Abstract
This review summarises the recent evidence on preoperative therapeutic strategies in pancreatic cancer and discusses the rationale for an imminent need for a personalised therapeutic approach in non-metastatic disease. The molecular diversity of pancreatic cancer and its influence on prognosis and treatment response, combined with the failure of ‘all-comer’ treatments to significantly impact on patient outcomes, requires a paradigm shift towards a genomic-driven approach. This is particularly important in the preoperative, potentially curable setting, where a personalised treatment allocation has the substantial potential to reduce pancreatic cancer mortality.
Key words: Pancreatic cancer, preoperative, neoadjuvant, precision medicine, prognostic biomarkers, predictive biomarkers
Highlights
-
•
Molecular diversity of pancreatic cancer requires a paradigm shift towards a genomic-driven therapeutic approach.
-
•
Unselected treatment strategies demonstrate only limited efficacy in early-stage pancreatic cancer.
-
•
Personalised treatment in non-metastatic disease has potential to reduce pancreatic cancer mortality.
-
•
It is fundamental to implement preoperative clinical studies enriched for potential prognostic/predictive biomarkers.
-
•
Novel models of therapeutic development are warranted to accelerate progress in pancreatic cancer care and research.
Introduction
Pancreatic cancer (PC) is one of the most lethal solid malignancies and is predicted to soon become the second leading cause of cancer mortality in developed countries.1 Estimates of temporal trends for PC incidence and mortality produced by GLOBOCAN 2018 indicate a worldwide trend towards a dramatic increase of both incidence (+77.7% with 356 358 new cases) and mortality (+79.9%, 345 181 deaths) from 2018 to 2040.2 This is mostly due to our inability to improve prevention and treatment approaches, despite major efforts in preclinical and clinical research that have marginally impacted patient outcomes over the past 50 years. In fact, this incremental progress translates to an increased 5-year survival rate from 6% to only 9% in the years 2014-2018, resulting in a mortality/incidence ratio of 94%.2 There is indeed an urgent need to reduce both PC incidence, by implementing research on primary and secondary prevention, and mortality, by accelerating therapeutic development.
Surgery with radical intent represents the only potential curative treatment option for PC patients; however, only 20% of cases are diagnosed with anatomically resectable disease.3 Notwithstanding substantial improvement in surgical techniques and post-operative outcomes, the overall recurrence rate after resection is approximately 85% and the 5-year survival less than 30%.4, 5, 6, 7 The best adjuvant chemotherapy regimen (modified FOLFIRINOX, i.e. 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan) likely adds modest survival benefit in all-comers at the expense of a considerable toxicity.7 The reasons for these poor outcomes stem from the inherent aggressiveness of PC, that lends it to be defined as ‘metastatic ab-initio’ disease, irrespective of the clinical stage. In fact, up to 26% of patients are found with occult metastases during surgical exploration,8 and approximately 70% of resected cases have nodal involvement on pathology after surgery.9 Furthermore, despite the importance of adjuvant therapy, studies demonstrated that up to 45% of patients are not able to receive the treatment after resection due to poor performance status, post-operative morbidity, or early progression of disease.10,11 Besides, fully completed adjuvant chemotherapy is an independent prognostic factor for survival after resection; however, only 55%-75% of those who initiate adjuvant therapy complete the treatment.12 In this context, increasing interest has been driven towards primary systemic treatments, initially investigated in borderline resectable and locally advanced PC with induction/cytoreductive intent13,14 and, more recently, applied to patients with resectable disease as a pure neoadjuvant (NAT) strategy.15,16 Preoperative treatment has been associated with several potential benefits including in-vivo chemosensitivity test, tumour shrinking with decreased nodal involvement, increased margin-negative resection rates, early treatment of occult micrometastases, improved compliance with chemotherapy, improved survival after curative resection, and better selection of patients who are more likely to benefit from surgery.17, 18, 19, 20 However, the role of NAT in PC is still debated due to a relative lack of robust clinical trial data supporting this approach.21, 22, 23 Particularly, several barriers have limited its application and data interpretation including the low response rate to chemotherapy in metastatic disease, the difficulty in assessing the impact of pathologic complete response (pCR) on survival in retrospective studies, the inaccuracy of radiological modalities to adequately define the therapeutic response, and the poor interobserver agreement in defining baseline resectability status.23, 24, 25
Preoperative therapy in PC: state of the art
Preoperative treatments, including chemotherapy and radiotherapy, have been investigated in the three different clinical scenarios of non-metastatic PC: borderline resectable, locally advanced unresectable, and resectable disease, as defined in Table 1.26, 27, 28, 29, 30, 31
Table 1.
Criteria defining resectability status at diagnosis and associated standard treatments
| Resectability statusa | NCCN31 | IAP consensus30 | Standard treatmentb |
|---|---|---|---|
| R |
|
|
Surgery followed by adjuvant treatment. Consider staging laparoscopy and neoadjuvant therapy, particularly in high-risk patients.c |
| BR |
Pancreatic head/uncinate process:
|
Subclassified according to SMV/PV involvement alone or arterial invasion. BR-PV (SMV/PV involvement alone)
|
Neoadjuvant therapy followed by surgery Consider staging laparoscopy |
| LA | Unreconstructible SMV/PV due to tumour involvement or occlusion (can be due to tumour or bland thrombus). Head/uncinate process:
|
|
Clinical trial (preferred). Induction chemotherapy (preferably 4-6 months) followed by chemoradiation or stereotactic body RT (SBRT) in selected patients (locally advanced without systemic metastases) or chemoradiation, or SBRT in selected patients who are not candidates for combination therapy. |
Different classifications, based on the anatomic contact on imaging of tumour and blood vessel, have been proposed and adapted over time [MD Anderson Cancer Center (MDACC) guidelines,30 the Americas Hepato-Pancreato-Biliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract (AHPBA/SSO/SSAT) expert consensus guidelines,26 the Intergroup Alliance,27 the International Study Group of Pancreatic Surgery (ISGPS) criteria,28 and NCCN guidelines31]. Recently, the consensus statement of the International Association of Pancreatology (IAP) added biological and conditional host-related factors to the classification based on imaging, including serum CA 19-9 of >500 IU/ml and/or positive regional lymph node metastases, and performance status of 2 or more.29
AO: aorta; BR: borderline resectable; CA: celiac axis; CHA: common hepatic artery; IVC: inferior vena cava; LA: locally advanced unresectable; PHA: proper hepatic artery; PV: portal vein; R; resectable; RT, radiotherapy; SMA: superior mesenteric artery; SMV: superior mesenteric vein.
Decisions about resectability status should be made in consensus at multidisciplinary discussions.
Participation in clinical trials is especially encouraged.
High-risk patients: CA 19-9 more than 500 IU/ml, regional lymph node metastasis (biopsy or PET-CT), poor performance status (Eastern Cooperative Oncology Group score = 2, or more).
In borderline resectable PC the use of NAT has been associated with increased radical resection rates and superior overall survival in meta-analysis including cohort studies, retrospective observations, and phase I/II clinical trials.32,33 More recently, the first randomised phase III trial conducted in this setting (the Dutch PREOPANC trial), comparing preoperative chemoradiotherapy versus immediate surgery in patients with resectable or borderline resectable PC, showed that patients with borderline resectable tumours had significantly improved overall survival (OS), disease-free survival (DFS), and locoregional failure-free interval (LFFI) for preoperative chemoradiotherapy.34
In resectable disease the effectiveness of NAT is still uncertain, with conflicting results on survival benefit compared with upfront surgery.3,35 In one of the largest retrospective studies comparing NAT followed by resection and upfront resection, the NAT group was associated with improved survival compared with standard strategy [median survival, 26 months versus 21 months, respectively, hazard ratio (HR) 0.72; 95% confidence interval (CI), 0.68-0.78]. Patients in the upfront resected group had statistically significant higher pathologic T stage, positive lymph nodes, and positive resection margin. Compared with a subset of upfront resected patients who received adjuvant therapy, NAT patients had a better survival (median survival, 26 months vs 23 months, respectively, HR 0.83; 95% CI, 0.73-0.89).18 In addition, two recent systematic reviews and meta-analyses investigated the survival gain of NAT over standard treatment in patients with resectable tumour. Despite the significant improvement of radical resection rate, and the reduction of lymph nodes involvement, these studies did not show sufficient evidence for survival benefit of NAT when compared with upfront surgery (HR 0.96; 95% CI, 0.82-1.1215 and HR 0.86; 95% CI, 0.73-1.0321). However, in Lee et al., the subgroup of patients who completed NAT with subsequent resection had significantly increased survival than surgery followed by adjuvant treatment (HR 0.82; 95% CI, 0.71-0.93).15 Thus, a marginal favourable outcome in patients treated with NAT compared with those treated with the standard strategy may support this approach in resectable tumours. Despite these promising data, further randomised prospective studies are necessary to clearly establish the role of NAT in resectable PC.
The situation is different for patients with locally advanced unresectable tumours where systemic cytotoxic therapy is considered the first-choice treatment modality.31 Conversion surgery should be considered at multidisciplinary meetings and proposed in selected cases with optimal response after induction treatment, and only in specialised institutions. In a patient-level meta-analysis conducted on patients with locally advanced PC who underwent surgical resection after induction FOLFIRINOX, the percentage of conversion surgery ranged from 0% to 43% with a pooled percentage of 26% and an R0 rate between 50% and 100%.36 Due to conflicting results, it is still debatable whether patients should receive further ‘local regional’ therapy such as sequential chemoradiation or stereotactic body radiation therapy (SBRT) following induction chemotherapy.37,38
To summarise, current guidelines recommend NAT for borderline resectable PC, while upfront surgery followed by adjuvant treatment is still the standard recommendation for resectable disease except in cases that are high-risk for major abdominal surgery or in patients with high-risk characteristics (i.e. suspicion of advanced disease based on imaging findings or on significantly elevated carbohydrate antigen 19-9, large primary tumours or regional lymph nodes involvement, uncontrolled pain or excessive weight loss)39, 40, 41 (Table 1).
In locally advanced unresectable PC, primary systemic therapy constitutes the initial choice, and in some cases the addition of locoregional therapy can be considered for local control36,42,43 (Table 1).
Guidelines suggest the following options for preoperative treatment: FOLFIRINOX, modified FOLFIRINOX (m-FOLFIRINOX), gemcitabine, or gemcitabine/nab-paclitaxel.39, 40, 41,44 Multimodal treatment with chemoradiotherapy can be considered in selected cases, but the conclusions about its efficacy are controversial.9,13,37,45, 46, 47 Additional strategies such as perioperative treatments showed early promising results but need further investigation.48
Importantly, when preoperative therapy is indicated, current guidelines advice to refer patients to high-volume centres and encourage participation in clinical trials considering the limited evidence to recommend specific neoadjuvant regimens off-study.39
It is worth highlighting that the aforementioned recommendations on preoperative treatment in non-metastatic PC produced by the most important international cancer societies, are based on systematic reviews of cohort studies (Oxford Levels of Evidence category 2A) due to the lack of large phase III randomised controlled trials conducted in this setting.39, 40, 41,44 Additionally, results of published studies are often confounded by low patient numbers and lack of consensus regarding the definition of what precisely constitutes resectable, borderline resectable, and locally advanced—unresectable—disease.49 Thus, considering the overall lack of high-quality data from randomised controlled trials, several queries still need to be addressed such as the optimal candidates for preoperative treatment, the optimal treatment and number of therapeutic cycles, the timing of surgery after treatment, the additional benefit of sequential post-operative therapy (perioperative strategy), as well as the role of radiotherapy.50
Clinical relevance of PC molecular subtyping
To date, all available evidence on preoperative treatment relies on studies with a ‘one-size-fits-all’ design, without the use of a prognostic or predictive biomarker-based selection process. This ‘all-comers’ approach has widely characterised the drug development process in PC and has been associated with a series of failures during the past 50 years, with only modest gain in survival obtained with polychemotherapy regimens in undefined patients subgroups.51 Recent insights from modern next-generation sequencing (NGS) technologies, shed light on the biological rationale of the disappointing results achieved so far. Indeed, PC is characterised by high molecular heterogeneity which results in different clinical behaviours among patients with similar tumour characteristics and presentation, including prognosis and treatment response/resistance.52, 53, 54 Over the last decade, several attempts to subtype PC based on commonly altered molecular networks have been made. This has led to the identification of subgroups based on genomic and transcriptomic analysis, sharing similar biological and clinical characteristics.
Genomic subtypes
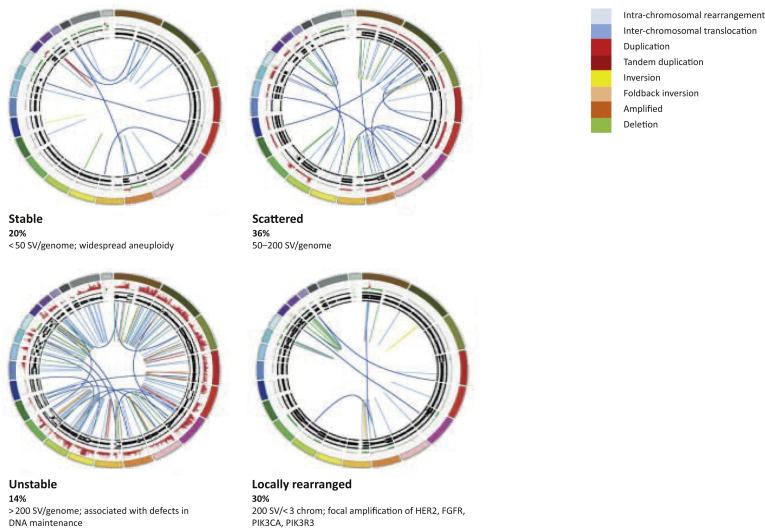
Whole-genome sequencing (WGS) allowed the classification of PC into four subtypes according to the frequency and distribution of structural variation of the genome: stable genomes (<50 structural variants per genome); scattered genomes (5-200 structural variants per genome); locally rearranged genomes (>200 structural variants clustered on <3 chromosomes); or unstable genomes (>200 structural variants distributed across the genome) (Figure 1).53 One of the most clinically meaningful subclasses resulting from this classification is represented by unstable tumours. Interestingly, in this group, a number of structural variants >558 was associated with significant defects in DNA damage response (DDR), particularly in homologous recombination repair (HRR) system. Additionally, genomic instability co-segregated with inactivation of DNA maintenance genes (BRCA1, BRCA2, or PALB2) and a mutational signature of DDR deficiency.53 Overall, alterations in DDR/HRR pathway were found in 24% of patients and were associated retrospectively with response to platinum-based chemotherapy.53 This finding had important clinical implications as it defined homologous recombination deficiency (HRD) as potential biomarker of therapeutic vulnerability to DNA damage agents, such as platinum and poly-ADP ribose polymerase inhibitors (PARPi).55, 56, 57, 58, 59, 60
Figure 1.
Whole-genome characterization of pancreatic cancer.
Subtypes of pancreatic cancer based on the number and pattern of chromosomal structural variants (SV). The coloured outer rings are chromosomes, the next ring represents copy-number changes (red = gain, green = loss), the inner rings represents allele frequency. The inner lines represent chromosome structural rearrangements detected by whole-genome paired sequencing and the legend indicates the type of rearrangement. Reprinted by permission from Macmillan Publishers Ltd.: Nature 518:495-501, copyright 2015.
Importantly, within HRD, germline mutations in BRCA1 and BRCA2 genes (which are the best characterised cause of HRD) have been associated with response to the PARP inhibitor olaparib in a phase III clinical trial (POLO) conducted in metastatic, platinum-sensitive, PC patients (first line setting).61 This was the first phase III trial that targeted a clinically relevant predictive biomarker in PC. This resulted in practice-changing governance with the approval of olaparib as maintenance strategy in platinum-sensitive advanced PC patients with BRCA germline mutations by the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA). Several other trials are investigating PARPi in metastatic PC patients including those with germline and somatic mutations not only in BRCA, but also in other HRD genes.59,60
It has also been documented that a small proportion of PC (1%-2%) has defects in the DNA response process resulting from dysfunctions in DNA mismatch repair (MMR). These tumours demonstrate microsatellite instability (MSI), which can be reliably detected by routine immunohistochemical assays for MSH1, PMS2, MLH1, and MSH6 expression, and can potentially be treated with immune checkpoint blockade therapy.62
Transcriptomic subtypes
More recently, PC has been classified by multiple groups using associated transcriptional networks that proposed several different but overlapping classifications (Table 2 and Figure 2).63,64 Collisson et al. identified three molecular subtypes using hybridisation array-based mRNA expression: classical, quasi-mesenchymal (QM-PDA) and exocrine-like.65 The classical subtype expressed GATA6 (the endodermal lineage-specifying transcription factor) and exhibited KRAS dependency while QM-PDA subtype correlated with high tumour grade and poor prognoses.65,66 Similarly, Moffitt et al., identified two tumour subtypes (basal-like and classical) and two stromal subtypes (normal and activated) as result of non-negative matrix factorisation (NMF) and virtual microdissection of microarray and RNAseq data from primary and metastatic PC tumours.67 This study showed that classical subtype was associated with better outcome compared with the basal one, instead characterised by worse survival and potentially larger benefit from adjuvant chemotherapy.
Table 2.
Molecular subtyping of pancreatic cancer
| Study | Histopathology and methodology | Subtypes | Biological insight | Clinical relevance |
|---|---|---|---|---|
| Moffitt et al.67 | (n = 206; 145 primary PDAC and 61 metastatic PDAC) mRNA expression microarray (n = 206; 134 normal sites) and RNAseq in 15 primary samples, 37 PDXs, 3 cell lines, and 6 CAFs |
Epithelial: Basal-like Classical Stromal: Activated Normal |
|
|
| Collisson et al.65 |
n = 85 primary untreated PDAC Microdissected (n = 27), whole PDAC (n = 39), and PDCLs (n = 19) Non-negative matrix factorization and consensus clustering |
Classical Quasi-mesenchymal Exocrine-like |
|
|
| Bailey et al.54 |
n = 266 primary untreated PDAC Consensus clustering to subtypes according to signatures defined by Moffitt et al.67 and Collisson et al.65 RNAseq (n = 96) and expression array (n = 266) |
Squamous Immunogenic Pancreatic progenitor ADEX |
|
|
ADEX: aberrantly differentiated endocrine exocrine; ATCC: American Type Culture Collection; CAF: cancer-associated fibroblast; IPMN: intraductal papillary mucinous neoplasm; PDAC: pancreatic ductal adenocarcinoma; PDCL: patient-derived cell line; PDX: patient-derived xenograft; RNAseq: RNA sequencing; TCGA: The Cancer Genome Atlas.
Adapted from Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol 2019; 16: 207-220,64 with permission from Springer Nature Limited. Copyright © 2019.
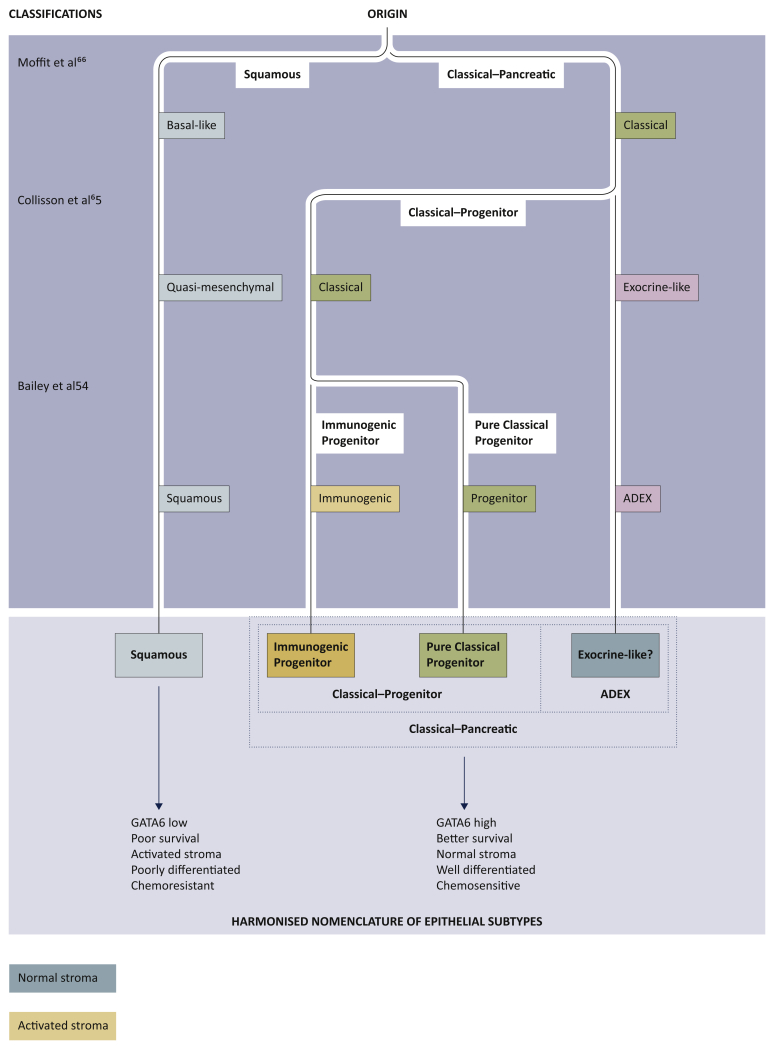
Figure 2.
Phylotranscriptomic tree of pancreatic cancer.
Two initial lineages are evident, largely driven by epigenetic events that separate pancreatic ductal adenocarcinoma into squamous (alternatively named basal-like and quasi-mesenchymal) and classical subtypes.
The classical-pancreatic subtype might contain a spectrum of tumours that resemble pancreatic precursors, paralleling lineages occurring during pancreatic development. We can then discern a classical-progenitor subtype and, although it is unclear as to whether more differentiated progenitor subtypes are due to contamination by normal epithelium, an aberrantly differentiated endocrine exocrine (ADEX) subtype. Although the immunogenic subtype is largely driven by the immune infiltrate of the tumour microenvironment, epithelial-specific mechanisms probably exist that generate such an immune response. Stromal subtypes have also been discerned and, currently, do not appear to be directly associated with epithelial subtypes. The harmonised nomenclature has two broad subtypes: squamous and classical-pancreatic, with the classical-progenitor and ADEX subtypes residing in the latter. The classical-progenitor subtype further subdivides into the immunogenic progenitor and pure classical progenitor subtypes. Adapted from Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol 2019; 16: 207-220,64 with permission from Springer Nature Limited. Copyright © 2019.
Additionally, the Australian PC Genome Initiative (APGI; http://www.pancreaticcancer.net.au), as part of the International Cancer Genome Consortium (ICGC), defined four subtypes of PC through an integrated genomic analysis of transcriptomes, methylome, and mutational and histopathology data: squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine (ADEX).54 In this study, the squamous subtype was enriched in gene programs typical of histologically squamous tumors of breast, bladder, lung, head and neck. These included biological pathways involved in inflammation, hypoxia response, metabolic programming, and TGF-β signalling.68 It overlapped with histopathologic adenosquamous tumours and was characterised by poor survival. Instead, the pancreatic progenitor subtype correlated with better outcome and expressed pathways involved in pancreatic endodermal differentiation. The ADEX group (a subclass of pancreatic progenitor tumours) was defined by transcriptional networks characterised by the simultaneous expression of transcriptional programs observed in the endocrine and exocrine pancreas, typically activated in the later stages of pancreatic development and differentiation.
Lastly, the immunogenic subtype, described by extending the analysis to the transcriptome of the immune infiltrate in the tumour microenvironment, was enriched for molecular signalling involved in immune cell infiltration and related immune response pathways.54
Despite discrepancies in nomenclatures and methods of identification, a substantial overlap exists between the different classifications with two main clinically relevant subgroups identified: squamous/basal-like and classical tumours (Figure 2). Squamous and basal-like (and QM-PDA) phenotypes share important aspects including the correlation with high tumour grade, metastatic disease, chemoresistance, and poor prognosis.57,67,69,70 On the other side, classical subtypes have generally a more favourable clinical outcome. These differences have also been documented in the recent genomics-driven COMPASS trial for advanced PC, which investigated the correlation between the therapeutic response to different treatment regimens and the transcriptomic profile obtained through tumour biopsy and RNA sequencing.70 The results of this study showed an overall response rate of 10% for basal-like and of 33% for classic tumours (P = 0.02).70 Notably, in patients treated with m-FOLFIRINOX, the progression rate was 60% in basal-like tumours compared with 15% in classic PC (P = 0.0002), with median OS of 5.9 months and 9.3 months for basal-like and classic, respectively (HR 0.47; 95% CI, 0.32-0.69, P = 0.0001).70 The expression of GATA6 has been proposed as a surrogate biomarker for the differentiation between basal-like and classic subtype, based on the observation that basal-like tumours have significantly lower levels of GATA6.57,69,70 However, whether GATA6-low can be used as a predictive biomarker of therapeutic response needs further investigation.57,70,71
An additional clinically relevant PC subtype is the immunogenic, enriched with infiltrating cytotoxic CD8+ T cells, regulatory T and B cells, and high expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) immune checkpoint proteins. In tumours with these molecular characteristics, there is a biological rationale for the investigation of immune modulation with checkpoint inhibitors.39
The rationale for a precision preoperative medicine approach
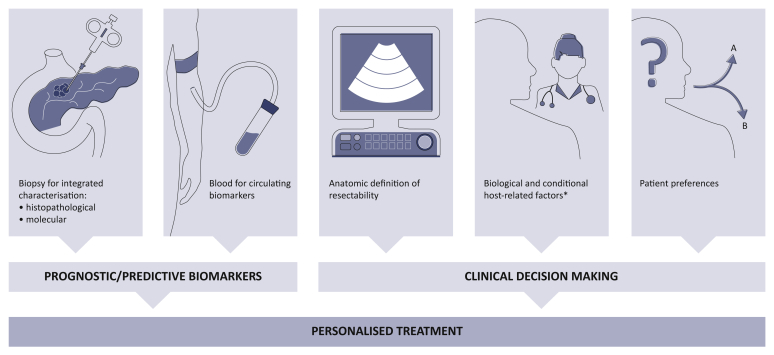
Considering the significant molecular heterogeneity of PC and the related prognostic and therapeutic implications, it is of utmost importance to implement biomarker-based preoperative clinical trials in order to validate prognostic and predictive factors, fundamental for an effective precision medicine approach. On one side, this would allow better risk stratification of candidates to upfront resection. On the other side, a genomic-driven precision approach may provide instruments useful for the choice of the optimal primary systemic treatment, by identifying biomarkers that could predict sensitivity or resistance to specific therapies. It is only through an integrated analysis of molecular prognostic/predictive biomarkers and clinical parameters that an individualised treatment path can be implemented in non-metastatic PC with the high potential to impact on therapeutic response, radical resection rate, and on survival (Figure 3).
Figure 3.
Ideal integration of clinical and biological information for personalised treatment of non-metastatic pancreatic cancer.
The integration of clinical and pathological features with molecular data from biopsy specimens and blood tests should be used to investigate and validate prognostic/predictive models for personalised treatment selection.
∗Serum CA 19-9 of >500 IU/ml, positive regional lymph node metastases, performance status of two or more, comorbidities.
As previously mentioned, the current decision algorithm in preoperative setting is predominantly based on imaging, clinical features, and blood tests and does not incorporate the tumour's biologic aggressiveness, chemoresistance, or metastatic propensity.39 Indeed, no biomarkers that predict treatment efficacy or resistance are currently available and robust prognostication models are still lacking. Recently, a preoperative prediction nomogram incorporating two biomarkers (S100A2 and S100A4), and clinical variables including age, tumour size, and location was developed and independently validated but its use in clinical practice is limited.72 Interestingly, preliminary results from a randomised phase II SWOG S1505 trial of perioperative m-FOLFIRINOX versus gemcitabine/nab-paclitaxel in resectable PC, showed similar results in terms of outcomes between the two therapeutic strategies thus indicating that in unselected populations it is almost impossible to see differences between different regimens as well as assess the relative role of platinum compounds versus other agents.48
It is therefore evident that precision medicine in non-metastatic PC remains an urgent and unmet need. Tailoring the therapeutic strategy on the molecular profile in preoperative setting is instead fundamental to improving outcome. Published studies reported exceptional responses after NAT, translating in long-term survival, in approximately 30% of patients,73, 74, 75, 76, 77, 78 while 17%-30% of cases progress during the therapy and up to 38% have no response.32,79 Progression during NAT likely reflects a more aggressive disease phenotype and has been empirically proposed as an indirect identifier of patients who will have limited benefit from curative surgery because of the high probability of relapse after resection.80 However, there is growing evidence that response to NAT is a crucial determinant of long-term prognosis and that primary chemoresistance reflects sub-optimal treatment for the majority of patients.76,81,82 The optimal treatment strategy for aggressive, chemotherapy-resistant tumours is challenging and needs to be defined. Data from a recent study showed that genetic or pharmacological depletion of histone methyltransferase enhancer of zeste homologue 2 (EZH2) can increase GATA6 expression, thus inducing a subtype-switching in favour of a less aggressive, and potentially more therapy-susceptible, classical PC subtype.83 This may represent a promising strategy to be further investigated in squamous/basal-like tumours.
Alternatively, improvements in response rates and in clinical outcomes observed in exceptional and major responders are due to the effects of small subgroups of chemotherapy-sensitive patients.32,84 For example, we can speculate that the response rate reported in patients treated with platinum salts (approximately 30%),14,85 reflects a molecular background characterised by DDR/HRD (reported in up to 35% of early-stage PC patients) that has been associated with higher sensitivity to platinum-based chemotherapy.53,55, 56, 57,86 The identification of these subjects is important as the treatment with a platinum-backbone regimen would be more appropriate for those patients than gemcitabine-based chemotherapy.58 Furthermore, even in the presence of locally advanced unresectable tumours, the goal of treatment should be curative surgery in patients with HRD genome as treatment with a platinum-backbone regimen in these subjects is likely to result in higher response rates than gemcitabine-based chemotherapy, and is thus more likely to result in better outcomes and higher surgical resection rates.53,58 The ability to undergo tumour resection after primary systemic therapy is important as it constitutes the best chance of long-term survival for locally advanced PC compared with either no surgery or local procedures (HR 0.39; 95% CI, 0.34-0.46; P < 0.001).87 Thus, maximising the identification of likely platinum responders is fundamental considering the potentially significant impact on prognosis. However, whether HRD can predict response to platinum in early-stage disease needs further investigation as the majority of evidence derives from metastatic setting. It is indeed possible that different biological features between early-stage and advanced PC may result in different therapeutic susceptibility among tumours with similar molecular profile.55
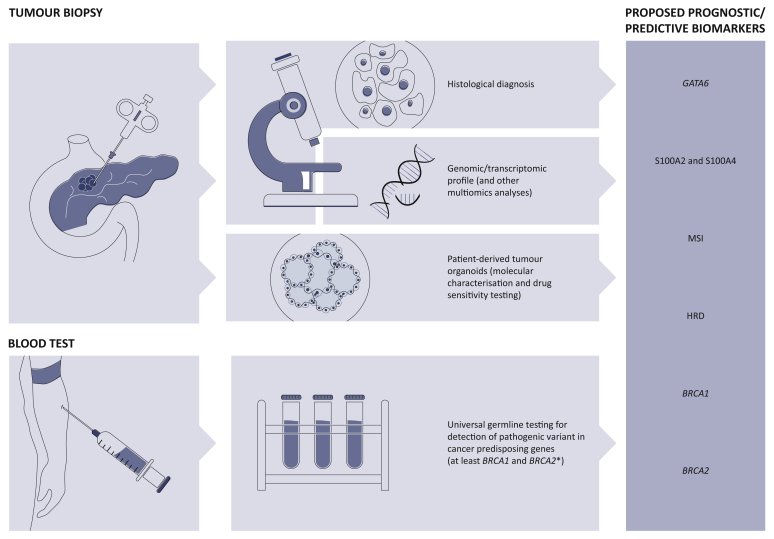
Several novel potential therapeutic targets are currently under investigation in metastatic PC and many others are on the horizon (Table 3).88 To date, the most clinically meaningful biomarkers that have potential to be successfully translated into the preoperative setting and incorporated in the design of future clinical trials are germline BRCA1/2 mutations, HRD (more in general), and MSI. Prognostic biomarkers, such as S100A2/S100A4 and GATA6 (to differentiate classical from squamous tumours), should be considered (Figure 4).
Table 3.
Therapeutic targets in pancreatic cancer
| Therapeutic target | Treatment | Study phase |
|---|---|---|
| BRCA, HRD | PARPi, platinum | Phase I-III |
| MMRd | Immunotherapya | Phase I, II |
| HER2/HER3 | Zenocutuzumab | Phase I, II |
| CDK4/6 | Palbociclib, PD-0332991 | Phase I |
| ALK | Ceritinib | Phase I |
| ERK1/2 | Ulixertinib, KO-947 | Phase I |
| TRK/ROS1 | Entrectinib, Larotrectinib | Phase II |
| KRASG12C | AMG 510 | Phase I |
| Metabolism | Devimistat, hydroxychloroquine | Phase I-III |
| TME | PEGPH20, VCN-01, FAK/BTK inhibitors, ATRA | Phase I-III |
| BRAF | MAPK signalling inhibitors | |
| ATM | ATM inhibitors | |
| ATR | ATR inhibitors | |
| STK11 | mTOR inhibitors | |
| FGFR | FGFR inhibitors | |
| Replication stress | ATR, WEE1 inhibitors |
Bold: Currently under clinical investigation
Roman: Preclinical evidence.
ALK, anaplastic lymphoma kinase; ATM, ataxia telangiectasia; ATR, ataxia telangiectasia and Rad3-related protein; ATRA, all-trans-retinoic-acid; BTK, Bruton's tyrosine kinase; FAK, Focal adhesion kinase; FGFR, fibroblast growth factor receptors; HRD, homologous recombination deficiency; MMRd, mismatch repair deficiency; PARPi, poly-ADP ribose polymerase inhibitors; PEGPH20, pegvorhyaluronidase-α; TME, extracellular tumour microenvironment.
Immune checkpoint inhibitors, cancer vaccines, and CAR T-cell therapy.
Figure 4.
Proposed biomarkers to be implemented in future neoadjuvant clinical trials.
HRD: homologous recombination deficiency, defined by germline or somatic mutations in HRD-related genes (BRCA1/2, ATM, PALB2, FANC-genes, RAD51, etc.), COSMIC3 signature, or genomic instability through structural variation patterns; MSI: microsatellite instability.
∗NCCN guidelines have been recently updated and recommend universal screening for germline variant in patients with PC, regardless of age, ethnicity, and family/personal history of cancer, including not only BRCA1/2 but also ATM, CDKN2A, PALB2, STK11, TP53, MLH1, MSH2, MSH6, and PMS2.31
Challenges in implementing precision medicine in early-stage PC
The clinical relevance of preclinical data supporting a precision oncology approach needs to be validated in the clinic through biomarker-driven clinical trials. However, several hurdles limit implementation, including technical, organizational, and economic barriers (Table 4).89,90 There is concern about the ability of local pancreatic biopsy, especially using endoscopic ultrasound with fine needle aspiration (EUS-FNA), to obtain sufficient tissue for molecular profiling.91 Furthermore, low cellularity and abundant stroma, typical of PC biopsies, often hamper the possibility to perform additional analysis beyond cyto/histopathology.92 The highly spatial intratumoural heterogeneity of PC also prevents obtaining a reliable molecular characterization, representative of the entire tumour.93,94 In addition to these technical challenges, a few other interrelated factors hinder the successful clinical implementation of precision medicine in PC. Health systems face an overall lack of bioinformatics capacity specialised in the analysis and interpretation of complex data obtained from tumour sequencing. Furthermore, despite evidence of better outcomes for PC patients managed in high-volume centres,95,96 the vast majority are still diagnosed and treated at community hospitals where access to molecular analysis is limited and practising oncologists have little or no training to successfully use the information for clinical decision making.89 Conversely, the centralisation of current clinical implementation of multiomics technologies in highly specialised tertiary cancer centres poses important considerations about disparities of access to cutting-edge cancer programs. The situation is further complicated by the lack of biomarker-based clinical trials for PC patients, challenges in conducting adequately powered clinical trials in small molecular subgroups, the turnaround timing, costs/effectiveness, and reimbursement of molecular analyses. Recently, innovative therapeutic development platforms have been developed with the aim of integrating molecular data in clinical trials and accelerating precision therapeutic development for PC patients. These include PRECISION-Panc in the UK, EPPIC (Enhanced Pancreatic Cancer Profiling for Individualized Care) in Canada, and Precision Promise in the USA, which represent a possible solution to overcome the challenges mentioned earlier. These platforms aim to integrate discovery with preclinical development and innovative clinical trial design, allowing forward and backward translation.73,97 As part of PRECISION-Panc, to facilitate real world personalised clinical trials, a dynamic and flexible tissue acquisition and molecular profiling pathway has been developed (the PRECISION-Panc Master Protocol). This approach, based on extra passes on EUS pancreatic biopsy and peripheral venous sampling of blood for integrated multiomic analysis, delivers molecular profiling in patients with all stages of PC with a success rate of over 80%.98 The molecular information may guide eligibility for enrolment in a PRIMUS trial (pancreatic cancer individualised multi-arm umbrella study), investigating different biomarker-based treatment options.
Table 4.
Barriers to implementation of precision medicine for pancreatic cancer
| Sample-specific | Technology-specific | Practical/organisational | Therapeutic development |
|---|---|---|---|
Tissue acquisition:
|
Inconsistency in molecular test selection:
|
Timing of molecular testing:
Lack of bioinformatic capacity Lack of provider awareness and education Lack of patient awareness Financial concerns:
|
Identification and validation of therapeutic targets Lack of specific molecular-targeted drugs Lack of biomarker-based clinical trials Challenges in conducting adequately powered clinical trials in small molecular subgroups Primary therapeutic resistance |
IHC, immunohistochemistry; NGS, next-generation sequencing; WES, whole exome sequencing; WGS, whole-genome sequencing.
A few other experiences have demonstrated the feasibility and the utility of molecular profiling in driving therapeutic choice in patients' metastatic PC, with positive impact on survival outcomes.56,57,99, 100, 101 It has been demonstrated that a molecular-driven precision medicine can be safely integrated into clinical management of PC patients with rapid turnaround time (<30 days).56,57,99, 100, 101 Thus, the incorporation of preclinical data for prognostic/predictive assessment in early-stage PC seems to be compatible with current standards. Indeed, the median waiting time from surgical consultation to surgery in high-volume centres is 29-31 days and potential delays in accessing surgery would seem not to negatively affect pathological features and survival of most patients.102,103 In addition, data from the US National Cancer Database (2003-2011), including 14 807 resected PC patients, indicate that an early allocation of surgery, within 12 weeks from diagnosis, is not associated with a survival benefit.104
From hypothesis generation to clinical applicability
The next step is to advance this promising strategy in the preoperative setting, where precision medicine is still an unmet and urgent need. Amongst the emerging plethora of potential therapeutic vulnerabilities in PC, the most promising target is represented by the homologous recombination deficiency (HRD) pathway. The clinical relevance of this molecular characteristic in patients with early-stage PC has been recently pointed out in two retrospective studies. Golan et al. showed that patients with borderline resectable PC carrying germline BRCA mutations have an increased chance for pCR than wild type after neoadjuvant FOLFIRINOX (44.4% versus 10%, respectively; P = 0.009). Furthermore, the median OS after surgery was not reached among patients with germline mutations at 32 months for BRCA non-carriers (P = 0.2).81 This is consistent with other data reported in literature in which pCR was associated with better DFS and OS after surgery.75 Similarly, Yu et al. retrospectively studied patients with resected PC and a pathogenic germline mutation in BRCA1, BRCA2, and PALB2.105 Median OS in mutation carriers exposed to platinum in the perioperative setting was not reached versus 23.1 months in wild type patients (HR 0.12; 95% CI, 0.01-1.00). Patients in the mutation-positive group who received perioperative treatment with platinum had a trend toward improved median OS compared with those who did not (HR 0.15; 95% CI, 0.02-1.23; P = 0.07). Despite the retrospective design, these studies highlight the importance of a biomarker-driven treatment in the preoperative setting as it can guide the therapeutic choice in a personalised manner and can significantly improve patient outcomes. However, to the best of our knowledge, there are no prospective neoadjuvant clinical trials evaluating BRCA mutations as predictive biomarkers in PC (aside from locally advanced unresectable disease, which is usually included in clinical trials for advanced PC).
Currently, only a few trials are using a biomarker-enriched design in the neoadjuvant setting (summarised in Table 5). An ongoing prospective trial (PRIMUS002, NCT04176952) conducted in the context of PRECISION-Panc97 is investigating the potential predictive role of DNA damage repair (DDR) deficiency in patients treated with NAT. This is an integrated, open label, non-randomised, phase II study examining two therapeutic regimens (FOLFOX-A, i.e. 5-fluorouracil/leucovorin, oxaliplatin, nab-paclitaxel, and gemcitabine/nab-paclitaxel) given for 3 months before surgery in resectable and borderline resectable PC, aimed at assessing efficacy and toxicity with integrated translational work. Indeed, the study is powered to test a proposed DDR-deficient biomarker for response rate in patients treated with FOLFOX-A regimen. Particularly, this biomarker is a candidate HRD signature hypothesised to be a predictor of response to platinum-based therapy, and derived from a specific pattern of genomic structural rearrangements seen in known HRD cancers, from published and unpublished data sets.73,106 An additional phase II randomised study (PRIMUS-005, STAR-PAC2) will soon be activated and will investigate all-trans-retinoic-acid (ATRA) as a stromal targeting agent in a novel drug combination in locally advanced unresectable PC.107
Table 5.
Current biomarker-enriched preoperative clinical trials in pancreatic cancer
| Trial ID | Biomarker | Therapeutic drugs | Phase | Status |
|---|---|---|---|---|
| NCT04176952 | HRD signature | FOLFOX-A Gemcitabine/nab-paclitaxel |
II non-randomised | Recruiting |
| NCT04005690 | Multiple, not specified | Cobimetinib Olaparib |
II non-randomised | Recruiting |
| NCT04481204 | Multiple, not specified | Multiple drugs | II randomised | Not yet recruiting |
Clinical trials including (not limited to) patients with resectable/borderline resectable pancreatic cancer. FOLFOX-A: 5-fluorouracil/leucovorin, oxaliplatin, nab-paclitaxel.
DDR, DNA damage response; HRD, homologous recombination system deficiency.
Another important biomarker-enriched study is investigating the association between mitogen-activated protein kinase inhibitor cobimetinib and PARP inhibitor olaparib in different clinical scenarios, including the NAT setting (NCT04005690). This is a phase II feasibility study in which validation of cobimetinib and olaparib molecular targets will be explored with tissue collection before and after therapy for biomarker evaluation. Several predictive biomarkers of therapeutic sensitivity/resistance are investigated; however, detailed information is not available from the study's description (https://clinicaltrials.gov/ct2/show/NCT04005690).
PARP inhibition is also being investigated in association with chemoradiotherapy in localised PC. The rationale for this approach is provided by preclinical studies, which showed remarkable synergy between radiotherapy and PARP1/2i veliparib in orthotopic animal models of non-metastatic PC.108 A recent phase I trial investigated safety and clinical efficacy of veliparib combined with gemcitabine-based chemoradiation in 30 locally advanced PC patients (NCT01908478) with translational analyses. The regimen was safe, tolerable, and clinically active.109 Median progression-free survival (PFS) and OS of the whole cohort were 9.8 months (95% CI: 8.4-18.6) and 14.6 months (95% CI: 11.6-21.8), respectively. Median OS was 19 months (95% CI: 6.2-27.2) in patients with impaired DDR tumours and 14 months (95% CI: 10.0-21.8) in patients with DDR proficient tumours. Expression of the DDR transcripts PARP3 and RBX1 were associated with improved OS.109 Despite the promising results showed in this study, further evidence is warranted to confirm the activity and efficacy of this multimodality strategy in potentially resectable patients.
Lastly, the PIONEER-Panc phase II randomised clinical trial (NCT04481204) will investigate novel therapeutic approaches in three clinical stage groups of localised PC based on Bayesian platform design. This trial entails exploratory translational multiomics analyses and organoids-based in vitro drug testing that will provide important information for the design of future biomarker-based phase III trials.
Conclusion
The current knowledge on the molecular heterogeneity of PC poses important considerations about the future management of patients with non-metastatic disease. Firstly, the clinical investigation and validation of putative molecular prognostic biomarkers is imperative to identify the subset of patients who would benefit most from preoperative treatment rather than from upfront surgery. In parallel, it is fundamental to design genomic-driven clinical trials in order to test predictive biomarkers necessary to match the candidates to primary systemic therapy, tailored on the tumour molecular profile, thus allowing the opportunity for better treatment and survival outcomes. Furthermore, considering the relative rarity of non-metastatic disease, the possibility to significantly impact on the natural disease history with an optimal treatment strategy, and the surgical challenges on the definition of borderline resectable/resectable/locally advanced disease, it is advisable to refer these patients to high-volume centres with extensive expertise. Besides, due to the lack of high-quality data from randomised controlled trials, every candidate for preoperative treatments should be evaluated for enrolment in randomised (ideally molecularly-driven) clinical trials to guarantee the best therapeutic opportunity. Lastly, novel models of therapeutic development are warranted to investigate multiple hypothesis in small molecular subgroups to accelerate the drug testing process and approval and maximise the networking of centres with available clinical protocols, with possible referral to central high-volume institutes. It is only through major efforts in implementing a precision medicine approach that we can improve survival of PC patients.
Acknowledgments
Funding
Cancer Research UK - Grant numbers: C29717/A17263, C29717/A18484, C596/A18076, C596/A20921, A23526. Wellcome Trust – Grant number: 103721/Z/14/Z.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Gillen S., Schuster T., Meyer Zum Buschenfelde C. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H., Neuhaus P., Hochhaus A. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 5.Groot V.P., Daamen L.A., Hagendoorn J. Use of imaging during symptomatic follow-up after resection of pancreatic ductal adenocarcinoma. J Surg Res. 2018;221:152–160. doi: 10.1016/j.jss.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Strobel O., Neoptolemos J., Jager D., Buchler M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Hammel P., Hebbar M. FOLFIRINOX or Gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Fu Y., Chen Q. Predictors of distant metastasis on exploration in patients with potentially resectable pancreatic cancer. BMC Gastroenterol. 2018;18:168. doi: 10.1186/s12876-018-0891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versteijne E., Suker M., Groothuis K. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo S.C., Gilson M.M., Herman J.M. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkow R.P., Bilimoria K.Y., Tomlinson J.S. Post-operative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–377. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 12.Valle J.W., Palmer D., Jackson R. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–512. doi: 10.1200/JCO.2013.50.7657. [DOI] [PubMed] [Google Scholar]
- 13.Jang J.Y., Han Y., Lee H. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268:215–222. doi: 10.1097/SLA.0000000000002705. [DOI] [PubMed] [Google Scholar]
- 14.Maggino L., Malleo G., Marchegiani G. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 2019;154(10):932–942. doi: 10.1001/jamasurg.2019.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y.S., Lee J.C., Yang S.Y. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: a systematic review and meta-analysis. Sci Rep. 2019;9:15662. doi: 10.1038/s41598-019-52167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley A., Van Der Meer R. Upfront Surgery versus neoadjuvant therapy for resectable pancreatic cancer: systematic review and bayesian network meta-analysis. Sci Rep. 2019;9:4354. doi: 10.1038/s41598-019-40951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla A., Ferrone C.R. Neoadjuvant therapy for resectable pancreatic cancer: an evolving paradigm shift. Front Oncol. 2019;9:1085. doi: 10.3389/fonc.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokdad A.A., Minter R.M., Zhu H. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35:515–522. doi: 10.1200/JCO.2016.68.5081. [DOI] [PubMed] [Google Scholar]
- 19.Roland C.L., Yang A.D., Katz M.H. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:1168–1175. doi: 10.1245/s10434-014-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurence J.M., Tran P.D., Morarji K. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–2069. doi: 10.1007/s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 21.Ye M., Zhang Q., Chen Y. Neoadjuvant chemotherapy for primary resectable pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 2020;22:821–832. doi: 10.1016/j.hpb.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Janssen Q.P., O'Reilly E.M., van Eijck C.H.J., Groot Koerkamp B. Neoadjuvant treatment in patients with resectable and borderline resectable pancreatic cancer. Front Oncol. 2020;10:41. doi: 10.3389/fonc.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni N.M., Soloff E.V., Tolat P.P. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology's disease-focused panel for pancreatic ductal adenocarcinoma: part I, AJCC staging system, NCCN guidelines, and borderline resectable disease. Abdom Radiol (NY) 2020;45:716–728. doi: 10.1007/s00261-019-02289-5. [DOI] [PubMed] [Google Scholar]
- 24.Oba A., Ho F., Bao Q.R. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10:245. doi: 10.3389/fonc.2020.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrone C.R., Marchegiani G., Hong T.S. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadhachary G.R., Tamm E.P., Abbruzzese J.L. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Dixon E., Abdalla E., Schwarz R.E., Vauthey J.N. AHPBA/SSO/SSAT sponsored consensus conference on multidisciplinary treatment of hepatocellular carcinoma. HPB (Oxford) 2010;12:287–288. doi: 10.1111/j.1477-2574.2010.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz M.H., Marsh R., Herman J.M. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bockhorn M., Uzunoglu F.G., Adham M. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Isaji S., Mizuno S., Windsor J.A. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Tempero M.A. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17:603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 32.Tang K., Lu W., Qin W., Wu Y. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. Pancreatology. 2016;16:28–37. doi: 10.1016/j.pan.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Janssen Q.P., Buettner S., Suker M. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. 2019;111:782–794. doi: 10.1093/jnci/djz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Versteijne E., Suker M., Groothuis K. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C.P., Xue X.J., Liang N. Effect of chemoradiotherapy and neoadjuvant chemoradiotherapy in resectable pancreatic cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:549–559. doi: 10.1007/s00432-013-1572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suker M., Beumer B.R., Sadot E. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleeff J., Ronellenfitsch U., Michalski C.W. Do we need sequential local therapy following neoadjuvant chemotherapy for locally advanced pancreatic cancer? EClinicalMedicine. 2019;17:100222. doi: 10.1016/j.eclinm.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung J., Yoon S.M., Park J.H. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One. 2019;14:e0214970. doi: 10.1371/journal.pone.0214970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tempero M.A., Malafa M.P., Chiorean E.G. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw. 2019;17:202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 40.Pentheroudakis G., Committee E.G. Recent eupdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann Oncol. 2019;30:1395–1397. doi: 10.1093/annonc/mdz180. [DOI] [PubMed] [Google Scholar]
- 41.Khorana A.A., McKernin S.E., Berlin J. Potentially curable pancreatic adenocarcinoma: ASCO Clinical Practice Guideline update. J Clin Oncol. 2019;37:2082–2088. doi: 10.1200/JCO.19.00946. [DOI] [PubMed] [Google Scholar]
- 42.Gulhati P., Prakash L., Katz M.H.G. First-line gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2019;26:619–627. doi: 10.1245/s10434-018-6807-9. [DOI] [PubMed] [Google Scholar]
- 43.Paiella S., De Pastena M., Romeo F. Ablation treatments in unresectable pancreatic cancer. Minerva Chir. 2019;74:263–269. doi: 10.23736/S0026-4733.18.07881-1. [DOI] [PubMed] [Google Scholar]
- 44.Ducreux M., Cuhna A.S., Caramella C. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 45.Pan L., Fang J., Tong C. Survival benefits of neoadjuvant chemo(radio)therapy versus surgery first in patients with resectable or borderline resectable pancreatic cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;18:1. doi: 10.1186/s12957-019-1767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reni M., Balzano G., Zanon S. Safety and efficacy of preoperative or post-operative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3:413–423. doi: 10.1016/S2468-1253(18)30081-5. [DOI] [PubMed] [Google Scholar]
- 47.Ghaneh P., Palmer D.H., Cicconi S. ESPAC-5F: four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol. 2020;38:4505. [Google Scholar]
- 48.Sohal D., Duong M.T., Ahmad S.A. SWOG S1505: results of perioperative chemotherapy (peri-op CTx) with mfolfirinox versus gemcitabine/nab-paclitaxel (Gem/nabP) for resectable pancreatic ductal adenocarcinoma (PDA) J Clin Oncol. 2020;38:4504. [Google Scholar]
- 49.Gilbert J.W., Wolpin B., Clancy T. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol. 2017;28:2067–2076. doi: 10.1093/annonc/mdx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinrich S., Besselink M., Moehler M. Opinions and use of neoadjuvant therapy for resectable, borderline resectable, and locally advanced pancreatic cancer: international survey and case-vignette study. BMC Cancer. 2019;19:675. doi: 10.1186/s12885-019-5889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreyer S.B., Chang D.K., Bailey P., Biankin A.V. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23:1638–1646. doi: 10.1158/1078-0432.CCR-16-2411. [DOI] [PubMed] [Google Scholar]
- 52.Biankin A.V., Waddell N., Kassahn K.S. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waddell N., Pajic M., Patch A.M. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey P., Chang D.K., Nones K. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 55.Pishvaian M.J., Blais E.M., Brody J.R. Outcomes in patients with pancreatic adenocarcinoma with genetic mutations in DNA damage response pathways: results from the know your tumour program. JCO Precision Oncology. 2019;3:1–10. doi: 10.1200/PO.19.00115. [DOI] [PubMed] [Google Scholar]
- 56.Aguirre A.J., Nowak J.A., Camarda N.D. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 2018;8:1096–1111. doi: 10.1158/2159-8290.CD-18-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aung K.L., Fischer S.E., Denroche R.E. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res. 2018;24:1344–1354. doi: 10.1158/1078-0432.CCR-17-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park W., Chen J., Chou J.F. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin Cancer Res. 2020;26:3239–3247. doi: 10.1158/1078-0432.CCR-20-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golan T., Varadhachary G.R., Sela T. Phase II study of olaparib for BRCAness phenotype in pancreatic cancer. J Clin Oncol. 2018;36:297. [Google Scholar]
- 60.Zhu H., Wei M., Xu J. PARP inhibitors in pancreatic cancer: molecular mechanisms and clinical applications. Mol Cancer. 2020;19:49. doi: 10.1186/s12943-020-01167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Golan T., Hammel P., Reni M. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahin I.H., Askan G., Hu Z.I., O'Reilly E.M. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol. 2017;28:2950–2961. doi: 10.1093/annonc/mdx503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martens S., Lefesvre P., Nicolle R. Different shades of pancreatic ductal adenocarcinoma, different paths towards precision therapeutic applications. Ann Oncol. 2019;30:1428–1436. doi: 10.1093/annonc/mdz181. [DOI] [PubMed] [Google Scholar]
- 64.Collisson E.A., Bailey P., Chang D.K., Biankin A.V. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207–220. doi: 10.1038/s41575-019-0109-y. [DOI] [PubMed] [Google Scholar]
- 65.Collisson E.A., Sadanandam A., Olson P. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagtegaal I.D., Odze R.D., Klimstra D. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moffitt R.A., Marayati R., Flate E.L. Virtual microdissection identifies distinct tumour- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoadley K.A., Yau C., Wolf D.M. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinelli P., Carrillo-de Santa Pau E., Cox T. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut. 2017;66:1665–1676. doi: 10.1136/gutjnl-2015-311256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Kane G.M., Grunwald B.T., Jang G.H. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4901–4910. doi: 10.1158/1078-0432.CCR-19-3724. [DOI] [PubMed] [Google Scholar]
- 71.Martinelli P., Carrillo-de Santa Pau E., Cox T. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut. 2017;66:1665–1676. doi: 10.1136/gutjnl-2015-311256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dreyer S.B., Pinese M., Jamieson N.B. Precision oncology in surgery: patient selection for operable pancreatic cancer. Ann Surg. 2020;272:366–376. doi: 10.1097/SLA.0000000000003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dreyer S.B., Jamieson N.B., Morton J.P. Pancreatic cancer: from genome discovery to PRECISION-Panc. Clin Oncol (R Coll Radiol) 2020;32:5–8. doi: 10.1016/j.clon.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Murphy J.E., Wo J.Y., Ryan D.P. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He J., Blair A.B., Groot V.P. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268:1–8. doi: 10.1097/SLA.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gemenetzis G., Groot V.P., Blair A.B. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270:340–347. doi: 10.1097/SLA.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Motoi F., Kosuge T., Ueno H. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Q., Rashid A., Gong Y. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. doi: 10.1016/j.anndiagpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khattab A., Patruni S., Abel S. Long-term outcomes by response to neoadjuvant chemotherapy or chemoradiation in patients with resected pancreatic adenocarcinoma. J Gastrointest Oncol. 2019;10:918–927. doi: 10.21037/jgo.2019.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Townend P., de Reuver P.R., Chua T.C. Histopathological tumour viability after neoadjuvant chemotherapy influences survival in resected pancreatic cancer: analysis of early outcome data. ANZ J Surg. 2018;88:E167–E172. doi: 10.1111/ans.13897. [DOI] [PubMed] [Google Scholar]
- 81.Golan T., Barenboim A., Lahat G. Increased rate of complete pathologic response after neoadjuvant FOLFIRINOX for BRCA mutation carriers with borderline resectable pancreatic cancer. Ann Surg Oncol. 2020;27:3963–3970. doi: 10.1245/s10434-020-08469-8. [DOI] [PubMed] [Google Scholar]
- 82.He J., Blair A.B., Groot V.P. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268(1):1–8. doi: 10.1097/SLA.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patil S., Steuber B., Kopp W. EZH2 regulates pancreatic cancer subtype identity and tumour progression via transcriptional repression of GATA6. Cancer Res. 2020;80(21):4620–4632. doi: 10.1158/0008-5472.CAN-20-0672. [DOI] [PubMed] [Google Scholar]
- 84.Chang D.K., Grimmond S.M., Evans T.R., Biankin A.V. Mining the genomes of exceptional responders. Nat Rev Cancer. 2014;14:291–292. doi: 10.1038/nrc3723. [DOI] [PubMed] [Google Scholar]
- 85.Rombouts S.J., Walma M.S., Vogel J.A. Systematic review of resection rates and clinical outcomes after FOLFIRINOX-based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol. 2016;23:4352–4360. doi: 10.1245/s10434-016-5373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park W., Wong W., Yu K.H. Homologous recombination deficiency (HRD): a biomarker for first-line (1L) platinum in advanced pancreatic ductal adenocarcinoma (PDAC) J Clin Oncol. 2019;37:4132. [Google Scholar]
- 87.Torgeson A., Lloyd S., Boothe D. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer. 2017;123:3816–3824. doi: 10.1002/cncr.30780. [DOI] [PubMed] [Google Scholar]
- 88.Nevala-Plagemann C., Hidalgo M., Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 89.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ersek J.L., Black L.J., Thompson M.A., Kim E.S. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188–196. doi: 10.1200/EDBK_200633. [DOI] [PubMed] [Google Scholar]
- 91.Kitano M., Yoshida T., Itonaga M. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19–32. doi: 10.1007/s00535-018-1519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baek H.W., Park M.J., Rhee Y.Y. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic lesions. J Pathol Transl Med. 2015;49:52–60. doi: 10.4132/jptm.2014.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cros J., Raffenne J., Couvelard A., Pote N. Tumour heterogeneity in pancreatic adenocarcinoma. Pathobiology. 2018;85:64–71. doi: 10.1159/000477773. [DOI] [PubMed] [Google Scholar]
- 94.Vietsch E.E., Graham G.T., McCutcheon J.N. Reprint of: circulating cell-free DNA mutation patterns in early and late stage colon and pancreatic cancer. Cancer Genet. 2018;228-229:131–142. doi: 10.1016/j.cancergen.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Ahola R., Sand J., Laukkarinen J. Centralization of pancreatic surgery improves results: review. Scand J Surg. 2020;109:4–10. doi: 10.1177/1457496919900411. [DOI] [PubMed] [Google Scholar]
- 96.Balzano G., Guarneri G., Pecorelli N. Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg. 2020;107:1510–1519. doi: 10.1002/bjs.11716. [DOI] [PubMed] [Google Scholar]
- 97.Dreyer S.B., Jamieson N.B., Cooke S.L. PRECISION-Panc: the next generation therapeutic development platform for pancreatic cancer. Clin Oncol (R Coll Radiol) 2020;32:1–4. doi: 10.1016/j.clon.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Dreyer S.B., Jamieson N.B., Evers L. Feasibility and clinical utility of endoscopic ultrasound guided biopsy of pancreatic cancer for next-generation molecular profiling. Chin Clin Oncol. 2019;8:16. doi: 10.21037/cco.2019.04.06. [DOI] [PubMed] [Google Scholar]
- 99.Lowery M.A., Jordan E.J., Basturk O. Real-time genomic profiling of pancreatic ductal adenocarcinoma: potential actionability and correlation with clinical phenotype. Clin Cancer Res. 2017;23:6094–6100. doi: 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 100.Chantrill L.A., Nagrial A.M., Watson C. Precision medicine for advanced pancreas cancer: the individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin Cancer Res. 2015;21:2029–2037. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 101.Lee M., Jones M.R., Williamson L. Comprehensive genomic analysis of metastatic pancreatic ductal adenocarcinoma (mPDAC) reveals a significant proportion of clinical actionable aberrations. J Clin Oncol. 2019;37:e15753. [Google Scholar]
- 102.Marchegiani G., Andrianello S., Perri G. Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB (Oxford) 2018;20:411–417. doi: 10.1016/j.hpb.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 103.Kirkegard J., Mortensen F.V., Hansen C.P. Waiting time to surgery and pancreatic cancer survival: a nationwide population-based cohort study. Eur J Surg Oncol. 2019;45:1901–1905. doi: 10.1016/j.ejso.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 104.Mirkin K.A., Hollenbeak C.S., Wong J. Time to surgery: a misguided quality metric in early stage pancreatic cancer. J Gastrointest Surg. 2018;22:1365–1375. doi: 10.1007/s11605-018-3730-0. [DOI] [PubMed] [Google Scholar]
- 105.Yu S., Agarwal P., Mamtani R. Retrospective survival analysis of patients with resected pancreatic ductal adenocarcinoma and a germline BRCA or PALB2 mutation. JCO Precision Oncology. 2019;3:1–11. doi: 10.1200/PO.18.00271. [DOI] [PubMed] [Google Scholar]
- 106.Dreyer S.B., Upstill-Goddard R., Paulus-Hock V. Targeting DNA damage response and replication stress in pancreatic cancer. Gastroenterology. 2021;160(1):362–377.e13. doi: 10.1053/j.gastro.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kocher H.M., Basu B., Froeling F.E.M. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11:4841. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuli R., Surmak A.J., Reyes J. Radiosensitization of pancreatic cancer cells in vitro and in vivo through poly (ADP-ribose) polymerase inhibition with ABT-888. Transl Oncol. 2014;7(3):439–445. doi: 10.1016/j.tranon.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tuli R., Shiao S.L., Nissen N. A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. EBioMedicine. 2019;40:375–381. doi: 10.1016/j.ebiom.2018.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]