Abstract
Objective
Immunoglobulin E (IgE) and its receptor, FcɛRI, importantly contribute to the pathophysiology of chronic spontaneous urticaria (CSU). Recent findings point to a possible role of total IgE as a marker of CSU disease activity, endotypes, and responses to treatment. The evidence in support of total IgE included in the diagnostic workup of patients with CSU has not yet been reviewed.
Methods
Publications were searched via PubMed. The search terms used were “chronic urticaria” and “total IgE.” Studies were screened by titles and abstracts, and 141 were used in the review.
Results
CSU patients frequently had elevated total IgE serum levels (up to 50%), but normal or very low total IgE levels also occurred. High total IgE may represent high disease activity, longer disease duration, high chance of responding to omalizumab treatment, quick relapse after stopping omalizumab, and lower chance of responding to cyclosporine. Low IgE, in contrast, may suggest Type IIb autoimmune CSU, poor response to treatment with omalizumab and a better chance to benefits from cyclosporine treatment. Furthermore, IgE in different CSU cohorts may have different physicochemical properties that could explain differences in treatment responses to IgE-directed therapies.
Conclusion
The results of our review suggest that total IgE is a valuable marker for CSU, and we recommend its assessment in the routine diagnostic workup of CSU patients.
Keywords: Urticaria, chronic spontaneous urticaria, immunoglobulin E, biomarkers, omalizumab, cyclosporine, therapeutics, diagnosis, receptor
INTRODUCTION
Chronic urticaria is a frequent disease with a broad spectrum of different clinical presentations.1,2 Chronic spontaneous urticaria (CSU), where recurrent itchy wheals and flare skin reactions occur spontaneously, i.e. without definite triggers, is the most common form of chronic urticaria. CSU Patients suffer from recurrent wheals, with or without angioedema, mostly with high quality of life impairment, insufficient control over their disease, and long disease duration.3
Skin mast cells are the key effector cells in the pathophysiology of CSU.4 Their activation, degranulation and release of mediators, including histamine, drive the clinical manifestation of CSU, the development of itchy wheals, angioedema or both. In many CSU patients, immunoglobulin E (IgE) and its high affinity receptor, FcɛRI, are involved in the activation and degranulation of mast cells, and the current urticaria guideline treatment algorithm recommends the use of omalizumab, an anti-IgE antibody, when the treatment with a second-generation antihistamine is not effective.3 Anti-IgE treatment, with omalizumab and ligelizumab, markedly reduces disease activity in many patients with CSU refractory to antihistamine therapy.5
Beyond its key role in the pathogenesis of CSU and as a target of treatment, IgE in CSU has been reported 1) to be elevated, 2) to reflect disease activity, 3) to be linked to disease endotypes, 4) to be biologically and functionally different, and 5) to predict the course of the disease and the response to treatment. In this review, we evaluate the role and relevance of total serum IgE as a diagnostic marker for CSU and discuss the rationale and value of measuring total serum IgE in the routine diagnostic workup of CSU patients.
ARE TOTAL SERUM IgE LEVELS ELEVATED IN CSU?
IgE levels of < 100 or >100 IU/mL are often considered normal or elevated, respectively.6 However, it is well documented that mean IgE levels are different between men and women, between children and adults, and among populations of different genetic background and exposure to environmental factors.7,8,9,10 In CSU, elevated total IgE levels were first reported more than 40 years ago.11 More recent publications have demonstrated higher than normal total IgE levels in about half of CSU patients, varying from 18% to 82% in different studies using different tests and cutoff levels (Supplementary Table S1).
The median or mean total IgE levels were reported to be between 100 and 300 IU/mL in most studies in CSU (20 of 32, 63%). Case control studies indicate that total IgE levels are higher in CSU patients as compared with healthy individuals.12,13,14,15,16 On the other hand, total IgE levels in patients with CSU are not so high as in patients with allergies or atopic diseases. In atopic dermatitis, for example, IgE levels often reach 1,000 IU/mL or more.17 Mean IgE values in CSU patients vary from 66 to 1,037 IU/mL (Supplementary Table S1), regardless of whether comorbid atopic dermatitis was excluded.18,19 The highest levels of IgE were found in atopic patients with CSU,20 CSU with concomitant gastroesophageal reflux disorder,21 and CSU patients with high interleukin (IL)-33 serum levels,22 indicating that atopic status and concomitant disorders may influence serum total IgE levels of CSU patients.
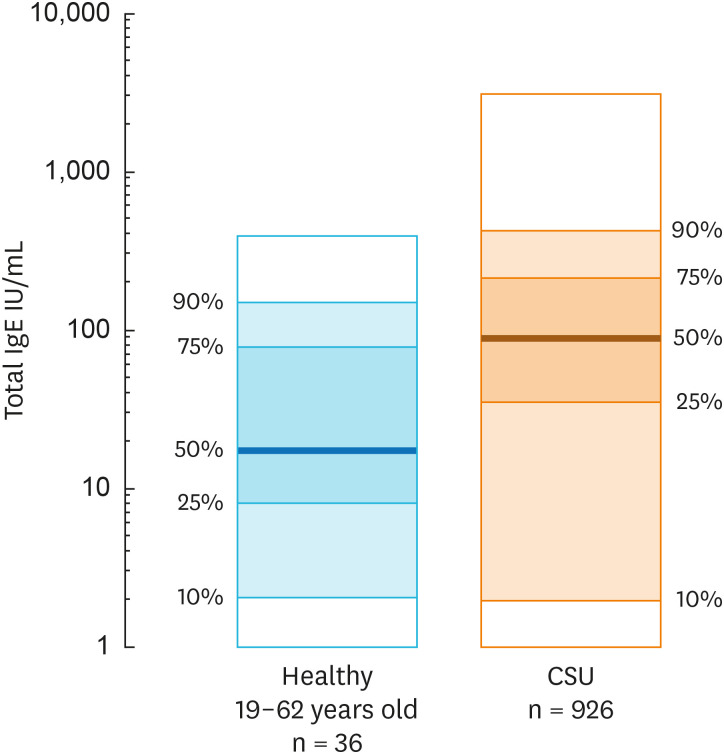
In our patient cohort with CSU, total IgE levels average 89 IU/ml (Fig. 1), with about 50% of patients showing serum total IgE levels > 100 IU/mL. Of note, our patients with CSU display a wide range of total IgE-low (< 30 IU/mL) levels in 25% of patients and very low levels (< 2 IU/mL) in 10% of patients. Several other studies reported subpopulations of CSU patients with low or very low levels of total IgE.23,24,25,26,27 Taken together, CSU comes with both elevated or low total IgE, the former being more common.
Fig. 1. Comparison of total serum IgE levels between 926 CSU patients and 36 healthy controls as measured by ImmunoCap Method (own data). Depicted are median (50%, prominent line), 25% and 75% percentile (dark shaded) as well as 10% and 90% percentile (light shaded) and extreme values (white shaded).
IgE, immunoglobulin E; CSU, chronic spontaneous urticaria.
IS CSU DIFFERENT AMONG PATIENTS WITH ELEVATED, NORMAL OR LOW LEVELS OF IgE?
Very few studies have compared CSU patients with different levels of IgE for differences in the clinical characteristics of their disease. Straesser et al. 23 reported that CSU patients with low total IgE levels are younger, 34 years old when total IgE is < 15 IU/mL, than patients with normal or higher total IgE levels, 44 and 53 years old, respectively; howeer, these differences did not reach significance. Kessel et al. 14 reported significantly higher rates of elevated IgE levels in patients who had their CSU for more than 25 months compared with those with shorter disease duration (40% [27/68] vs. 23% [31/132]). In 2 studies, serum total IgE levels correlated with CSU disease severity,14,28 but not 1 study.29
Further studies are needed. Importantly, they should compare patients with high and normal IgE levels as well as those with low and normal IgE levels, as correlation analyses across all patients may fail to detect how IgE levels are linked to clinical features of CSU.
ARE TOTAL IgE LEVELS LINKED TO DIFFERENCES IN LABORATORY MARKERS FOR CSU?
Basopenia and eosinopenia, markers for high CSU disease activity, are found in up to 14% and 10% of CSU patients, respectively.30,31,32 While there are no studies on the link between total IgE and basopenia, a recent report showed a weak, but highly significant, positive correlation between total IgE levels and eosinophil numbers (r = 0.275, P < 0.01).32
Previous studies reported significant positive correlations between serum total IgE and basophil FcɛRI expression levels.18,33
Elevated levels of D-Dimer and CRP, also markers of high inflammatory activity in CSU, were reported not to be linked to total IgE. IgE levels and rates of elevated IgE levels were similar in CSU patients with elevated and normal D-Dimer levels29,34 and in patients with elevated and normal CRP.29,35,36
THE ROLE OF IgE IN THE PATHOGENESIS OF CSU
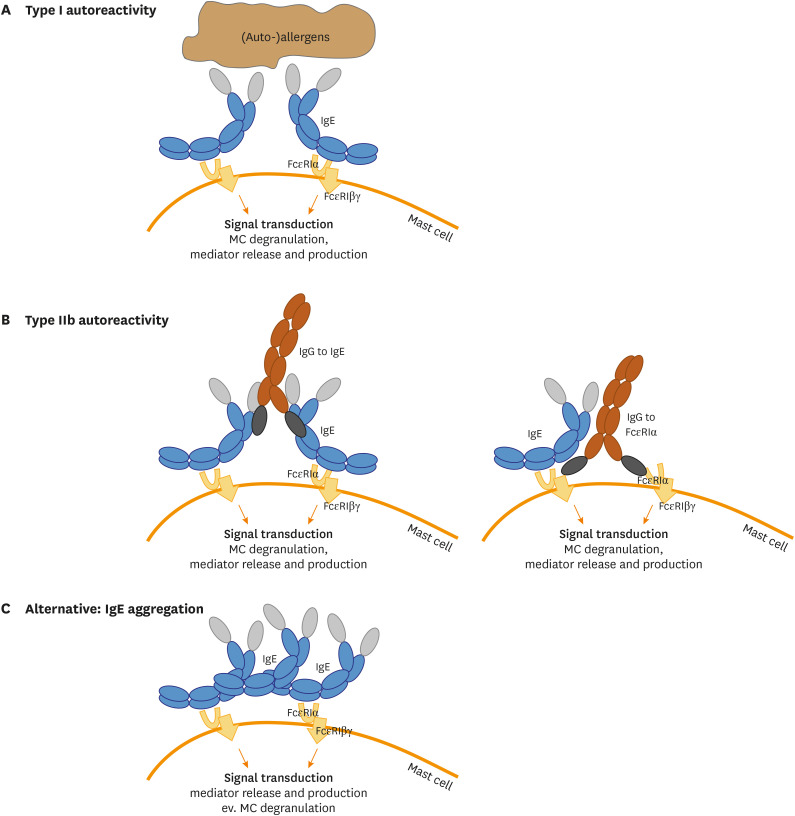
Our current understanding of the underlying pathophysiology of CSU suggests that there are at least 2 endotypes of CSU.37,38,39 In Type I autoimmune CSU (TIaiCSU; also referred to as autoallergic CSU) autoreactive IgE antibodies directed against autoantigens are thought to degranulate skin mast cells via the classical activation of the high affinity IgE receptor FcɛRIα.40 In Type IIb autoimmune CSU (TIIbaiCSU), mast cell degranulation is caused by IgG and/or IgM autoantibodies directed against FcɛRIα or FcɛRIα-bound IgE (Fig. 2A and B).41 IgE, therefore, plays a key role in the pathogenesis of CSU and is an important driver of mast cell degranulation. Other mast cell-activating and -priming signals and mechanisms that are held to contribute to the pathogenesis of CSU include IgE stacking (Fig. 2C) and aggregation, alarmins, ligands of MRGPRX2, activated complement components as well as cytokines, but their roles are less well defined and understood.
Fig. 2. IgE can exert MC activation via different mechanisms in CSU. (A) IgE bound to the high-affinity receptor subunit alpha on the surface of MC can detect (auto-)allergens and by recognition of 2 IgE molecules in close proximity downstream signal transduction is exerted, leading to MC degranulation and subsequent mediator production (type I CSU). (B) In CSU the occurrence of IgG autoantibodies directed against IgE (left) or against the high affinity receptor subunit alpha (right) can result in high affinity receptor cross-linking and MC activation (type IIb CSU). (C) Further alternative mechanisms could also lead to MC activation, e.g. unspecific IgE antibody stacking has been shown to lead to mediator production and release.
IgE, immunoglobulin E; MC, mast cell; CSU, chronic spontaneous urticaria.
THE ROLE OF IgE IN TIaiCSU
Patients with TIaiCSU are thought to have autoreactive IgE antibodies to self-antigens such as thyroid peroxidase (TPO),42,43,44 tissue factor (TF), thyroglobulin (TG),45 double stranded DNA (dsDNA),46 and IL-24.40 The rate of TIaiCSU patients has not been established because commercially available and standardized and validated assays are not yet available. Different studies show different rates of patients with IgE to individual autoallergens, ranging, for TPO for example, from 8%47 to 54%,42 possibly depending on the used ELISA detection method. As of now, more than 200 autoallergens have been reported to be targets of IgE in CSU patients40 and most of the IgE of CSU patients have autoreactive IgE,48 very different from the IgE in healthy controls, where less than 1% of all IgE binds to autoantigens.
Is the presence of auto-IgE in patients with TIaiCSU linked to elevated serum total IgE levels? Three studies showed that this is indeed the case reporting significantly higher total IgE levels in patients with IgE to TPO compared to those without,43,44,47 but a study did not.42
THE ROLE OF IgE IN TIIbaiCSU
Antigen-IgE interactions are not involved in the degranulation of mast cells that drive the development of wheals and angioedema in TIIbaiCSU. In TIIbaiCSU, mast cell degranulation is due to IgG or IgM autoantibodies to the high-affinity IgE receptor FcεRIα,49,50 or IgE bound to it.51 The ability of these autoantibodies to degranulate mast cells is linked to the expression levels of its targets, FcεRIα and IgE, on the surface of mast cells.52 Total IgE levels are known to regulate FcεRI and FcεRI-bound IgE expression levels, with low and high IgE levels linked to low/high FcεRI and bound IgE expression levels, respectively. Also, IgE bound to FcεRI can inhibit the activation of mast cells by autoantibodies directed to FcεRI.53 Accordingly, total IgE levels could modulate TIIbaiCSU disease activity.
TIIbaiCSU is identified by the combination of positive results of autologous serum skin testing, basophil testing, and tests for FceRI/IgE autoantibodies.54 Also, in the only study that used all the 3 tests in CSU patients, less than 10% had TIIbaiCSU, but many tested patients were positive for one or 2 markers.38 In this study, patients with TIIbaiCSU had markedly lower total IgE levels, 22 IU/mL, as compared with 102 IU/mL in non-TIIbaiCSU patients (P < 0.001).38 TIIbaiCSU patients also had a higher rate of low total IgE (< 40 IU/mL), 86%, than non-TIIbaiCSU patients (26%, P < 0.01).38
In support of these findings, most studies on total IgE in ASST-positive CSU patients also reported to be lower levels,20,38,55 but one study each found similar36 and higher levels.14 Also, Baioumy et al.13 reported lower total IgE levels in CSU patients with IgG to FcεRIα, although the difference in CSU patients without these autoantibodies was not significant. Why total IgE levels are reduced in patients with TIIbaiCSU is currently unknown.
Taken together, TIaiCSU is thought to be linked to normal/high total IgE levels, and there is strong evidence that TIIbaiCSU is linked to low and very low total IgE levels. Are these 2 endotypes also linked to differences in the biological and functional properties of IgE?
DIFFERENCES IN THE BIOLOGY AND FUNCTION OF IgE BETWEEN TIaiCSU AND TIIbaiCSU
In a recent study, CSU patients had more lipophilic IgE than healthy controls, and IgE lipophilicity correlated with autoreactivity (r = 0.8; P < 0.0001).48 It is currently unclear why IgE in patients with TIaiCSU is more lipophilic; however, glycosylation patterns may be involved. IgE is heavily glycosylated (Supplementary Fig. S1),56 and recent publications showed that modifications of the glycosylation pattern affect IgE-receptor binding and allergenic pathogenicity.57,58 The glycosylation of IgE is thought to be important for its 3-dimensional folding and bending and its lipophilicity (Supplementary Fig. S1B).56 Higher lipophilicity may promote IgE aggregation and stacking, as is seen with highly cytokinergic IgE,59 which can crosslink FcɛRI without antigen binding, initiate cytokine production in mast cells (MCs) (Fig. 2C),60 and shows polyreactivity to various self-antigens (β-galactosidase, dsDNA, TG, ssDNA and histamine releasing factor) that induce MC degranulation.61,62,63
The IgE in CSU patients with low levels often fails to increase upon treatment with omalizumab. Omalizumab usually results in a rapid and sustained increase in total IgE due to the prolongation of the half-life of IgE after binding to omalizumab. In a recent study, 60 of 63 (95%) CSU patients with IgE higher than 43 IU/mL, but only 17 of 33 (52%) patients with low IgE (≤ 43 IU/mL), increased their IgE level by at least 100% during the first 4 weeks of omalizumab treatment.27 Failure to double IgE levels by week 4 predicted non-response to treatment. This indicates that the IgE in patients with TIIbaiCSU is not only often low but also functionally different in regard to its binding to omalizumab, and that this difference is linked to impaired treatment responses. Further studies need to clarify, in detail, how IgE in patients with TIaiCSU and TIIbaiCSU differs from IgE in healthy individuals.
IS TOTAL IgE A MARKER AND PREDICTOR FOR THE RESPONSE OF CSU PATIENTS TO THERAPY?
A second-generation antihistamine (sgAH) at a standard dose is the first-line treatment of CSU. Updosing can improve the response to sgAH treatment in some patients.64 If this fails, omalizumab treatment is needed and recommended, with ciclosporin as a fourth-line fall back option.
Total IgE levels were similar in responders and non-responders to sgAH treatment in 4 CSU studies with at least 95 patients each.20,65,66,67 In contrast, most studies on total IgE and omalizumab treatment showed that low IgE levels predict non-response in patients with CSU (Supplementary Table S2). For example, Straesser et al.23 reported that 48% of patients with low total IgE (≤15 IU/mL), but more than 85% of those with IgE levels higher than 15 IU/mL, benefitted from omalizumab treatment.23 In another study, low IgE levels (≤ 43 IU/mL) were linked to benefit in 66% of treated patients as compared with 95% in those with higher IgE levels.27 Weller and colleagues26 reported low IgE levels in 40% of CSU patients who showed no response to omalizumab treatment, as compared with 27% and 3% of patients with normal and complete response, respectively.
Total IgE does not appear to be linked to the onset time of treatment responses to omalizumab in CSU patients. A large-scale retrospective study found no difference in total IgE levels between early complete, late complete and late partial responders to omalizumab.68 Another more recent and smaller study reported a tendency to higher IgE in early responders.69 In contrast, high elevated IgE levels in 1 study were linked to a shorter time to relapse in CSU patients who stopped treatment.70 However, 2 subsequent studies failed to confirm this.68,69
Two smaller studies reported that IgE levels are also linked to the outcome of cyclosporine treatment. In 1 study, serum IgE levels were significantly lower in cyclosporine responders vs. non-responders and were negatively correlated with the benefit of treatment.71 The other study showed that patients with high IgE levels had lower rates of response than those with low IgE.72
Taken together, serum total IgE levels can help predict the outcome of omalizumab and cyclosporine treatment. There is strong evidence that low levels are linked to poor response to omalizumab but good response to cyclosporine, whereas some reports point towards a connection of normal and high IgE levels and good response to omalizumab and maybe a poor response to cyclosporine (Supplementary Table S2).
DO IgE LEVELS CHANGE IN RESPONSE TO TREATMENT OR SPONTANEOUS REMISSION OF CSU?
In CSU, changes in total IgE levels, as of yet, have only been studied in patients treated with omalizumab, quilizumab, or acupuncture. Omalizumab leads to an increase in total IgE in most CSU patients (Supplementary Table S3). Patients with low IgE before treatment, in 1 study, showed lower rates of elevated total IgE in week 4 of omalizumab treatment, and this was linked to non-response.27 In a proof-of-concept study, quilizumab, which targets the M1-prime segment of membrane-expressed IgE, reduced median serum total IgE level by 30%, but did not result in clinically meaningful improvements.73 One small acupuncture study in CSU reported significant drops in total IgE levels of about 40%,74 but did not report the clinical effects of treatment. As of now, there is no study on the change of total IgE levels after spontaneous remission of CSU (supplementary Table S3).
Autoallergen-specific IgE, i.e., IgE-anti-TPO, was reported to be increased in CSU patients who experience symptom exacerbation.44 Serum IgE-anti-IL-24 levels, but not IgG-anti-IL-24 serum levels, were significantly decreased in responders, but not in non-responders to autologous serum therapy.75,76
DISCUSSION AND SUMMARY: VALUE OF MEASURING TOTAL IgE IN CSU
High total IgE levels may point to high disease activity, longer disease duration, TIaiCSU, a high chance to respond to omalizumab treatment, a quick relapse after stopping omalizumab, and a lower chance of responding to cyclosporine. Low IgE, in turn, may point to TIIbaiCSU, a reduced chance to respond to omalizumab and a better chance to benefit from cyclosporine treatment. This makes total IgE a valuable marker, and we recommend including its assessment in the routine diagnostic work up of patients with CSU.
Does the knowledge of a patient's total IgE level help decide which treatment is best? At present, it does not. Total IgE does not appear to predict the response to first- and second-line treatment, a standard and higher than standard-dose antihistamine, respectively. Omalizumab is the best treatment option for patients who fail to respond to antihistamine treatment, regardless of their total IgE levels. However, low total IgE, especially in combination with elevated IgG-anti-TPO38 should have us suspect that a patient has TIIbaiCSU and should, therefore, be monitored closely.
CONCLUSIONS
Taken together, there is strong circumstantial evidence that total IgE is currently one of the best generally available surrogate markers for the discrimination of TIaiCSU and TIIbaiCSU. However, many questions remain to be answered. These include, but are not limited to: What exactly are the clinical differences in CSU patients at different total IgE serum levels? Why is total IgE reduced in TIIbaiCSU? Does total IgE, in patients with low and high levels, return to normal after CSU remission occurs? Are normal and high levels of total IgE a marker of response to omalizumab and cyclosporine? What is an optimal cutoff value for low total IgE? Further studies are needed to address these questions, and, answering them will help advance our understanding of CSU and improve its treatment.
ACKNOWLEDGMENTS
We thank Beate Schinzel for help with manuscript submission.
Pavel Kolkhir, Polina Pyatilova and Sherezade Moñino-Romero were supported by a GA2LEN fellowship. This project benefitted from the support of the GA2LEN network of urticaria centers of references and excellence (UCAREs; www.ga2len-ucare.com) and the “Russian Academic Excellence Project 5-100”. Carolin Steinert was supported by the DGF Grant KFO 339.
Footnotes
Disclosure: Sabine Altrichter is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, AstraZeneca, Moxie, Sanofi and ThermoFisher.
Pavel Kolkhir is or recently was a speaker for Novartis and Roche.
Frank Siebenhaar is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Aralez, Blueprint, Glenmark, Novartis, Pediapharm and Uriach.
Martin Church has been a speaker or consultant for Almirall, FAES Pharma, Menarini, Moxie, MSD, Novartis, UCB Pharma, Sanofi-Aventis and Uriach.
Marcus Maurer is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Gilead, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach.
Jie Shen Fok, Jörg Scheffel, Qingqing Jiao, Polina Pyatilova, Carolin Steinert, Dorothea Terhorst-Molawi, Sherezade Moñino-Romero, and Yi-Kui Xiang have no conflict of interests.
SUPPLEMENTARY MATERIALS
Total serum IgE in CSU
IgE as a marker for therapeutic response
Changes of IgE upon treatment or natural cession
Secreted human IgE has 7 glycosilation sites and is the most glycosylated immunoglobuline in the human system except IgA2. Glycosilation sites are within proximity of the important high and low affinity IgE receptor binding sites (A). Naturally in solution IgE occurs in a bended U-shaped form (B).
References
- 1.Fricke J, Ávila G, Keller T, Weller K, Lau S, Maurer M, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75:423–432. doi: 10.1111/all.14037. [DOI] [PubMed] [Google Scholar]
- 2.Saini S, Shams M, Bernstein JA, Maurer M. Urticaria and angioedema across the ages. J Allergy Clin Immunol Pract. 2020;8:1866–1874. doi: 10.1016/j.jaip.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 4.Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282:232–247. doi: 10.1111/imr.12632. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381:1321–1332. doi: 10.1056/NEJMoa1900408. [DOI] [PubMed] [Google Scholar]
- 6.Williams P, Sewell WA, Bunn C, Pumphrey R, Read G, Jolles S. Clinical immunology review series: an approach to the use of the immunology laboratory in the diagnosis of clinical allergy. Clin Exp Immunol. 2008;153:10–18. doi: 10.1111/j.1365-2249.2008.03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HY, Choi J, Ahn K, Hahm MI, Lee SY, Kim WK, et al. Reference values and utility of serum total immunoglobulin E for predicting atopy and allergic diseases in Korean schoolchildren. J Korean Med Sci. 2017;32:803–809. doi: 10.3346/jkms.2017.32.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott H, Stanzel S, Ocklenburg C, Merk HF, Baron JM, Lehmann S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta Derm Venereol. 2009;89:257–261. doi: 10.2340/00015555-0627. [DOI] [PubMed] [Google Scholar]
- 9.Dodig S, Richter D, Benko B, Zivcić J, Raos M, Nogalo B, et al. Cut-off values for total serum immunoglobulin E between non-atopic and atopic children in north-west Croatia. Clin Chem Lab Med. 2006;44:639–647. doi: 10.1515/CCLM.2006.092. [DOI] [PubMed] [Google Scholar]
- 10.Campos A, Reyes J, Blanquer A, Liñares T, Torres M. Total serum IgE: adult reference values in Valencia (1981-2004). Usefulness in the diagnosis of allergic asthma and rhinitis. Allergol Immunopathol (Madr) 2005;33:303–306. doi: 10.1016/s0301-0546(05)73247-x. [DOI] [PubMed] [Google Scholar]
- 11.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy. 1974;4:265–271. doi: 10.1111/j.1365-2222.1974.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 12.Demirkan S, Baççıoğlu A. Rationale for the autologous serum skin test in acute versus chronic urticaria. Postepy Dermatol Alergol. 2019;36:703–706. doi: 10.5114/ada.2019.91421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baioumy SA, Esawy MM, Shabana MA. Assessment of circulating FCεRIa in chronic spontaneous urticaria patients and its correlation with clinical and immunological variables. Immunobiology. 2018;223:807–811. doi: 10.1016/j.imbio.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Kessel A, Helou W, Bamberger E, Sabo E, Nusem D, Panassof J, et al. Elevated serum total IgE--a potential marker for severe chronic urticaria. Int Arch Allergy Immunol. 2010;153:288–293. doi: 10.1159/000314370. [DOI] [PubMed] [Google Scholar]
- 15.Staubach P, Vonend A, Burow G, Metz M, Magerl M, Maurer M. Patients with chronic urticaria exhibit increased rates of sensitisation to Candida albicans, but not to common moulds. Mycoses. 2009;52:334–338. doi: 10.1111/j.1439-0507.2008.01601.x. [DOI] [PubMed] [Google Scholar]
- 16.Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59:869–873. doi: 10.1111/j.1398-9995.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 17.Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7. doi: 10.1016/j.jdermsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Deza G, Bertolin-Colilla M, Sanchez S, Soto D, Pujol RM, Gimeno R, et al. Basophil FcɛRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;141:2313–2316.e1. doi: 10.1016/j.jaci.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;73:57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.de Montjoye L, Darrigade AS, Gimenez-Arnau A, Herman A, Dumoutier L, Baeck M. Correlations between disease activity, autoimmunity and biological parameters in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2020 doi: 10.23822/EurAnnACI.1764-1489.132. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 21.Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS One. 2018;13:e0207602. doi: 10.1371/journal.pone.0207602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017;7:17797. doi: 10.1038/s41598-017-18187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2018;6:1386–1388.e1. doi: 10.1016/j.jaip.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salman A, Comert E. The real-life effectiveness and safety of omalizumab updosing in patients with chronic spontaneous urticaria. J Cutan Med Surg. 2019;23:496–500. doi: 10.1177/1203475419847956. [DOI] [PubMed] [Google Scholar]
- 25.Frossi B, De Carli S, Bossi F, Pucillo C, De Carli M. Co-occurrence of chronic spontaneous urticaria with immunoglobulin a deficiency and autoimmune diseases. Int Arch Allergy Immunol. 2016;169:130–134. doi: 10.1159/000445058. [DOI] [PubMed] [Google Scholar]
- 26.Weller K, Ohanyan T, Hawro T, Ellrich A, Sussman G, Koplowitz J, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 27.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73:705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 28.Gao C, Chen WC, Liu W, Chen Q, Chen S, Xu Y, et al. Pathogenic role of circulating CD4+CXCR5+ cell subpopulations in patients with chronic spontaneous urticarial. Am J Transl Res. 2020;12:4434–4444. [PMC free article] [PubMed] [Google Scholar]
- 29.Baek YS, Jeon J, Kim JH, Oh CH. Severity of acute and chronic urticaria correlates with D-dimer level, but not C-reactive protein or total IgE. Clin Exp Dermatol. 2014;39:795–800. doi: 10.1111/ced.12413. [DOI] [PubMed] [Google Scholar]
- 30.Rauber MM, Pickert J, Holiangu L, Möbs C, Pfützner W. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy. 2017;72:1904–1911. doi: 10.1111/all.13215. [DOI] [PubMed] [Google Scholar]
- 31.Saini SS. Basophil responsiveness in chronic urticaria. Curr Allergy Asthma Rep. 2009;9:286–290. doi: 10.1007/s11882-009-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolkhir P, Church MK, Altrichter S, Skov PS, Hawro T, Frischbutter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8:318–325.e5. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Deza G, Bertolín-Colilla M, Pujol RM, Curto-Barredo L, Soto D, García M, et al. Basophil FcεRI Expression in Chronic Spontaneous Urticaria: A Potential Immunological Predictor of Response to Omalizumab Therapy. Acta Derm Venereol. 2017;97:698–704. doi: 10.2340/00015555-2654. [DOI] [PubMed] [Google Scholar]
- 34.Asero R, Marzano AV, Ferrucci S, Genovese G, Cugno M. Baseline D-dimer plasma levels correlate with disease activity but not with the response to omalizumab in chronic spontaneous urticaria. Allergy. 2019;74:2538. doi: 10.1111/all.13936. [DOI] [PubMed] [Google Scholar]
- 35.Ghazanfar MN, Holm JG, Thomsen SF. Effectiveness of omalizumab in chronic spontaneous urticaria assessed with patient-reported outcomes: a prospective study. J Eur Acad Dermatol Venereol. 2018;32:1761–1767. doi: 10.1111/jdv.15045. [DOI] [PubMed] [Google Scholar]
- 36.Ye YM, Park JW, Kim SH, Ban GY, Kim JH, Shin YS, et al. Prognostic factors for chronic spontaneous urticaria: a 6-month prospective observational study. Allergy Asthma Immunol Res. 2016;8:115–123. doi: 10.4168/aair.2016.8.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer M, Eyerich K, Eyerich S, Ferrer M, Gutermuth J, Hartmann K, et al. Urticaria: Collegium Internationale Allergologicum (CIA) update 2020. Int Arch Allergy Immunol. 2020;181:321–333. doi: 10.1159/000507218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoepke N, Asero R, Ellrich A, Ferrer M, Gimenez-Arnau A, E H Grattan C, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST study. Allergy. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 39.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772–1781.e1. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 40.Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;142:876–882. doi: 10.1016/j.jaci.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CE, et al. Classification of anti-FcεRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002;110:492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 42.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez J, Sánchez A, Cardona R. Causal relationship between anti-TPO IgE and chronic urticaria by in vitro and in vivo tests. Allergy Asthma Immunol Res. 2019;11:29–42. doi: 10.4168/aair.2019.11.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez J, Sánchez A, Cardona R. Clinical characterization of patients with chronic spontaneous urticaria according to anti-TPO IgE levels. J Immunol Res. 2019;2019:4202145. doi: 10.1155/2019/4202145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cugno M, Asero R, Ferrucci S, Lorini M, Carbonelli V, Tedeschi A, et al. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy. 2018;73:2408–2411. doi: 10.1111/all.13587. [DOI] [PubMed] [Google Scholar]
- 46.Hatada Y, Kashiwakura J, Hayama K, Fujisawa D, Sasaki-Sakamoto T, Terui T, et al. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int Arch Allergy Immunol. 2013;161(Suppl 2):154–158. doi: 10.1159/000350388. [DOI] [PubMed] [Google Scholar]
- 47.Shin YS, Suh DH, Yang EM, Ye YM, Park HS. Serum specific IgE to thyroid peroxidase activates basophils in aspirin intolerant urticaria. J Korean Med Sci. 2015;30:705–709. doi: 10.3346/jkms.2015.30.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakin E, Church MK, Maurer M, Schmetzer O. On the lipophilic nature of autoreactive IgE in chronic spontaneous urticaria. Theranostics. 2019;9:829–836. doi: 10.7150/thno.29902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschläger M, et al. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altrichter S, Zampeli V, Ellrich A, Zhang K, Church MK, Maurer M. IgM and IgA in addition to IgG autoantibodies against FcɛRIα are frequent and associated with disease markers of chronic spontaneous urticaria. Allergy. 2020 doi: 10.1111/all.14412. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 51.Fiebiger E, Stingl G, Maurer D. Anti-IgE and anti-Fc epsilon RI autoantibodies in clinical allergy. Curr Opin Immunol. 1996;8:784–789. doi: 10.1016/s0952-7915(96)80005-7. [DOI] [PubMed] [Google Scholar]
- 52.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 53.Kawakami T, Kitaura J, Xiao W, Kawakami Y. IgE regulation of mast cell survival and function. Novartis Found Symp. 2005;271:100–107. [PubMed] [Google Scholar]
- 54.Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27–36. doi: 10.1111/all.12056. [DOI] [PubMed] [Google Scholar]
- 55.Abd El-Azim M, Abd El-Azim S. Chronic autoimmune urticaria: frequency and association with immunological markers. J Investig Allergol Clin Immunol. 2011;21:546–550. [PubMed] [Google Scholar]
- 56.Shade KT, Conroy ME, Anthony RM. IgE glycosylation in health and disease. Curr Top Microbiol Immunol. 2019;423:77–93. doi: 10.1007/82_2019_151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Epp A, Sullivan KC, Herr AB, Strait RT. Immunoglobulin glycosylation effects in allergy and immunity. Curr Allergy Asthma Rep. 2016;16:79. doi: 10.1007/s11882-016-0658-x. [DOI] [PubMed] [Google Scholar]
- 58.Shade KC, Conroy ME, Washburn N, Kitaoka M, Huynh DJ, Laprise E, et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature. 2020;582:265–270. doi: 10.1038/s41586-020-2311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bax HJ, Bowen H, Beavil RL, Chung R, Ward M, Davies AM, et al. IgE trimers drive SPE-7 cytokinergic activity. Sci Rep. 2017;7:8164. doi: 10.1038/s41598-017-08212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bax HJ, Bowen H, Dodev TS, Sutton BJ, Gould HJ. Mechanism of the antigen-independent cytokinergic SPE-7 IgE activation of human mast cells in vitro . Sci Rep. 2015;5:9538. doi: 10.1038/srep09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashiwakura J, Okayama Y, Furue M, Kabashima K, Shimada S, Ra C, et al. Most highly cytokinergic IgEs have polyreactivity to autoantigens. Allergy Asthma Immunol Res. 2012;4:332–340. doi: 10.4168/aair.2012.4.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MA, Park HS. Highly cytokinergic IgE antibodies and autoimmune mechanisms. Allergy Asthma Immunol Res. 2012;4:311–312. doi: 10.4168/aair.2012.4.6.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sánchez-Borges M, Capriles-Hulet A, Caballero-Fonseca F, González-Aveledo L. Justification for IgE as a therapeutic target in chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2017;49:148–153. doi: 10.23822/eurannaci.1764-1489.02. [DOI] [PubMed] [Google Scholar]
- 64.Guillén-Aguinaga S, Jáuregui Presa I, Aguinaga-Ontoso E, Guillén-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1153–1165. doi: 10.1111/bjd.14768. [DOI] [PubMed] [Google Scholar]
- 65.Ulambayar B, Yang EM, Cha HY, Shin YS, Park HS, Ye YM. Increased platelet activating factor levels in chronic spontaneous urticaria predicts refractoriness to antihistamine treatment: an observational study. Clin Transl Allergy. 2019;9:33. doi: 10.1186/s13601-019-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trinh HK, Pham DL, Ban GY, Lee HY, Park HS, Ye YM. Altered systemic adipokines in patients with chronic urticaria. Int Arch Allergy Immunol. 2016;171:102–110. doi: 10.1159/000452626. [DOI] [PubMed] [Google Scholar]
- 67.Magen E, Mishal J, Zeldin Y, Schlesinger M. Clinical and laboratory features of antihistamine-resistant chronic idiopathic urticaria. Allergy Asthma Proc. 2011;32:460–466. doi: 10.2500/aap.2011.32.3483. [DOI] [PubMed] [Google Scholar]
- 68.Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol. 2019;33:918–924. doi: 10.1111/jdv.15350. [DOI] [PubMed] [Google Scholar]
- 69.Grieco T, Dies L, Sernicola A, Chello C, Gagliostro N, Carnicelli G, et al. Potential clinical and serological predictors of chronic spontaneous urticaria relapse in patients under omalizumab treatment. Immunotherapy. 2020;12:1173–1181. doi: 10.2217/imt-2020-0088. [DOI] [PubMed] [Google Scholar]
- 70.Ertas R, Ozyurt K, Ozlu E, Ulas Y, Avci A, Atasoy M, et al. Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;140:1749–1751. doi: 10.1016/j.jaci.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Santiago L, Ferreira B, Ramos L, Gonçalo M. IgE levels are negatively correlated with clinical response to ciclosporin in chronic spontaneous urticaria. Br J Dermatol. 2019;180:199–200. doi: 10.1111/bjd.17005. [DOI] [PubMed] [Google Scholar]
- 72.Endo T, Toyoshima S, Kanegae K, Izaki S, Nishimori N, Ito M, et al. Identification of biomarkers for predicting the response to cyclosporine A therapy in patients with chronic spontaneous urticaria. Allergol Int. 2019;68:270–273. doi: 10.1016/j.alit.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Harris JM, Cabanski CR, Scheerens H, Samineni D, Bradley MS, Cochran C, et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol. 2016;138:1730–1732. doi: 10.1016/j.jaci.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 74.Jianli C. The effect of acupuncture on serum IgE level in patients with chronic urticaria. J Tradit Chin Med. 2006;26:189–190. [PubMed] [Google Scholar]
- 75.Yu L, Buttgereit T, Stahl Skov P, Schmetzer O, Scheffel J, Kocatürk E, et al. Immunological effects and potential mechanisms of action of autologous serum therapy in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2019;33:1747–1754. doi: 10.1111/jdv.15640. [DOI] [PubMed] [Google Scholar]
- 76.Kocatürk E, Aktaş S, Türkoğlu Z, Kavala M, Zindanci I, Koc M, et al. Autologous whole blood and autologous serum injections are equally effective as placebo injections in reducing disease activity in patients with chronic spontaneous urticaria: a placebo controlled, randomized, single-blind study. J Dermatolog Treat. 2012;23:465–471. doi: 10.3109/09546634.2011.593485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total serum IgE in CSU
IgE as a marker for therapeutic response
Changes of IgE upon treatment or natural cession
Secreted human IgE has 7 glycosilation sites and is the most glycosylated immunoglobuline in the human system except IgA2. Glycosilation sites are within proximity of the important high and low affinity IgE receptor binding sites (A). Naturally in solution IgE occurs in a bended U-shaped form (B).