Abstract
Purpose
After adolescence, asthma is more frequent in females than in males due to different hormonal, immunologic, and occupational/environmental factors. The higher prevalence and severity of the disease in females have already been reported in international registries. The aim of this study was to explore the difference in terms of clinical, functional, and biological characteristics between male and female patients with severe asthma in a real-life, registry-based setting.
Methods
Baseline data from the Severe Asthma Network in Italy registry were analyzed in 1,123 patients with severe asthma, according to sex.
Results
Almost 2/3 of severe asthmatics were female. Late-onset asthma, obesity and gastro-esophageal reflux were more frequent in females than in males, while previous smoking habits and nasal polyposis were more frequent in males. Females had poor asthma control and a higher number of severe exacerbations leading to hospitalization, in comparison to males. Biomarkers of type 2 inflammation (blood eosinophil, exhaled nitric oxide, and serum immunoglobulin E levels) were significantly higher in males than in females. The type 2 profile (defined by a combination of these 3 biomarkers) was significantly more frequent in males than in females. In multivariate analysis, late-onset asthma and a normal body mass index were only independent variables associated with the type 2 profile, while male sex and age showed only a trend toward the association with the type 2 profile.
Conclusions
Significant differences may be observed between male and female patients with severe asthma, influencing the asthma pheno-endotyping in both sexes.
Keywords: Severe asthma, sex, male, female, type 2, biomarkers, obesity, smoking
INTRODUCTION
The current worldwide prevalence of asthma in the general population is 5%, with significant differences among countries and continents.1 Overall, asthma is more prevalent in women and the sex difference has been stable over the last decade.2,3 However, the female/male ratio changes throughout life, because asthma is more prevalent among boys at younger ages and becomes more common in females after puberty. This sex disparity disappears after menopause.4 A complex interplay of hormonal, immunological, and occupational/environmental factors may account for this difference.
Besides higher prevalence, asthma is more severe in females; being female is a risk factor for higher health care utilization, hospitalizations, and emergency department admissions.4,5 Moreover, even if reduction in asthma mortality has been observed in the last 15 years, a gap between both sexes still exists, with a higher prevalence of deaths due to asthma in women.2 In some severe asthma registries, the prevalence of females ranged from 57% to 71%6,7,8,9,10,11,12,13,14; the female sex was also associated with some comorbidities (such as obesity and gastroesophageal reflux) and poorer asthma control. However, no differences between both sexes in terms of biomarkers were reported in these studies.
The aim of the present study was to assess the sex difference in the Severe Asthma Network in Italy (SANI), a recently developed registry recruiting patients with severe asthma from several Italian centers with particular expertise in the diagnosis and the management of severe asthma.15 The preliminary data on the main clinical and functional characteristics of these patients have already been published, showing a higher prevalence of females than males as expected.16 In this paper, we characterized males and females in terms of clinical, functional, and biologic pheno-endotypes to assess potential different “treatable traits” for both sexes.
MATERIALS AND METHODS
Study population
The Italian Network SANI is a web-based observatory collecting demographic, clinical, functional, and inflammatory data from patients aged > 12 years affected by severe asthma, according to the European Respiratory Society/American Thoracic Society definition.17 Patients were recruited in reference centers homogeneously distributed on the national territory. They were selected according to the recommendations of the Global Initiative for Asthma (GINA) guidelines,18 and a final diagnosis of “severe asthma” was made only after a long follow-up period during which all major factors potentially responsible for uncontrolled asthma (adherence, inhaler technique, comorbidities, etc.) have been appropriately managed.
For each patient, the following data were collected: (1) demographic data (age, sex, height, weight, and body mass index [BMI]), (2) clinical data (onset age of asthma [early: <12 years; intermediate: 12–40 years; late: > 40 years], presence of allergies and other comorbidities such as bronchiectasis, gastro-esophageal reflux [GERD], and nasal polyposis, acute exacerbations, and unscheduled visits), (3) functional features, (4) asthma control in the previous month (according to standardized questionnaires (Asthma Control Test [ACT]; Asthma Control Questionnaire [ACQ]), and (5) inflammatory markers (fractional exhaled nitric oxide [FeNO], blood eosinophils, and serum immunoglobulin E [IgE]). All the data were categorized for males and females.
Comorbidities were defined as: a) confirmed if specific diagnostic tests were available (e.g., computerized tomography for bronchiectasis, gastro-esophageal endoscopy and/or pH-manometry for GERD, nasal endoscopy for polyposis); and b) suspected if patients reported symptoms, with a positive response to the appropriate treatment. Rhinitis was defined as previously reported in the past and no longer present, or currently still present.
All patients underwent a regular treatment with an inhaled corticosteroids (ICSs)/long-acting beta-agonists combinations; they were associated with anticholinergic drugs in 36% of the cases and with leukotriene receptor antagonists in a few cases; and a regular or frequent treatment with oral corticosteroids (OCS) was reported in 40% of the patients. Most patients received biologic drugs (anti-IgE or anti-interleukin 5 [IL5]/IL5 receptor) during the follow-up period, but the evaluation of asthma control, pulmonary function, and biomarkers was based on data collected at the beginning of biologic therapies. All measurements, including biomarkers, were taken in a stable phase of the disease, out of any asthma exacerbation since at least 4 weeks, and without any withdrawal of the current pharmacological treatment.
Spirometric and exhaled nitric oxide tests were performed in each center according to their standard procedures.
Ethical issues
The study was carried out according to the Helsinki and Oviedo Declaration. The SANI registry was set up according to the third edition recommendation on registries for evaluating patient outcomes published by the Effective Health Care Program of the Agency for Healthcare Research and Quality (https://effectivehealthcare.ahrq.gov/topics/registries-guide-3rd-edition/research/). The protocol was designed following the principles and procedures of the Good Clinical Practice (ICH Harmonized Tripartite Guidelines for Good Clinical Practice 1996; Directive 91/507. EEC, The Rules Governing Medical Products in the European Community) and according to Italian law (D.L.vo n.211 24 June 2003;D.L.n.200 6 November 2007; MD, 21 December 2007).
The SANI initiative is supported by several pharmaceutical companies listed in the funding statement, which provided unrestricted grants and played no role in study design and planned analysis.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (SPSS, Chicago, IL. USA). The Mann-Whitney 2-sample statistic for non-normal distributed or ordinal variables, or the Student's t-test for continuous normal distributed variables were used to compare variables between both sexes. Categorical variables were compared with the Fisher's exact test. A P value of <0.05 was considered statistically significant.
Multiple logistic regression analysis with robust standard error estimate was used to analyze the relationship between both definitions of type 2 endotype as dependent and sex (female as reference category)/age classes/age of asthma onset (as class i.e., early as reference, intermediate and late or linear effects)/smoking habits (no smoker as reference, past or current smokers)/BMI classes (< 25 vs. ≥ 25)/comorbidities (presence vs. absence) as predictive variables.
RESULTS
Overall, 1,123 patients with severe asthma were evaluated. The demographic and clinical characteristics of the patients are shown in Table 1: 61.8% of the patients were female, mean age was approximately 56 years in both sexes. The onset age of the asthma was comparable in both sexes, but intermediate onset (between 12 and 40 years) and late onset (> 40 years) were more frequent in females. The number of ex-smokers was significantly larger in males. Less than 50% of patients had a normal BMI (< 25); the number of overweight patients (BMI 25–30) was larger in males than in females, whereas obesity (BMI > 30) was more common in females. Other comorbidities such as rhinitis and bronchiectasis were equally distributed in both sexes, whereas a significant prevalence of GERD (both confirmed and suspected) was observed in females. Nasal polyposis was slightly more prevalent in males. The prevalence of atopy (defined by a positive response to skin prick tests to common inhalant allergens) was similar in both sexes.
Table 1. Main clinical features of severe asthmatics according to sex.
| Male | Female | P value | |||
|---|---|---|---|---|---|
| No. | 433 (38.2) | 690 (61.8) | |||
| Mean age (yr) | 56.1 ± 13.1 | 55.6 ± 13.2 | NS | ||
| Age of asthma onset (yr) | 35.1 ± 16.8 | 33.4 ± 16.7 | NS | ||
| Onset of asthma | |||||
| Early onset | 12.9 | 13.9 | |||
| Intermediate onset | 47.2 | 52.7 | |||
| Late onset | 39.9 | 33.4 | NS | ||
| Smoking habits | |||||
| Non smokers | 69.1 | 76.2 | |||
| Ex-smokers | 27.3 | 20.4 | |||
| Current smokers | 3.6 | 3.4 | < 0.004 | ||
| BMI | |||||
| < 25 | 45.0 | 47.9 | |||
| 25–30 | 42.3 | 30.0 | |||
| > 30 | 12.8 | 22.1 | < 0.0001 | ||
| Comorbities | |||||
| Bronchiectasis (conf + susp) | 16.9 | 15.1 | NS | ||
| GERD (conf + susp) | 28.5 | 39.9 | < 0.002 | ||
| Polyposis (conf + susp) | 47.5 | 41.5 | < 0.05 | ||
| Rhinitis (prev + curr) | 64.3 | 61.6 | NS | ||
| Atopy (by SPT) | 69.2 | 67.7 | NS | ||
| Treatment | |||||
| ICS-LABA | 95.5 | 93.7 | NS | ||
| Mean ICS dose | |||||
| LAMA | 35.3 | 36.9 | NS | ||
| Montelukast | 60.9 | 64.6 | NS | ||
| OCS (regular or frequent courses) | 42.2 | 41.1 | NS | ||
Data are reported as mean ± standard deviation or number (%).
BMI, body mass index; GERD, gastro-esophageal reflux; conf + susp, confirmed and suspected; prev + curr, previous and current; SPT, skin prick tests; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist; LAMA, long-acting anti-muscarinic agent; OCS, oral corticosteroids; NS, not significant.
Table 2 shows the level of asthma control (according to ACT, ACQ, and exacerbation rate in the previous year) in these patients. Symptom control was significantly poorer in females than in males, while the mean number of severe exacerbations (requiring OCS treatment for at least 3 days) and Emergency Department accesses for asthma attacks were similar in both sexes. One out of 5 patients reported at least 1 hospitalization for asthma in the previous year, with a slightly greater prevalence in females. The prebronchodilator forced expiratory volume in 1 second (FEV1) was similarly reduced in both sexes.
Table 2. Asthma control, PB-FEV1 and biomarkers in severe asthmatics according to sex.
| Male | Female | P value | |
|---|---|---|---|
| ACT (median [IQR]) | 19 (8) | 18 (8) | NS |
| ACQ (median [IQR]) | 2.3 (2.3) | 2.4 (2.2) | NS |
| Severe exacerbations (No./yr, mean) | 2.3 (3.3) | 2.5 (3.6) | NS |
| ED attendency (No./yr, mean) | 0.32 (1.4) | 0.3 (0.8) | NS |
| Hospitalization (No./yr, mean) | 0.17 (.61) | 0.22 (.61) | 0.07 |
| PB-FEV1 (L, mean) | 2.54 (0.87) | 1.83 (0.66) | 0.005 |
| PB-FEV1 (%pred) | 72.4 (21) | 76.0 (23) | NS |
| Blood eosinophils (median [IQR]) | 360 (510) | 300 (490) | NS |
| Blood eosinophils | 6.06 | 5.69 | NS |
| Blood eosinophils > 150/µL | 74.3 | 66.0 | < 0.002 |
| Blood eosinophils > 300/µL | 56.3 | 49.8 | NS |
| Blood eosinophils > 400/µL | 41.5 | 40.9 | NS |
| FeNO | 49.9 ± 44.5 | 40.3 ± 38.7 | 0.001 |
| FeNO > 25 ppb | 62.8 | 53.9 | 0.05 |
| FeNO > 50 ppb | 35.1 | 27.9 | < 0.005 |
| Serum IgE (median [IQR]) | 508 (686) | 352 (503) | 0.02 |
| Serum IgE > 150 U/µL | 67.3 | 56.1 | 0.006 |
| Serum IgE > 700 U/µL | 22.05 | 12.96 | < 0.005 |
Data are shown as mean ± standard deviation or number (%).
ACT, asthma control test; ACQ, asthma control questionnaire; IQR, interquartile range; ED, emergency department; PB-FEV1, pre-bronchodilator forced expiratory volume in the first second; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; NS, not significant.
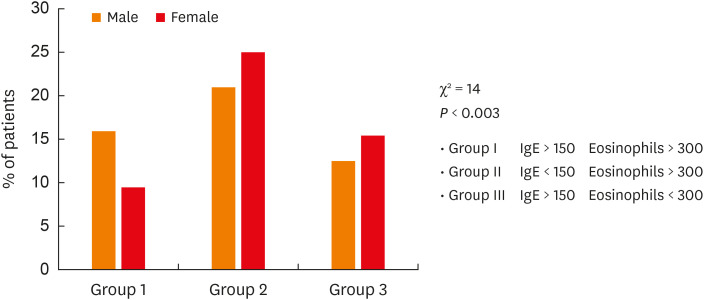
As far as biomarkers are concerned, a large number and a high percentage of blood eosinophils were noted, without any significant difference between both sexes. This was probably due to the large variability of this evaluation among patients. Mean FeNO values and the rate of patients with FeNO > 25 ppb as well as mean serum IgE levels and the rate of patients with IgE > 150 U/L were significantly higher in males. When a different combination of serum IgE and blood eosinophils were considered, the rate of patients with high levels of serum IgE and high levels of blood eosinophils was significantly higher in males than in females (Figure).
Figure. Prevalence of severe asthmatic patients with a different combination of serum IgE and blood eosinophil abnormalities, according to sex. The distribution among the 3 groups was significantly different (P < 0.003) between both sexes by the chi square test.
IgE, immunoglobulin E.
We attempted to characterize these patients according to the type 2 endotype, using the biomarkers available, i.e., a combination of high serum IgE (> 150 U/µL), high blood eosinophils (> 150 or 300 cell/µL), and high FeNO levels (> 25 ppb), according to the GINA guidelines.14 Using both criteria (criterium No.1: IgE >150 and/or BE > 300 and/or FeNO > 25; criterium No.2: IgE > 150 and/or BE > 150 and/or FeNO >25), the prevalence of the type 2 endotype was significantly higher in males than in females (74.3% vs. 69.3%, P < 0.02, and 76.1% vs. 70.1%, P < 0.02, respectively). Due to the fact that the lower prevalence of the type 2 endotype in females could also be explained by a different prevalence of comorbidities in both sexes, which can modify inflammatory patterns (smoking habits and comorbidities such as obesity, GERD, and nasal polyposis), we performed a multivariate analysis including not only sex, age, and onset of asthma, but also comorbidities, in order to identify which variables were independently associated to the type 2 phenotype as defined according to the 2 criteria (Tables 3 and 4). BMI (< 25) and late-onset asthma (with a positive linear trend toward age of onset) were only independent factors significantly associated with the type 2 endotype, while there was a trend toward age and male sex with both the first and second criteria.
Table 3. Multivariate analysis for type 2 endotype (defined as criterion No.1: IgE > 150 and/or BE > 300 and/or FeNO > 25) in severe asthmatic patients.
| Variable | OR | 95% CI | P value | ||
|---|---|---|---|---|---|
| Sex (male vs. female) | 1.28 | 0.96–1.69 | 0.087 | ||
| BMI (< 25 vs. ≥ 25) | 1.82 | 1.37–2.42 | 0.001 | ||
| Age (yr) | |||||
| 41–60 vs. < 40 | 1.23 | 0.81–1.89 | 0.329 | ||
| > 60 vs. < 40 | 1.69 | 1.08–2.65 | 0.022 | ||
| Age of onset (vs. early < 12) | |||||
| Intermediate (12–40) | 1.39 | 0.91–2.10 | 0.122 | ||
| Late (> 40) | 1.89 | 1.17–3.04 | 0.009 | ||
| (trend) | 0.008 | ||||
IgE, immunoglobulin E; BE, blood eosinophils; FeNO, fractional exhaled nitric oxide; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Table 4. Multivariate analysis for the type 2 endotype (defined as criterium No.2: IgE > 150 and/or BE > 150 and/or FeNO > 25) in severe asthmatic patients.
| Variable | OR | 95% CI | P value | ||
|---|---|---|---|---|---|
| Sex (male vs. female) | 1.30 | 0.96–1.76 | 0.087 | ||
| BMI (< 25 vs. ≥ 25) | 1.62 | 1.20–2.18 | 0.002 | ||
| Age (yr) | |||||
| 41–60 vs. < 40 | 1.06 | 0.67–1.66 | 0.801 | ||
| > 60 vs. < 40 | 1.60 | 0.99–2.60 | 0.055 | ||
| Age of onset (vs. early < 12) | |||||
| Intermediate (12–40) | 1.33 | 0.86–2.06 | 0.199 | ||
| Late (> 40) | 1.63 | 1.00–2.68 | 0.050 | ||
| (trend) | 0.017 | ||||
IgE, immunoglobulin E; BE, blood eosinophils; FeNO, fractional exhaled nitric oxide; OR, odds ratio; CI, confidence interval; BMI, body mass index.
DISCUSSION
According to several networks, severe asthma is more common in females.6,7,8,9,10,11,12,13,14 Many reasons may account for this difference: hormonal factors, environmental exposure, and type and rate of comorbidities. The results of our study which was performed in a large database of severe asthmatic patients showed that the female to male ratio is about 2:1.
It is well known that hormonal status may favor sex difference because ovarian hormones seem to increase and testosterone seems to decrease bronchial inflammation with still unclear mechanisms; however, fluctuations in estrogens are likely to be involved.19 The role of the oral contraceptive pill is still debated, whereas replacement therapy seems to increase the risk of asthma. Indeed, estrogen receptors (ERs) are constitutively expressed in the lungs, including smooth muscle cells, without any difference between males and females, but the relative reciprocal role of the ERα and ERβ, based on the microenvironment and the hormonal level in terms of bronchoconstriction/dilatation, has recently been highlighted.20 In animal models of asthma following allergic sensitization (ovalbumin or house dust mite), ovariectomy clearly reduced eosinophilia, IgE production and airway hyperreactivity.21 Our study confirmed the higher prevalence of asthma in adult females with severe asthma, along with other observations about a lower rate of remission.22 A possible explanation may come from the recently demonstrated effects of estrogens on type 2 innate lymphoid cells, which would be increased into the circulation of severe asthmatic women.23 Alternatively, women can show a more aggressive phenotype of asthma simply because the protective activity of male sex hormones, known as potent downregulators of both innate and adaptive immune responses, is lacking.23,24,25 Interestingly, in vivo experimental models showed that ovariectomy strongly reduces production of both type 2 innate lymphoid cells and IL-17A-secreting cells, involved in eosinophilic and neutrophilic phenotypes, respectively.26 Our real-life observational data are in partial agreement with these experimental reports, suggesting that several interactions between biochemical, clinical, and environmental factors are involved in determining the inflammatory profile of severe asthma.
In addition, several other factors can explain this higher asthma prevalence in females. Occupational risks may play an important role because women are more commonly busy in Health Care, retails, and education fields as well as in the house environment; work-related asthma is more frequently reported in females.27 Women are more likely to own pets at home and allow them into the house. As previously reported,2 obesity was more common in females in our series. A higher BMI was observed in women with severe asthma compared to women with not severe asthma; this figure was not reported in men.6,28 It is unclear if this finding is related to sex hormones or other sex-specific factors. The presence of obesity and a higher BMI may account for the significant prevalence of gastroesophageal reflux in females in our study.
Our study also showed that females with severe asthma have a poorer control of symptoms and a higher number of hospitalizations for asthma attacks than males, despite a similar level of FEV1 and a similar rate of severe exacerbations. These data are partially concordant with those reported in other national or international registries on severe asthma: in effect, some studies suggest that females have greater limitations to daily life activities due to asthma29,30; on the contrary, other studies show that males have poorer pulmonary function than females.12 In our study, the poorer control of asthma symptoms in females compared to males may have been attributed to the higher prevalence of comorbidities (obesity and GERD) in females, which may increase asthma-like symptoms and reduce the efficacy of anti-asthma treatments.2,31
Finally, we demonstrated that the type 2 pattern of inflammation is more frequent in males than in females. We used 2 different criteria for the definition of type 2 inflammation: serum IgE levels, blood eosinophil levels (with 2 different cutoffs), and FeNO levels. A similar approach has been suggested by some recent publications,7,16 and it is in agreement with the concept that the eosinophilic inflammation of the airways observed in severe asthma (which can be directly measured only using induced sputum or bronchial biopsy, both of which are not feasible in current practice) may be driven by both IgE-mediated and non-IgE-mediated inflammatory pathways, and may be easily measured through simple, albeit not invasive, tests. A lower type 2 inflammatory profile in females may be explained by the higher prevalence of some comorbidities, such as obesity and GERD, which are often associated with a noneosinophilic inflammation,32 and by the lower prevalence of nasal polyposis, typically associated with a high degree of eosinophilic inflammation.20,33 The lower prevalence of type 2 inflammation (which responds better to ICSs) in females may in part explain the poorer asthma control in females than in males.
We also performed a multivariate analysis in order to evaluate which of these potential confounding factors could be an independent predictor of type 2 inflammation. As expected, our analysis showed that late-onset asthma and normal BMI were independent predictors of type 2 asthma, while age and male sex showed only a trend.
In summary, the analysis of our Italian database of severe asthmatics showed some significant differences between males and females, involving both clinical characteristics and levels of biomarkers of type 2 inflammation. These differences between both sexes should be considered for implementing personalized management programs of patients with severe asthma.
SANI Network: E. Bacci. M. Bonavia, P. Caiaffa, C. Calabrese, G. Camiciottoli, C. Caruso, S. Centanni, ME. Conte, AG. Corsico, L. Cosmi, MT. Costantino, N. Crimi, S. D'Alò, M. D'Amato, S. Del Giacco, E. Favero, A. Farsi, BPM. Foschino, G. Guarnieri, G. Guida, C. Lombardi, L. Macchia, F. Menzella, M. Milanese, P. Montuschi, E. Nucera, R. Parente, G. Passalacqua, V. Patella, G. Pelaia, L. Pini, FLM. Ricciardolo, L. Ricciardi, L. Richeldi, E. Ridolo, G. Rolla, P. Santus, N. Scichilone, P. Solidoro, G. Spadaro, A. Spanevello, A. Vianello, MR. Yacoub, MC. Zappa.
ACKNOWLEDGMENTS
This study was supported by unconditioned grants from AstraZeneca, GlaxoSmithKline, Novartis and Sanofi, which supported the activity of the SANI Initiative.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zein JG, Denson JL, Wechsler ME. Asthma over the adult life course: gender and hormonal influences. Clin Chest Med. 2019;40:149–161. doi: 10.1016/j.ccm.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy Asthma Immunol Res. 2018;10:387–396. doi: 10.4168/aair.2018.10.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schatz M, Camargo CA., Jr The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91:553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- 5.Zein JG, Udeh BL, Teague WG, Koroukian SM, Schlitz NK, Bleecker ER, et al. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011–2012. PLoS One. 2016;11:e0157301. doi: 10.1371/journal.pone.0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest. 2013;143:406–414. doi: 10.1378/chest.12-0872. [DOI] [PubMed] [Google Scholar]
- 7.Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR) Respir Med. 2014;108:1723–1732. doi: 10.1016/j.rmed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SAA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chipps BE, Zeiger RS, Borish L, Wenzel SE, Yegin A, Hayden ML, et al. Key findings and clinical implications from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012;130:332–342.e10. doi: 10.1016/j.jaci.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maio S, Baldacci S, Bresciani M, Simoni M, Latorre M, Murgia N, et al. RItA: the Italian severe/uncontrolled asthma registry. Allergy. 2018;73:683–695. doi: 10.1111/all.13342. [DOI] [PubMed] [Google Scholar]
- 11.Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157:790–804. doi: 10.1016/j.chest.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Milger K, Korn S, Buhl R, Hamelmann E, Herth FJ, Gappa M, et al. Age- and sex-dependent differences in patients with severe asthma included in the German Asthma Net cohort. Respir Med. 2020;162:105858. doi: 10.1016/j.rmed.2019.105858. [DOI] [PubMed] [Google Scholar]
- 13.van Bragt JJMH, Adcock IM, Bel EHD, Braunstahl GJ, Ten Brinke A, Busby J, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J. 2020;55:1901163. doi: 10.1183/13993003.01163-2019. [DOI] [PubMed] [Google Scholar]
- 14.Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, et al. Characteristics of adult severe refractory asthma in Korea analyzed from the severe asthma registry. Allergy Asthma Immunol Res. 2019;11:43–54. doi: 10.4168/aair.2019.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senna G, Guerriero M, Paggiaro PL, Blasi F, Caminati M, Heffler E, et al. SANI-severe asthma network in Italy: a way forward to monitor severe asthma. Clin Mol Allergy. 2017;15:9. doi: 10.1186/s12948-017-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019;7:1462–1468. doi: 10.1016/j.jaip.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2019. [cited 2020 Mar 31]. Available from: www.ginasthma.org. [Google Scholar]
- 19.De Martinis M, Sirufo MM, Suppa M, Di Silvestre D, Ginaldi L. Sex and gender aspects for patient stratification in allergy prevention and treatment. Int J Mol Sci. 2020;21:1535. doi: 10.3390/ijms21041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhallamudi S, Connell J, Pabelick CM, Prakash YS, Sathish V. Estrogen receptors differentially regulate intracellular calcium handling in human nonasthmatic and asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2020;318:L112–24. doi: 10.1152/ajplung.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda M, Tanabe M, Ito W, Ueki S, Konnno Y, Chihara M, et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology. 2013;18:797–806. doi: 10.1111/resp.12078. [DOI] [PubMed] [Google Scholar]
- 22.Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents' Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504.e1. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Reports. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, et al. A major population of functional KLRG1− ILC2s in female lungs contributes to a sex bias in ILC2 numbers. Immunohorizons. 2018;2:74–86. doi: 10.4049/immunohorizons.1800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214:1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol. 2018;201:1843–1854. doi: 10.4049/jimmunol.1800293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talini D, Ciberti A, Bartoli D, Del Guerra P, Iaia TE, Lemmi M, et al. Work-related asthma in a sample of subjects with established asthma. Respir Med. 2017;130:85–91. doi: 10.1016/j.rmed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–1348. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 29.Colombo D, Zagni E, Ferri F, Canonica GW PROXIMA study centers. Gender differences in asthma perception and its impact on quality of life: a post hoc analysis of the PROXIMA (Patient Reported Outcomes and Xolair® In the Management of Asthma) study. Allergy Asthma Clin Immunol. 2019;15:65. doi: 10.1186/s13223-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.To T, Gray N, Ryckman K, Zhu J, Fong I, Gershon A. Sex differences in health services and medication use among older adults with asthma. ERJ Open Res. 2019;5:1–8. doi: 10.1183/23120541.00242-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novelli F, Bacci E, Latorre M, Seccia V, Bartoli ML, Cianchetti S, et al. Comorbidities are associated with different features of severe asthma. Clin Mol Allergy. 2018;16:25. doi: 10.1186/s12948-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]