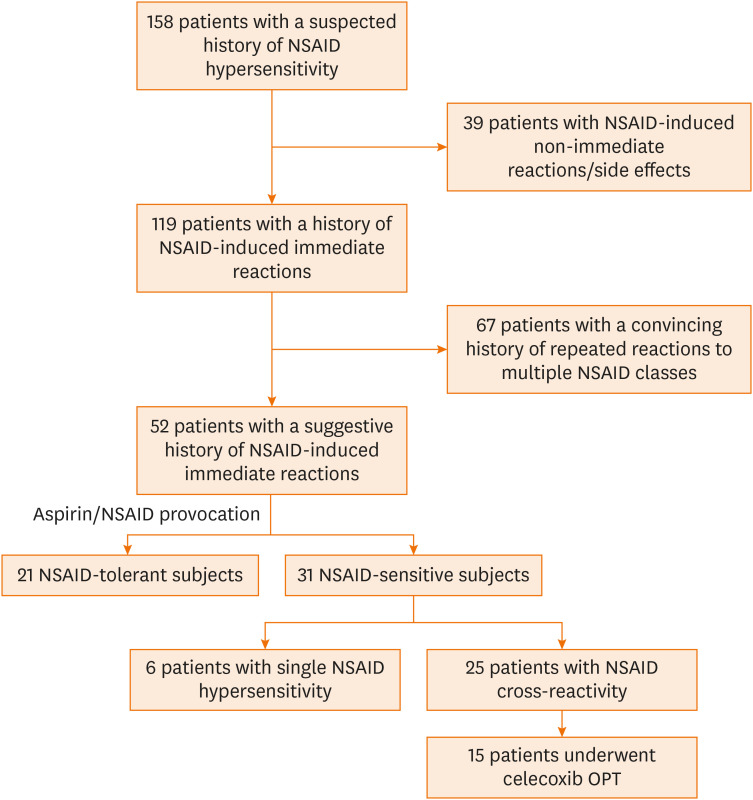
A total of 158 adult patients with a suspected history of NSAID hypersensitivity between 2015 and 2018 at King Chulalongkorn Memorial Hospital were initially recruited into this study as shown in Fig. 1. Thirty-five patients with a history compatible with a non-immediate hypersensitivity reaction and 4 subjects with clinical symptoms compatible with NSAID-related side effects were later excluded.
Fig. 1. Schematic diagram of patient selection for this study.
A total of 158 patients with a suspected history of NSAID hypersensitivity were initially recruited into this study. Cross-reactive NSAID hypersensitivity was clinically diagnosed in 67 patients with a well-documented drug allergy history and verified by a positive drug provocation test in 25 patients with a suggestive history of NSAID reactions.
NSAID, non-steroidal anti-inflammatory drug.
Cross-reactive NSAID hypersensitivity was clinically diagnosed in 67 patients with a convincing drug allergy history as defined in the methods section. The remaining 52 patients with a suggestive history of NSAID hypersensitivity underwent an OPT to confirm NSAID cross-reactivity status. Fifteen out of 25 patients with confirmed NSAID cross-reactivity were subsequently provoked with celecoxib to find a safe alternative NSAID. Single NSAID/acetaminophen hypersensitivity was verified in 6 patients who tolerated aspirin provocation but developed an allergic reaction upon re-challenge with acetaminophen or diclofenac.
Demographic data of adult patients with cross-reactive NSAID hypersensitivity
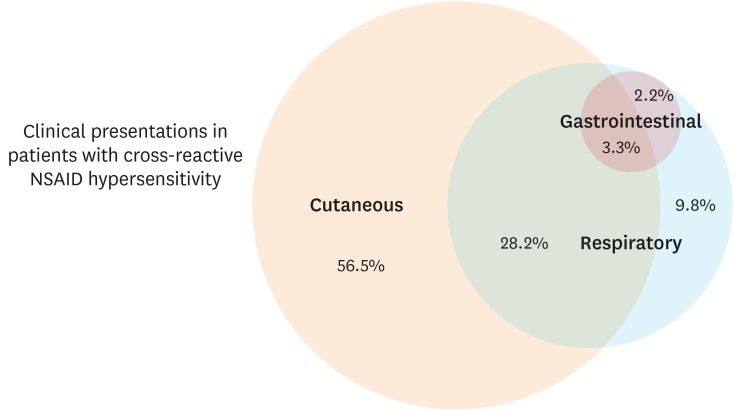
The clinical characteristics of adult patients with cross-reactive NSAID hypersensitivity are shown in Table 1 (n = 92). Most patients were females (77.2%) with an average age of 45.7 ± 13.4 years. The average age of onset was 32.2 ± 13.3 years and about half of them had atopic family history. The majority of them (56.5%) reported only cutaneous symptoms such as facial angioedema and/or acute urticarial rash, after NSAID exposure, while 9.8% of them developed only respiratory symptoms. Interestingly, about 33.7% of the patients experienced a combination of symptoms in multiple organ systems as shown in Fig. 2. The groups of NSAIDs frequently responsible for hypersensitivity reactions were propionic acid derivatives and acetic acid derivatives. Although inflammatory airway diseases (chronic sinusitis, nasal polyposis, and asthma) were significantly more common in patients who experienced only respiratory reactions after exposure to an NSAID compared to the other phenotypes, a significant portion of the patients reporting blended reactions had underlying airway diseases and chronic urticaria as well. Acetaminophen intolerance was also reported in about one-third (35.9%) of NSAID-sensitive subjects.
Table 1. Clinical characteristics among different phenotypes of patients with cross-reactive NSAID hypersensitivity (n = 92).
| Phenotypes | NECD/NIUA (n = 52) | NERD (n = 9) | NIBR (n = 31) | Total (n = 92) | |
|---|---|---|---|---|---|
| Gender (female/male) | 37/15 | 6/3 | 28/3 | 71/21 | |
| Age (yr) | 43.6 ± 12.3 | 47.3 ± 11.9 | 48.7 ± 15.3 | 45.7 ± 13.4 | |
| Age of onset (yr) | 31.3 ± 13.8 | 34.6 ± 11.0 | 33.1 ± 13.3 | 32.2 ± 13.3 | |
| Underlying disease (%) | |||||
| Chronic urticaria | 13.5 | 11.1 | 29.0 | 18.5 | |
| Chronic sinusitis* | 13.5 | 55.6 | 32.3 | 23.9 | |
| Nasal polyposis* | 7.7 | 66.7 | 16.1 | 16.3 | |
| Asthma* | 17.3 | 88.9 | 35.5 | 30.4 | |
| Atopic family history (%) | 42.3 | 55.6 | 61.3 | 50.0 | |
| Drug exposure time to symptom onset (min) | 58.8 ± 31.9 | 48.3 ± 34.3 | 47.6 ± 29.9 | 54.0 ± 31.6 | |
| Presenting symptoms (%)* | |||||
| Facial/periorbital angioedema | 71.2 | 0.0 | 74.2 | 65.2 | |
| Acute urticaria | 73.1 | 0.0 | 61.3 | 62.0 | |
| Naso-ocular reaction | 0.0 | 33.3 | 61.3 | 23.9 | |
| Acute asthma | 0.0 | 88.9 | 67.7 | 31.5 | |
| Gastrointestinal symptoms | 0.0 | 0.0 | 16.1 | 5.4 | |
| Implicated NSAIDs (%)† | |||||
| Salicylic acids | 23.1 | 55.6 | 35.5 | 30.4 | |
| Propionic acids | 44.2 | 22.2 | 54.8 | 45.7 | |
| Acetic acids | 23.1 | 22.2 | 48.4 | 31.5 | |
| Enolic acids | 7.7 | 0.0 | 6.5 | 6.5 | |
| Anthranilic/fenamic acids | 28.8 | 11.1 | 29.0 | 27.2 | |
| Acetaminophen intolerance (%)§ | 28.8 | 22.2 | 51.6 | 35.9 | |
Values are presented as number (%) or mean ± standard deviation. Each patient could present with multiple symptoms and from more than one implicated drug.
NSAID, non-steroidal anti-inflammatory drug; NECD, non-steroidal anti-inflammatory drug-exacerbated cutaneous (urticaria/angioedema) disease in patients with underlying chronic urticaria; NIUA, non-steroidal anti-inflammatory drug-induced urticaria/angioedema in patients without underlying chronic urticaria; NERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; NIBR, non-steroidal anti-inflammatory drug-induced blended reactions.
*P < 0.05 among different phenotypes. †Examples of commonly implicated drugs in this study based on NSAID classification: 1) Salicylic acids: acetylsalicylic acid (aspirin), salicylic acid, diflunisal, salsalate; 2) Propionic acids: ibuprofen, naproxen, ketoprofen, fenoprofen, flurbiprofen; 3) Acetic acids: indomethacin, ketorolac, diclofenac, sulindac; 4) Enolic acids: piroxicam, meloxicam, tenoxicam; 5) Anthranilic/fenamic acids: mefenamic acid, flufenamic acid. §Acetaminophen intolerance: cannot tolerate acetaminophen higher than 500 mg.
Fig. 2. Clinical presentations in patients with cross-reactive NSAID hypersensitivity.
A proportional Venn diagram shows that about half of patients with cross-reactive NSAID hypersensitivity in Thailand reported only cutaneous symptoms after NSAID exposure, while one-third of them experienced NSAID-induced blended reactions.
NSAID, non-steroidal anti-inflammatory drug.
Clinical respiratory parameters and uLTE4 levels in different phenotypes of patients with cross-reactive NSAID hypersensitivity confirmed by positive OPT
NSAID hypersensitivity was confirmed in 59.6% (31/52) of the tested patients with a history of NSAID-induced immediate reactions. The majority of patients with cross-reactive NSAID hypersensitivity (17/25) according to OPT results were atopic individuals. The common phenotypes of patients with confirmed cross-reactive NSAID sensitivity were NECD/NIUA, NIBR, and NERD, respectively, as shown in Supplementary Table S1.
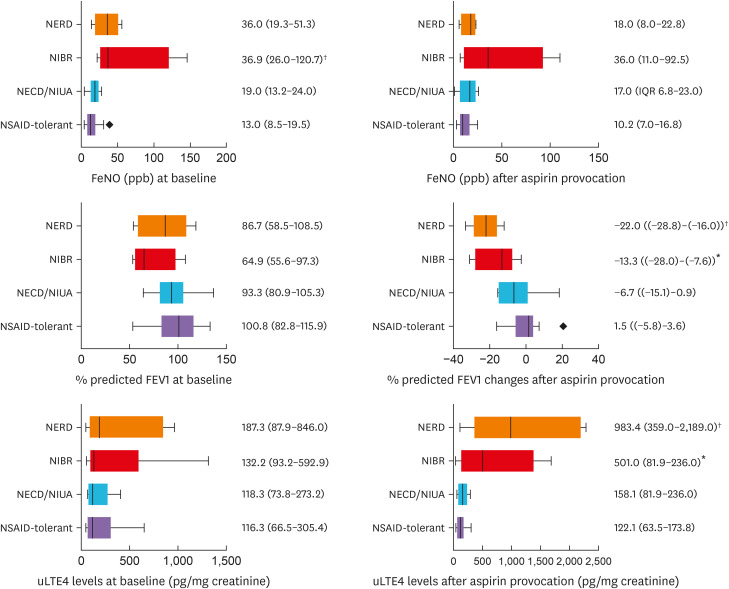
The average FeNO, %predicted FEV1, and uLTE4 levels were measured at baseline and after provocation with aspirin/NSAID in 25 NSAID cross-reactive and 21 NSAID-tolerant subjects as shown in Table 2. At baseline, patients with confirmed NSAID cross-reactivity (NSAID-sensitive group) had higher FeNO (P < 0.01) and slightly less %predicted FEV1 compared to the NSAID-tolerant subjects, while basal uLTE4 levels between NSAID-sensitive and NSAID-tolerant groups were comparable. After provocation, %predicted FEV1 was substantially reduced in NSAID-sensitive subjects, whereas uLTE4 levels were significantly elevated compared to the NSAID-tolerant group (P < 0.01). Celecoxib provocation was well tolerated in all NSAID-sensitive subjects. No statistical differences in the average FeNO, %predicted FEV1, or uLTE4 levels in 15 NSAID-sensitive patients (7 NECD/NIUA, 4 NIBR, 4 NERD) after celecoxib provocation were observed compared to those in NSAID-tolerant subjects.
Table 2. Respiratory parameters and uLTE4 levels in NSAID-sensitive subjects upon provocation with aspirin or celecoxib compared to NSAID-tolerant subjects.
| Characteristics | Baseline | After aspirin (OPT) | P value* | |
|---|---|---|---|---|
| NSAID tolerant (n = 21) | ||||
| FeNO (ppb) | 13.0 (8.5–19.5) | 10.2 (7.0–16.8) | 0.03 | |
| %predicted FEV1 | 100.8 (82.8–115.9) | 99.0 (75.2–118.2) | 0.90 | |
| uLTE4 (pg/mg creatinine) | 116.3 (66.5–305.4) | 122.1 (63.5–173.8) | 0.17 | |
| NSAID sensitive (n = 25) | ||||
| FeNO (ppb) | 27.0 (17.8–40.9)† | 18.0 (10.5–31.0) | <0.01 | |
| %predicted FEV1 | 86.7 (63.3–104.9) | 74.6 (52.4–89.8)† | <0.01 | |
| uLTE4 (pg/mg creatinine) | 118.3 (93.2–407.2) | 204.9 (90.6–817.8)† | <0.01 | |
uLTE4, urinary leukotriene E4; NSAID, non-steroidal anti-inflammatory drug; OPT, oral provocation test; ppb, parts per billion; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second.
*P values before vs. after provocation test, Wilcoxon signed-rank test; †P < 0.01 compared to the NSAID-tolerant subjects.
According to the subgroup analysis, 10 NECD/NIUA, 9 NIBR, and 6 NERD were categorized as shown in Fig. 3. At baseline, %predicted FEV1 was not significantly different among different types of cross-reactive NSAID hypersensitivity, although those in patients with confirmed NIBR were lowest (64.9%, IQR 56.9–97.3). The significant differences in basal FeNO levels (P < 0.01) among the 3 different phenotypes were observed. The basal FeNO levels were significantly higher in NIBR (36.9%, IQR 27.0–95.4) than in NSAID-tolerant subjects (P < 0.01). After aspirin OPT, the reduction of %FEV1 was considerably higher in patients with confirmed NERD (22.0%, IQR 16.0-28.8), followed by NIBR (13.3%, IQR 7.6–28.0), compared to those in NSAID-tolerant groups (P < 0.01). Basal uLTE4 levels were not significantly different among the 3 phenotypes. Interestingly, uLTE4 levels after aspirin provocation were significantly higher in NERD and NIBR patients than in the NECD/NIUA and NSAID-tolerant groups (P < 0.05 and < 0.01, respectively).
Fig. 3. Respiratory parameters and uLTE4 levels in different phenotypes of NSAID-sensitive subjects compared to NSAID-tolerant subjects.
Baseline FeNO in NIBR and NERD were higher than those in the NSAID-tolerant group. After aspirin provocation, % predicted FEV1 in NERD and NIBR were significantly reduced from the baseline while uLTE4 levels were much higher than those in NSAID-tolerant subjects.
uLTE4, urinary leukotriene E4; NSAID, non-steroidal anti-inflammatory drug; NIBR, non-steroidal anti-inflammatory drug-induced blended reactions; NERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; FEV1, forced expiratory volume in 1 second.
*P values < 0.05, †P values < 0.01.
Effects of aspirin desensitization on uLTE4 levels in patients diagnosed with NERD and NIBR
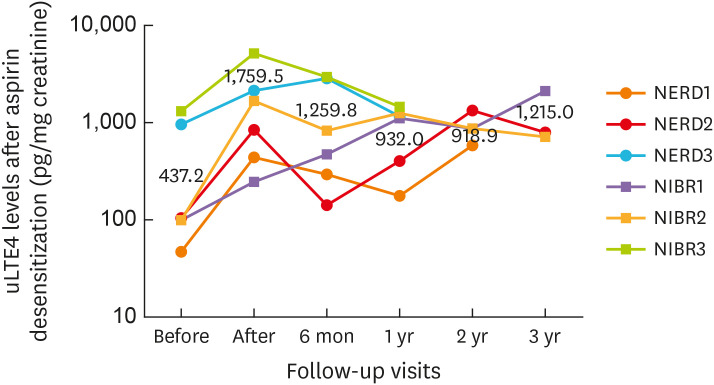
Aspirin desensitization was performed in selected patients (3 NERD and 3 NIBR) who had pre-existing inflammatory airway diseases and a history of recurrent nasal polyposis after surgery as shown in Table 3. The maintenance dose of aspirin was 650 mg/day during the first 6 months and reduced to 325 mg/day afterward. Levels of uLTE4 were measured at baseline after aspirin desensitization and serially followed up for 2–3 years as demonstrated in Fig. 4. Other than respiratory symptoms, acute urticarial rash and gastrointestinal symptoms (abdominal cramp/nausea/diarrhea) were also aggravated in NIBR subjects during aspirin desensitization as shown in Supplementary Fig. S1, but gradually relieved with supportive treatment. No sinus surgery was required for nasal polyp recurrence, and asthma symptoms were under control after aspirin desensitization in all 6 cases. Up to 3 years after aspirin desensitization, however, uLTE4 levels were significantly increased and remained elevated throughout the follow-up period, although urticarial rash and gastrointestinal symptoms were no longer observed while taking aspirin.
Table 3. Details of patients with cross-reactive NSAID hypersensitivity undergoing aspirin desensitization in this study.
| Patients (sex/year) | Underlying diseases | History of adverse drug reactions | Indication for aspirin desensitization | Follow-up duration (months) | Asthma control before/after aspirin desensitization | Recurrent nasal polyposis after aspirin desensitization | Skin symptoms after aspirin desensitization | Gastrointestinal symptoms after aspirin desensitization |
|---|---|---|---|---|---|---|---|---|
| 1. NERD (M/46) | Chronic sinusitis, nasal polyposis, asthma | Acute asthma 20 min after taking aspirin | 2 recurrent nasal polyps | 74 | Partly controlled with high dose ICS-LABA and add-on LTRA/well controlled with low dose ICS | None | NA | NA |
| 2. NERD (M/54) | Chronic rhinitis, nasal polyposis, asthma | Acute asthma, blocked nose 1 hr after taking aspirin | 3 recurrent nasal polyps | 24 | Uncontrolled with high dose ICS-LABA and add-on LTRA/well controlled with medium dose ICS-LABA | None | NA | NA |
| 3. NERD (M/56) | Chronic sinusitis, nasal polyposis, asthma | Acute asthma, blocked nose 1 hr after taking ibuprofen | 6 recurrent nasal polyps | 32 | Partly controlled asthma with high dose ICS-LABA and add-on LTRA/well controlled with medium dose ICS-LABA | None | NA | NA |
| 4. NIBR (M/48) | Chronic sinusitis, nasal polyposis, asthma | Acute asthma, blocked nose, conjunctival injection, urticarial rash 2 hr after taking aspirin | 3 recurrent nasal polyps | 72 | Partly controlled with medium dose ICS-LABA/well controlled with low dose ICS-LABA | None | No rash after taking aspirin | NA |
| 5. NIBR (F/65) | Chronic sinusitis, nasal polyposis, chronic urticaria, asthma (history of cardiac arrest from severe asthma) | Urticarial rash, periorbital angioedema, blocked nose, conjunctival injection, rhinorrhea, bronchospasm/wheezing, hoarseness of voice 1 hr after taking aspirin, ibuprofen, mefenamic acid, acetaminophen | 2 recurrent nasal polyps | 48 | Uncontrolled with high dose ICS-LABA and add-on LTRA/well controlled with low dose ICS-LABA | None | No rash after taking aspirin, chronic urticaria resolved | NA |
| 6. NIBR (F/38) | Chronic sinusitis, nasal polyposis, asthma | Acute asthma, blocked/running nose, stomach cramp, diarrhea, periorbital angioedema, urticaria 30 min after taking ibuprofen, indomethacin, mefenamic acid | 2 recurrent nasal polyps | 21 | Partly controlled with medium dose ICS-LABA/well controlled, with medium dose ICS-LABA | None | No rash after taking aspirin | No gastrointestinal symptoms after taking aspirin |
NSAID, non-steroidal anti-inflammatory drug; NERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor antagonist; NA, not applicable (no symptoms prior to aspirin desensitization); NIBR, non-steroidal anti-inflammatory drug-induced blended reactions.
Fig. 4. uLTE4 levels in NSAID-sensitive subjects after aspirin desensitization.
Our study demonstrated that uLTE4 levels (pg/mg creatinine) in 3 NERD and 3 NIBR patients were increased and remained elevated up to 3 years after aspirin desensitization even though recurrent nasal polyposis was successfully prevented in all cases.
uLTE4, urinary leukotriene E4; NSAID, non-steroidal anti-inflammatory drug; NERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; NIBR, non-steroidal anti-inflammatory drug-induced blended reactions.
DISCUSSION
Most studies on NSAID hypersensitivity have focused on NERD, although worldwide epidemiological data suggest that NECD/NIUA might be more common. Recent data have demonstrated that some patients cannot be categorized into the current classification of NSAID hypersensitivity, for example those with NSAID-induced periorbital angioedema and NIBR.14,19 These patients may also have underlying chronic airway diseases; however, the role of aspirin desensitization in the prevention of recurrent nasal polyposis and control of airway inflammation in NSAID-sensitive subjects other than the NERD phenotype has never been reported.
The leading presentations in NSAID-sensitive subjects in this study were periorbital angioedema, acute urticaria, bronchial symptoms, and naso-ocular reactions, respectively. Even those confirmed with NSAID exposure, a significant number of patients in our cohort also showed clinical responses in more than 1 organ system, indicating that the prevalence of NIBR was higher than previously thought. Although patients with IgE-mediated anaphylaxis might also present with multiple organ involvement, the fact that most of the reactions took about 1–2 hours to occur and the ability to react to multiple NSAIDs with dissimilar chemical structures made them more compatible with the non-immunologic effect of the drugs.
Facial angioedema around the periorbital area was the most common presenting symptom in Thai patients. Upon NSAID provocation, however, the predominant periorbital angioedema often accompanied by blocked nose, running nose, and itchy/red eyes. After a thorough examination, small urticarial rash on the trunk and extremities, and expiratory wheezing were occasionally detected, although frequently asymptomatic. It is noteworthy that periorbital angioedema may persist for hours or days while urticarial rash and respiratory symptoms are quickly resolved after treatment. As a result, NIBR could be underdiagnosed if history taking is not thoroughly reviewed, since symptoms with lesser severity in other organ systems may be unnoticed. In addition, the COX-1 inhibitor properties of the exposed NSAIDs could affect the phenotype of NSAID sensitivity reported by patients as some patients experienced both asthmatic attack and periorbital angioedema/generalized urticaria after taking aspirin but reported only periorbital edema after taking acetaminophen.
It is practically difficult to clearly distinguish between NSAID-induced periorbital angioedema and NSAID-induced naso-ocular reactions (upper airway manifestations of NERD), since patients occasionally experienced periorbital swelling, red/swollen eyes with tearing, and nasal congestion at the same time. In our opinion, the majority of NSAID hypersensitivity in the Thai population would be described as “NSAID-induced blended reaction with predominant periorbital angioedema” and probably related to “NSAID-induced isolated periorbital angioedema” phenotype as proposed by the Spanish group. It is interesting to note that the prevalence of atopic disease was high in this patient group. We speculate that the increased leukotriene production after NSAID consumption could lead to the worsening of pre-existing mucosal inflammation around the nose and eyes in patients who already suffered from the late-phase reaction of persistent allergic rhinitis.
Our study did not find any difference in terms of basal uLTE4 levels between NSAID-sensitive and NSAID-tolerant subjects. However, uLTE4 levels were higher in NSAID-sensitive patients, particularly in NERD and NIBR subjects, after the NSAID challenge compared to those in the NSAID-tolerant group. Though basal uLTE4 levels may not be a suitable marker to screen NSAID sensitivity status, elevated uLTE4 levels after aspirin OPT could be the supporting evidence for the diagnosis of cross-reactive NSAID hypersensitivity in cases where the clinical response is equivocal. Our study demonstrated that the degree of defective leukotriene homeostasis after exposure to drugs affecting the cyclo-oxygenase pathway might be related to the severity of respiratory involvement upon NSAID exposure.35 The fact that uLTE4 levels were barely changed after celecoxib provocation in patients with NSAID cross-reactivity confirms that celecoxib is a safe alternative NSAID in these patient groups.
According to our study, the patterns of NSAID hypersensitivity in southeast Asians are similar to those in southern Europeans and Latin Americans, and somewhat different from those reported in patients of northern European descent. Previous data suggested that aspirin nasal provocation could potentially diagnose some NSAID-sensitive patients presenting with predominant cutaneous reactions.36,37 Retrospectively, some of these patients might be re-classified as the NIBR phenotype, since a decreased nasal volume was also observed. In other words, patients diagnosed with an NSAID-induced cutaneous reaction who positively reacted to NSAID nasal provocation, especially those with a periorbital reaction, might be NIBR subjects with subclinical airway involvement.
The novelties of this study were the findings that NIBR accounted for about one-third of adult patients with cross-reactive NSAID hypersensitivity and that aspirin desensitization could successfully prevent the recurrence of nasal polyposis and alleviate respiratory symptoms not only in NERD, but also in NIBR, even though urine LTE4 levels remained elevated. However, since the reactions in some NIBR patients could lead to serious adverse events, aspirin desensitization in NIBR subjects should be performed only when it is strongly indicated in selected patients with no previous severe reactions to NSAIDs. In NIBR subjects who experienced previous severe reactions in multiple organ systems, the procedure of aspirin desensitization, if necessary, should be carefully monitored and the administration of biological agents such as dupilumab, if available, should be considered a safer therapeutic option in these cases.
Interestingly, uLTE4 levels were increased after aspirin desensitization and remained elevated throughout the follow-up period. It should be emphasized that urticarial rash and stomach-ache in NIBR patients also disappeared while patients were taking daily aspirin. These data suggest that the pathogenesis of nasal polyposis cannot be explained by cysLT overproduction alone and that aspirin desensitization could be useful to alleviate both respiratory and non-respiratory symptoms in NIBR subjects.
Interestingly, a recent study also found that the clinical benefit of high-dose aspirin desensitization in NERD was independent of a reduction of mast cell activation and cysLT production. They reported paradoxically increased uLTE4 levels 8 weeks after aspirin desensitization compared to the baseline levels and concluded that high-dose aspirin therapy did not restore the impaired eicosanoid pathway.38 According to their study findings and ours, the therapeutic effect of aspirin desensitization was not directly correlated with a change of arachidonic acid metabolism.
There are some limitations to this study. Aspirin desensitization was performed in only 6 patients in our cohort; therefore, the comparative success rates and potential adverse reactions of aspirin desensitization between NERD and NIBR patients could not be analyzed. Further studies with a larger sample size are needed to compare the long-term prognosis between NERD and NIBR, to understand the mechanism of aspirin desensitization for the improvement of respiratory and extra-respiratory symptoms and to evaluate its risk-benefit ratio of aspirin desensitization in NIBR subjects.
In conclusion, NIBR is not uncommon among patients diagnosed with NSAID hypersensitivity in Thailand. Increased uLTE4 levels upon aspirin provocation suggested a diagnosis of NSAID cross-reactivity, while baseline uLTE4 levels were not distinguishable between NSAID-tolerant and NSAID-sensitive subjects. Our study indicates that aspirin desensitization is effective not only in NERD but also in NIBR. The persistently high levels of uLTE4 in spite of clinical improvement in NSAID-sensitive patients after successful aspirin desensitization suggest that elevated leukotrienes alone cannot explain the pathogenesis of NSAID hypersensitivity reactions.
ACKNOWLEDGMENTS
This study was supported by the Ratchadaphiseksomphot fund, Faculty of Medicine, Chulalongkorn University, grant No. RA55/19 and the Skin and Allergy Research Unit, Chulalongkorn University, Bangkok, Thailand. Study data were collected and managed using Research Electronic Data Capture (REDCap) hosted at HIV Netherlands Australia Thailand Research Collaboration. The authors thank Thitima Kantachatvanich, MD for providing patient information.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Clinical characteristics of patients with confirmed NSAID-induced immediate reactions
Representative photos of skin rash demonstrated during aspirin desensitization in NIBR patients.
References
- 1.Blanca-Lopez N, Soriano V, Garcia-Martin E, Canto G, Blanca M. NSAID-induced reactions: classification, prevalence, impact, and management strategies. J Asthma Allergy. 2019;12:217–233. doi: 10.2147/JAA.S164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JU, Park JS, Chang HS, Park CS. Complementary participation of genetics and epigenetics in development of NSAID-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2019;11:779–794. doi: 10.4168/aair.2019.11.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill KN, Laidlaw TM. Pathogenesis of aspirin-induced reactions in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016;36:681–691. doi: 10.1016/j.iac.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46:2201–2206. doi: 10.1002/art.10426. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Laidlaw T. Cross-reactivity and tolerability of celecoxib in adult patients with NSAID hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2891–2893.e4. doi: 10.1016/j.jaip.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–1070. doi: 10.1056/NEJMra1712125. [DOI] [PubMed] [Google Scholar]
- 8.Wong JT, Nagy CS, Krinzman SJ, Maclean JA, Bloch KJ. Rapid oral challenge-desensitization for patients with aspirin-related urticaria-angioedema. J Allergy Clin Immunol. 2000;105:997–1001. doi: 10.1067/mai.2000.104571. [DOI] [PubMed] [Google Scholar]
- 9.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 11.Philpott CM, Erskine S, Hopkins C, Kumar N, Anari S, Kara N, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK National Chronic Rhinosinusitis Epidemiology Study. Respir Res. 2018;19:129. doi: 10.1186/s12931-018-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasper L, Sladek K, Duplaga M, Bochenek G, Liebhart J, Gladysz U, et al. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy. 2003;58:1064–1066. doi: 10.1034/j.1398-9995.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 13.Jares EJ, Sánchez-Borges M, Cardona-Villa R, Ensina LF, Arias-Cruz A, Gómez M, et al. Multinational experience with hypersensitivity drug reactions in Latin America. Ann Allergy Asthma Immunol. 2014;113:282–289. doi: 10.1016/j.anai.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Quiralte J, Blanco C, Delgado J, Ortega N, Alcntára M, Castillo R, et al. Challenge-based clinical patterns of 223 Spanish patients with nonsteroidal anti-inflammatory-drug-induced-reactions. J Investig Allergol Clin Immunol. 2007;17:182–188. [PubMed] [Google Scholar]
- 15.Fan Y, Feng S, Xia W, Qu L, Li X, Chen S, et al. Aspirin-exacerbated respiratory disease in China: a cohort investigation and literature review. Am J Rhinol Allergy. 2012;26:e20–2. doi: 10.2500/ajra.2012.26.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidon MI, Kang LW, Chin CW, Hoon LS, See Y, Goh A, et al. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005;116:e675–80. doi: 10.1542/peds.2005-0969. [DOI] [PubMed] [Google Scholar]
- 17.Kidon MI, Liew WK, Chiang WC, Lim SH, Goh A, Tang JP, et al. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007;144:51–56. doi: 10.1159/000102614. [DOI] [PubMed] [Google Scholar]
- 18.Ruxrungtham K, Chantaphakul H, Tiyasatapon S, Phanupak P. Acetamenophen cross-sensitivity is common in Thai patients with aspirin/NSAIDs sensitivity and may be life-threatening. J Allergy Clin Immunol. 2002;109(Supplement 1):S141. [Google Scholar]
- 19.Doña I, Pérez-Sánchez N, Eguiluz-Gracia I, Cano-Muñoz R, Bartra J, María José T, et al. Progress in understanding hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Allergy. 2020;75:561–575. doi: 10.1111/all.14032. [DOI] [PubMed] [Google Scholar]
- 20.Doña I, Barrionuevo E, Salas M, Laguna JJ, Agúndez J, García-Martín E, et al. NSAIDs-hypersensitivity often induces a blended reaction pattern involving multiple organs. Sci Rep. 2018;8:16710. doi: 10.1038/s41598-018-34668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wöhrl S. NSAID hypersensitivity - recommendations for diagnostic work up and patient management. Allergo J Int. 2018;27:114–121. doi: 10.1007/s40629-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagan JB, Laidlaw TM, Divekar R, O'Brien EK, Kita H, Volcheck GW, et al. Urinary leukotriene E4 to determine aspirin intolerance in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017;5:990–997.e1. doi: 10.1016/j.jaip.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Bochenek G, Stachura T, Szafraniec K, Plutecka H, Sanak M, Nizankowska-Mogilnicka E, et al. Diagnostic accuracy of urinary LTE4 measurement to predict aspirin-exacerbated respiratory disease in patients with asthma. J Allergy Clin Immunol Pract. 2018;6:528–535. doi: 10.1016/j.jaip.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Comhair SA, Bochenek G, Baicker-McKee S, Wang Z, Stachura T, Sanak M, et al. The utility of biomarkers in diagnosis of aspirin exacerbated respiratory disease. Respir Res. 2018;19:210. doi: 10.1186/s12931-018-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setkowicz M, Mastalerz L, Podolec-Rubis M, Sanak M, Szczeklik A. Clinical course and urinary eicosanoids in patients with aspirin-induced urticaria followed up for 4 years. J Allergy Clin Immunol. 2009;123:174–178. doi: 10.1016/j.jaci.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Zembowicz A, Mastalerz L, Setkowicz M, Radziszewski W, Szczeklik A. Safety of cyclooxygenase 2 inhibitors and increased leukotriene synthesis in chronic idiopathic urticaria with sensitivity to nonsteroidal anti-inflammatory drugs. Arch Dermatol. 2003;139:1577–1582. doi: 10.1001/archderm.139.12.1577. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66:818–829. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 28.Kowalski ML, Makowska JS. Seven steps to the diagnosis of NSAIDs hypersensitivity: How to apply a new classification in real practice? Allergy Asthma Immunol Res. 2015;7:312–320. doi: 10.4168/aair.2015.7.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczyńska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62:1111–1118. doi: 10.1111/j.1398-9995.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- 30.Cook KA, Modena BD, Wineinger NE, Woessner KM, Simon RA, White AA. Use of a composite symptom score during challenge in patients with suspected aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2017;118:597–602. doi: 10.1016/j.anai.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Celikel S, Stevenson D, Erkorkmaz U, White AA. Use of nasal inspiratory flow rates in the measurement of aspirin-induced respiratory reactions. Ann Allergy Asthma Immunol. 2013;111:252–255. doi: 10.1016/j.anai.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Asero R. Multiple nonsteroidal anti-inflammatory drug-induced cutaneous disease: what differentiates patients with and without underlying chronic spontaneous urticaria? Int Arch Allergy Immunol. 2014;163:114–118. doi: 10.1159/000356702. [DOI] [PubMed] [Google Scholar]
- 33.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 34.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105:130–135. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–564. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 36.Wismol P, Putivoranat P, Buranapraditkun S, Pinnobphun P, Ruxrungtham K, Klaewsongkram J. The values of nasal provocation test and basophil activation test in the different patterns of ASA/NSAID hypersensitivity. Allergol Immunopathol (Madr) 2012;40:156–163. doi: 10.1016/j.aller.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Campo P, Ayuso P, Salas M, Plaza MC, Cornejo-García JA, Doña I, et al. Mediator release after nasal aspirin provocation supports different phenotypes in subjects with hypersensitivity reactions to NSAIDs. Allergy. 2013;68:1001–1007. doi: 10.1111/all.12187. [DOI] [PubMed] [Google Scholar]
- 38.Cahill KN, Cui J, Kothari P, Murphy K, Raby BA, Singer J, et al. Unique effect of aspirin therapy on biomarkers in aspirin-exacerbated respiratory disease. A prospective trial. Am J Respir Crit Care Med. 2019;200:704–711. doi: 10.1164/rccm.201809-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of patients with confirmed NSAID-induced immediate reactions
Representative photos of skin rash demonstrated during aspirin desensitization in NIBR patients.