Abstract
This study aimed to compare the accuracy of novel lipid indices, including the visceral adiposity index (VAI), lipid accumulation product (LAP), triglycerides and glucose (TyG) index, TyG-body mass index (TyG-BMI), and TyG-waist circumference (TyG-WC), in identifying insulin resistance and establish valid cutoff values. This cross-sectional study used the data of 11,378 adults, derived from the United States National Health and Nutrition Examination Survey (1999–2016). Insulin resistance was defined as a homeostasis model assessment-insulin resistance value above the 75th percentile for each sex and race/ethnicities. The area under the curves (AUCs) were as follows: VAI, 0.735; LAP, 0.796; TyG index, 0.723; TyG-BMI, 0.823, and; TyG-WC, 0.822. The AUCs for TyG-BMI and TyG-WC were significantly higher than those for VAI, LAP, and TyG index (vs. TyG-BMI, p < 0.001; vs. TyG-WC, p < 0.001). The cutoff values were as follows: VAI: men 1.65, women 1.65; LAP: men 42.5, women 42.5; TyG index: men 4.665, women 4.575; TyG-BMI: men 135.5, women 135.5; and TyG-WC: men 461.5, women 440.5. Given that lipid indices can be easily calculated with routine laboratory tests, these values may be useful markers for insulin resistance risk assessments in clinical settings.
Subject terms: Endocrinology, Endocrine system and metabolic diseases, Epidemiology
Introduction
Insulin resistance (IR) is a pathological situation, in which there is a lack of physiological response to insulin acting on peripheral tissues1,2. Insulin resistance reduces glucose utilization in the muscles and fats and increases gluconeogenesis in the liver, leading to metabolic and hemodynamic disturbances known as metabolic syndrome, which is a major risk factor for coronary heart disease and cerebrovascular disease1–7. Considering the prevalence of insulin resistance and metabolic syndromes, it would be necessary to detect insulin resistance early even in healthy individuals8.
Insulin resistance was initially evaluated using the pancreatic suppression test, hyperinsulinemic euglycemic clamp technique (HIEG clamp), or minimal model approximation of the metabolism of glucose (MMAMG)9–11. However, these methods are invasive, complicated, expensive, and difficult to use clinically12. For these reasons, indices that measure insulin resistance indirectly have been developed. The homeostasis model for IR (HOMA-IR), which uses fasting blood glucose levels and insulin concentration as variables, was developed in 1985 and has been widely used to estimate IR13. However, a significant drawback of HOMA-IR is the lack of a standard assay for the measurement of fasting insulin concentration14. Therefore, considering these concerns regarding standardization, the HOMA-IR has a significant limitation in establishing an overall acceptable reference value. Furthermore, while several studies have defined IR as a value greater than the 75th percentile value of the HOMA-IR in individuals without diabetes mellitus, the reported cutoff values vary widely, ranging from 2.0 to 3.812,15–19. Given that the measurement of fasting insulin concentration is cumbersome and expensive, the HOMA-IR is not routinely measured in the clinical setting20.
Therefore, insulin-free equations for estimating IR, such as lipid indices, were developed. Lipid indices include visceral adiposity index (VAI), lipid accumulation product (LAP), and triglycerides and glucose (TyG) index21–25. These parameters were proposed as a useful surrogate measure of insulin resistance25–28. In addition, several studies have evaluated modified indices that combine TyG index and obesity indices such as body mass index (BMI) and waist circumference (WC)29–31. However, limited evidence is available regarding the discriminatory accuracy and cutoff values of these novel lipid indices for detecting insulin resistance.
Therefore, this study aimed to compare the accuracy of novel lipid indices in identifying insulin resistance using a representative sample of the US population and establish valid cutoff values for IR.
Results
The study included 11,378 adults (men 5478, women 7900; mean age, 40 years) from the National Health and Nutrition Examination Survey (NHANES) 1999–2016 (Fig. 1). Participants’ demographic and clinical characteristics were compared based on the presence or absence of IR, and the results are shown in Table 1. Age, BMI, WC, and blood pressure were higher in participants with insulin resistance. In addition, blood tests demonstrated high values of fasting glucose, hemoglobin A1C, fasting insulin, total cholesterol, and triglycerides, and low value of high-density lipoprotein (HDL) cholesterol, in participants with IR. Data (median with interquartile range) of each parameter according to race/ethnicity and sex are summarized in Table 2.
Figure 1.
Flowchart showing the final selection process.
Table 1.
Baseline characteristics of the participants in NHANES 1999–2016.
| Characteristics | Normal (N = 8524) | Insulin resistance* (N = 2854) | p-value |
|---|---|---|---|
| Age | 40.0 ± 14.8 | 39.6 ± 14.3 | 0.239 |
| Sex (men) | 4106 (48.2%) | 1372 (48.1%) | 0.946 |
| Race | 0.999 | ||
| Hispanics | 2537 (29.8%) | 847 (29.7%) | |
| Non-Hispanic Whites | 3548 (41.6%) | 1189 (41.7%) | |
| Non-Hispanic Blacks | 1644 (19.3%) | 550 (19.3%) | |
| Other race† | 795 (9.3%) | 268 (9.4%) | |
| BMI, kg/m2 | 26.1 ± 4.9 | 32.6 ± 7.1 | < 0.001 |
| WC, cm | 90.6 ± 12.4 | 106.5 ± 15.2 | < 0.001 |
| Smoking (≥ 100 cigarettes in life) | 3613 (42.4%) | 1156 (40.5%) | 0.081 |
| Systolic blood pressure, mmHg | 116.7 ± 17.3 | 121.1 ± 17.1 | < 0.001 |
| Diastolic blood pressure, mmHg | 68.6 ± 12.0 | 71.8 ± 12.8 | < 0.001 |
| Fasting glucose, mg/dL | 93.8 ± 9.2 | 100.9 ± 9.7 | < 0.001 |
| Fasting insulin, μU/mL | 7.5 ± 3.3 | 23.0 ± 13.6 | < 0.001 |
| HbA1c, % | 5.3 ± 0.4 | 5.4 ± 0.4 | < 0.001 |
| Total cholesterol, mg/dL | 195.4 ± 41.3 | 200.3 ± 40.7 | < 0.001 |
| Triglycerides, mg/dL | 112.5 ± 89.7 | 164.0 ± 115.0 | < 0.001 |
| HDL-cholesterol, mg/dL | 56.9 ± 16.2 | 47.4 ± 13.2 | < 0.001 |
| HOMA-IR | 1.6 ± 0.7 | 5.5 ± 3.5 | < 0.001 |
| VAI | 1.6 ± 2.0 | 2.9 ± 2.7 | < 0.001 |
| LAP | 39.4 ± 41.5 | 83.5 ± 61.2 | < 0.001 |
| TyG index | 4.5 ± 0.3 | 4.8 ± 0.3 | < 0.001 |
| TyG-BMI | 119.0 ± 24.8 | 155.4 ± 34.3 | < 0.001 |
| TyG-WC | 413.0 ± 68.5 | 509.0 ± 79.5 | < 0.001 |
Data are presented as mean ± standard deviation or number (%).
BMI body mass index, WC waist circumference, BP blood pressure, HbA1c hemoglobin A1c, HDL high-density lipoprotein; HOMA-IR homeostasis model assessment-insulin resistance, VAI visceral adiposity index, LAP lipid accumulation product, TyG index triglycerides and glucose index.
*Insulin resistance was defined as a HOMA-IR value above the 75th percentile for each sex and race/ethnicity.
†Other race included non-Hispanic Asian and multi-racial Americans.
Table 2.
Distribution of indirect parameters for insulin resistance according to race/ethnicity and sex.
| Indirect parameters for insulin resistance | Total | Hispanic | Non-Hispanic white | Non-Hispanic black | Other race* |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| HOMA-IR | |||||
| Men | 1.98 (1.27–3.24) | 2.34 (1.49–3.62) | 1.86 (1.20–3.07) | 1.82 (1.14–3.18) | 1.94 (1.18–2.96) |
| Women | 1.87 (1.21–3.06) | 2.24(1.46–3.44) | 1.60 (1.05–2.53) | 2.17 (1.40–3.59) | 1.59 (1.05–2.53) |
| VAI | |||||
| Men | 1.4 (0.8–2.3) | 1.6 (1.0–2.7) | 1.4 (0.9–2.5) | 1.0 (0.6–1.5) | 1.3 (0.8–2.3) |
| Women | 1.4 (0.9–2.2) | 1.7 (1.0–2.6) | 1.4 (0.9–2.3) | 1.0 (0.7–1.7) | 1.2 (0.8–2.1) |
| LAP | |||||
| Men | 38 (20–66) | 45 (26–72) | 41 (23–72) | 26 (14–47) | 30 (14–56) |
| Women | 35 (20–63) | 43 (24–71) | 34 (19–63) | 31 (18–52) | 25 (14–47) |
| TyG index | |||||
| Men | 4.64 (4.45–4.85) | 4.70 (4.52–4.90) | 4.65 (4.47–4.85) | 4.52 (4.34–4.7) | 4.64 (4.46–4.87) |
| Women | 4.535 (4.35–4.74) | 4.62 (4.42–4.81) | 4.55 (4.37–4.75) | 4.41 (4.26–4.59) | 4.50 (4.33–4.72) |
| TyG-BMI | |||||
| Men | 125 (108–144) | 131 (114–147) | 125 (108–144) | 120 (104–143) | 115 (100–133) |
| Women | 122 (103–147) | 129 (111–151) | 118 (100–143) | 130 (108–155) | 106 (91–127) |
| TyG-WC | |||||
| Men | 446 (391–499) | 456 (409–503) | 455 (400–510) | 416 (364–483) | 416 (365–466) |
| Women | 415 (362–477) | 431 (381–488) | 408 (358–473) | 421 (365–483) | 374 (332–436) |
IQR interquartile range, HOMA-IR homeostasis model assessment-insulin resistance, VAI visceral adiposity index, LAP lipid accumulation product, TyG index triglycerides and glucose index, BMI body mass index, WC waist circumference.
*Other race included non-Hispanic Asian and multi-racial Americans.
The receiver operating characteristic (ROC) curve for IR is presented in Fig. 2. The AUC was 0.723 for TyG index and 0.735 for VAI (Table 3). The AUC of LAP (0.796) was significantly higher than that of TyG index (p < 0.001). However, the AUCs of TyG-BMI (0.823) and TyG-WC (0.822) were significantly higher than that of LAP (vs. TyG-BMI, p < 0.001; vs. TyG-WC, p < 0.001). Subgroup analysis according to sex and race/ethnicities showed that TyG-BMI and TyG-WC had the highest AUC in every subgroup. Further analysis using 1:1 propensity score matching (PSM) data with age, sex, and race/ethnicities showed similar results (Table 3). The cutoff values of each lipid index were as follows: VAI: men 1.65, women 1.65; LAP: men 42.5, women 42.5; TyG index: men 4.665, women 4.575; TyG-BMI: men 135.5, women 135.5; and TyG-WC: men 461.5, women 440.5. The cutoff values with their corresponding sensitivity, specificity, and odds ratio (OR) of insulin resistance according to sex and race/ethnicities are summarized in Table 4.
Figure 2.
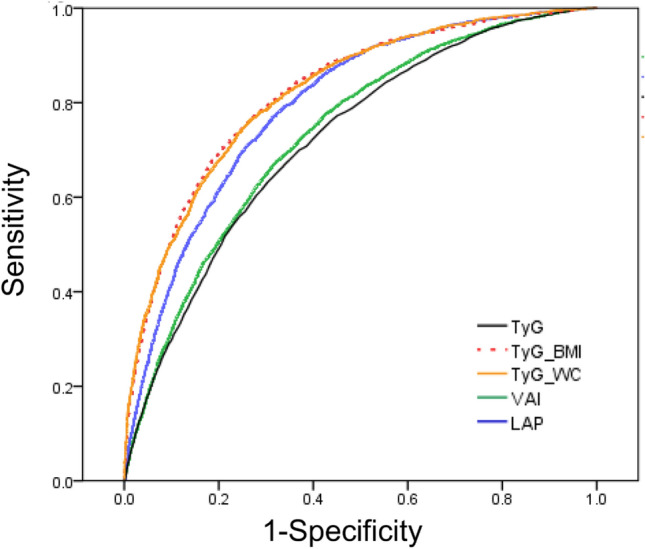
Receiver operating characteristic (ROC) curves of lipid indices for insulin resistance.
Table 3.
Area under the curve for each parameter for insulin resistance according to race/ethnicity and sex.
| VAI | LAP | TyG index | TyG_BMI | TyG_WC | |
|---|---|---|---|---|---|
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |
| Total | 0.735 (0.725–0.746) | 0.796 (0.787–0.805) | 0.723 (0.712–0.733) | 0.823 (0.814–0.832) | 0.822 (0.813–0.831) |
| Men | 0.735 (0.720–0.750) | 0.800 (0.787–0.813) | 0.727 (0.712–0.742) | 0.829 (0.816–0.841) | 0.828 (0.816–0.841) |
| Women | 0.735 (0.721–0.750) | 0.793 (0.780–0.806) | 0.726 (0.711–0.741) | 0.819 (0.807–0.832) | 0.825 (0.813–0.837) |
| Hispanics | |||||
| Men | 0.733 (0.706–0.760) | 0.788 (0.764–0.812) | 0.730 (0.702–0.757) | 0.824 (0.801–0.847) | 0.818 (0.795–0.841) |
| Women | 0.718 (0.691–0.745) | 0.773 (0.749–0.797) | 0.711 (0.684–0.738) | 0.818 (0.796–0.840) | 0.814 (0.792–0.837) |
| Non-Hispanic Whites | |||||
| Men | 0.754 (0.731–0.776) | 0.82 (0.801–0.839) | 0.742 (0.720–0.765) | 0.847 (0.829–0.865) | 0.852 (0.833–0.870) |
| Women | 0.759 (0.737–0.780) | 0.814 (0.795–0.833) | 0.753 (0.731–0.774) | 0.840 (0.822–0.858) | 0.842 (0.824–0.860) |
| Non-Hispanic Blacks | |||||
| Men | 0.733 (0.698–0.768) | 0.815 (0.785–0.845) | 0.730 (0.696–0.765) | 0.823 (0.793–0.853) | 0.829 (0.801–0.858) |
| Women | 0.722 (0.689–0.755) | 0.778 (0.748–0.809) | 0.710 (0.676–0.744) | 0.795 (0.765–0.825) | 0.810 (0.781–0.839) |
| Other race* | |||||
| Men | 0.734 (0.687–0.781) | 0.794 (0.750–0.837) | 0.712 (0.663–0.761) | 0.808 (0.761–0.855) | 0.830 (0.787–0.872) |
| Women | 0.773 (0.729–0.818) | 0.824 (0.783–0.864) | 0.758 (0.711–0.805) | 0.846 (0.808–0.885) | 0.847 (0.808–0.885) |
| PSM data | |||||
| Total | 0.732 (0.719–0.745) | 0.790 (0.778–0.802) | 0.716 (0.703–0.729) | 0.818 (0.807–0.829) | 0.818 (0.807–0.828) |
| Men | 0.726 (0.707–0.745) | 0.789 (0.772–0.805) | 0.718 (0.698–0.737) | 0.819 (0.803–0.835) | 0.818 (0.802–0.833) |
| Women | 0.737 (0.719–0.755) | 0.792 (0.776–0.809) | 0.722 (0.704–0.740) | 0.817 (0.802–0.833) | 0.827 (0.812–0.842) |
HOMA-IR homeostasis model assessment-insulin resistance, VAI visceral adiposity index, LAP lipid accumulation product, TyG index triglycerides and glucose index, BMI body mass index, WC waist circumference, AUC area under the curve, PSM propensity score matching with age, sex, and race/ethnicity.
*Other race included non-Hispanic Asian and multi-racial Americans.
Table 4.
Cutoff values for each parameter and their corresponding sensitivity, specificity, and odds ratios for insulin resistance.
| Indirect parameters for insulin resistance | Men | Women | ||
|---|---|---|---|---|
| Cutoff value (sensitivity, Specificity, %) | Odds ratio* (95% CI) | Cutoff value (sensitivity, Specificity, %) | Odds ratio* (95% CI) | |
| VAI | 1.65 (67.3, 69.0) | 4.71 (4.08–5.44) | 1.65 (66.0, 68.2) | 4.27 (3.71–4.90) |
| Hispanics | 1.65 (76.0, 62.0) | 5.22 (3.98–6.84) | 1.95 (64.0, 66.5) | 3.51 (2.75–4.48) |
| Non-Hispanic Whites | 1.65 (72.1, 66.9) | 4.76 (3.81–5.94) | 1.55 (73.0, 66.2) | 4.98 (4.01–6.19) |
| Non-Hispanic Blacks | 1.25 (62.5, 73.3) | 4.61 (3.32–6.40) | 1.15 (69.6, 64.3) | 4.09 (2.96–5.65) |
| Other race† | 1.35 (73.8, 61.1) | 4.54 (2.84–7.25) | 1.35 (77.9, 65.8) | 6.04 (3.72–9.81) |
| LAP | 42.5 (80.0, 66.4) | 8.31 (7.06–9.78) | 42.5 (74.4, 70.1) | 6.97 (6.01–8.08) |
| Hispanics | 49.5 (76.3, 66.8) | 6.40 (4.86–8.43) | 48.5 (75.9, 66.2) | 5.99 (4.59–7.83) |
| Non-Hispanic Whites | 42.5 (85.4, 63.4) | 9.34 (7.14–12.23) | 43.5 (75.2, 73.6) | 7.90 (6.31–9.89) |
| Non-Hispanic Blacks | 33.5 (78.3, 74.3) | 10.27 (7.11–14.84) | 36.5 (73.6, 68.8) | 6.48 (4.61–9.12) |
| Other race† | 32.5 (82.8, 66.5) | 9.94 (5.84–16.94) | 28.5 (86.3, 68.3) | 13.06 (7.40–23.03) |
| TyG index | 4.665 (71.9, 61.8) | 4.26 (3.68–4.92) | 4.575 (69.2, 63.2) | 4.10 (3.56–4.72) |
| Hispanics | 4.755 (70.3, 66.8) | 4.80 (3.71–6.22) | 4.665 (66.3, 64.2) | 3.33 (2.60–4.26) |
| Non-Hispanic Whites | 4.665 (76.6, 60.7) | 4.51 (3.59–5.68) | 4.605 (71.5, 66.6) | 4.67 (3.77–5.78) |
| Non-Hispanic Blacks | 4.565 (68.4, 66.2) | 4.07 (2.93–5.65) | 4.455 (68.6, 64.2) | 4.30 (3.12–5.93) |
| Other race† | 4.675 (66.7, 61.1) | 3.44 (2.21–5.35) | 4.565 (71.2, 67.7) | 4.83 (3.06–7.63) |
| TyG-BMI | 135.5 (72.5, 77.2) | 8.62 (7.42–10.00) | 135.5 (71.9, 76.9) | 9.10 (7.84–10.57) |
| Hispanics | 139.5 (72.7, 77.4) | 8.32 (6.36–10.88) | 135.5 (78.2, 70.6) | 7.85 (5.98–10.31) |
| Non-Hispanic Whites | 137.5 (72.6, 79.9) | 9.17 (7.29–11.54) | 132.5 (73.9, 79.8) | 10.8 (8.64–13.58) |
| Non-Hispanic Blacks | 132.5 (71.5, 77.9) | 8.49 (6.04–11.94) | 144.5 (68.6, 76.7) | 7.18 (5.19–9.93) |
| Other race† | 124.5 (72.1, 77.6) | 8.52 (5.34–13.59) | 111.5 (79.1, 73.5) | 10.87 (6.60–17.91) |
| TyG-WC | 461.5 (80.4, 70.2) | 10.33 (8.74–12.20) | 440.5 (76.4, 73.6) | 9.38 (8.05–10.94) |
| Hispanics | 477.5 (75.6, 73.8) | 9.05 (6.82–12.00) | 462.5 (72.2, 76.0) | 8.10 (6.22–10.55) |
| Non-Hispanic Whites | 485.5 (76.0, 78.5) | 10.59 (8.32–13.47) | 440.5 (76.6, 76.6) | 10.13 (8.03–12.78) |
| Non-Hispanic Blacks | 456.5 (72.3, 80.0) | 9.72 (6.84–13.80) | 462.5 (69.6, 78.4) | 8.15 (5.83–11.40) |
| Other race† | 451.5 (75.4, 80.4) | 13.08 (7.88–21.70) | 401.5 (77.9, 76.7) | 11.69 (6.96–19.62) |
HOMA-IR homeostasis model assessment-insulin resistance, VAI visceral adiposity index, LAP lipid accumulation product, TyG index triglycerides and glucose index, BMI body mass index, WC waist circumference.
*Adjusted for Age, race, smoking status, and blood pressure.
†Other race included non-Hispanic Asian and multi-racial Americans.
Discussion
In this study, we investigated the discriminatory accuracy of novel lipid indices for IR and confirmed that LAP showed significantly higher AUC than TyG index and VAI. There was a significant increase in AUC when BMI or WC was combined with TyG index, exhibiting an even higher discriminatory accuracy than that of LAP. Another important aspect of this study is that the cutoff value of each parameter for IR was presented using large-scale data, facilitating the clinical application of each parameter.
Although the mechanism through which lipid indices cause IR remains unclear, numerous studies have reported that glucolipotoxicity is a key mechanism in the modulation of IR32,33. Ectopic lipid accumulation in the liver and skeletal muscle tissue activates pathways that are associated with IR, leading to a decrease in the glucose uptake in muscle tissue and glycogen synthesis in the liver34–37. Insulin resistance in muscle tissue due to ectopic lipid accumulation increases hepatic lipogenesis and leads to IR in the liver and hyperlipidemia38–40. In addition, macrophage infiltration into white adipose tissue increases lipolysis, which stimulates hepatic triglyceride synthesis, thereby, promoting hyperlipidemia33. Macrophage-induced lipolysis in white adipose tissue also leads to increased hepatic gluconeogenesis and results in hyperglycemia through increased fatty acid delivery to the liver, which results in increased glycerol conversion to glucose41–43.
VAI uses BMI, WC, and triglyceride and HDL cholesterol levels to evaluate IR and was proposed by Amato et al. in 201021. First proposed by Kahn et al. using the NHANES data22,23, LAP is calculated using WC and fasting triglyceride levels. In a previous study conducted by Amato et al., VAI showed a significant inverse correlation with insulin sensitivity measured using a HIEG clamp, providing evidence that VAI can be a surrogate marker for IR21. In addition, VAI was reported to be associated with the glucose distribution rate evaluated through the HIEG clamp test in a study on patients with type 1 diabetes mellitus (DM)26, and it was shown to be inversely correlated with HIEG clamp tested insulin sensitivity in studies conducted on women with polycystic ovary syndrome (PCOS) in South Korea and China27,44. In the case of LAP, a small-scaled study with PCOS patients demonstrated a modest inverse correlation with insulin sensitivity measured through HIEG clamp test27. However, most of these studies using the HIEG clamp test had a small sample, and the clinical application of VAI and LAP requires an investigation of appropriate cutoff values through large scale population-based studies. In addition to studies measuring insulin resistance directly, there are numerous studies on the accuracy of VAI and LAP in assessing HOMA-IR defined insulin resistance. However, studies on cutoff values were mainly conducted on a small number of PCOS patients45–47. Thus, a study with a larger population with healthy adults is necessary. The current study is significant as it presents the cutoff values of VAI and LAP by sex, using a large and healthy population. Interestingly, the VAI cutoff value identified in this study is similar to the VAI cutoff value of 1.6–1.8 reported in the previous studies with PCOS patients27,45,46. However, in the case of LAP, previous studies have reported diverse cutoff values ranging from 18.5 to 33.8, and this study shows a higher cutoff value than that reported in the preceding studies27,45,46.
TyG index was proposed as a useful surrogate measure of IR by Guerrero-Romero et al. in 200824. Despite the small scale of previous studies, TyG index displayed an inverse correlation with insulin sensitivity measured through HIEG clamp and MMAMG25,28. Furthermore, various epidemiological studies have reported that TyG index is associated with the incidences of cardiovascular disease (CVD) and DM, indicating that TyG index is able to predict diseases that result from IR48–50. However, there are a few studies on the estimation of the cutoff value of TyG index for IR. Guerrero-Romero et al. suggested that the best value of the TyG index for the diagnosis of IR was 4.68 using the HIEG clamp test with a small sample size25. In a study with Korean NHANES, the cutoff values for metabolic syndrome, which is a pathological condition associate with IR, were 4.76 in men and 4.71 in women19. In a prospective cohort study with Korean population, the cutoff value to predict DM was 4.6949. In our study, the cutoff value of TyG index was 4.66 in men and 4.57 in women, which is similar to those of previous studies.
Recently, there have been studies on indices that combine adiposity status with the TyG index29–31. Considering that adipose tissues secrete inflammatory cytokines, adipokines, and reactive oxygen species, contributing to a variety of metabolic problems51–53, compound indices with TyG index and obesity parameters such as BMI and WC might be better indicators of IR than TyG index alone. Several studies reported that the compound indices were significantly associated with metabolic abnormalities such as high blood pressure, nonalcoholic fatty liver disease, prediabetes, DM, and hyperuricemia29–31,54–56. In addition, recent studies have indicated that TyG-BMI or TyG-WC is more effective in the identification of IR than VAI, LAP, and TyG index29,31. Er et al. reported the AUCs for IR were 0.734 for VAI, 0.761 for LAP, 0.708 for TyG index, 0.801 for TyG-BMI, and 0.772 for TyG-WC and proposed TyG-BMI as a clinically useful surrogate marker for the early identification of IR29. Lim et al. reported that the AUCs for TyG-BMI and TyG-WC (0.748 and 0.731, respectively) were larger than that for TyG index (0.690)31. However, such studies were conducted only in Asian populationss, and no studies have been conducted on other ethnic populations. Therefore, the current study is meaningful as it confirms using large-scale data that TyG-BMI or TyG-WC can be an effective surrogate marker for IR in the US population with various races/ethnicities. Nonetheless, to accurately assess the correlation of IR with TyG-BMI and TyG-WC, verification through the HIEG clamp test is required as has been performed for HOMA-IR and TyG index.
The present study has several strengths. This study is the largest to evaluate the performance of the novel lipid indices to identify insulin resistance in the general US population. In addition, this study conducted various subgroup analyses of IR, with age, sex, and PSM data, to minimize the bias caused by heterogeneity due to demographic characteristics. Moreover, it is important to propose valid cutoff values for each lipid index so that they can be used as a reference in clinical settings for identifying groups at risk for IR. To the best of our knowledge, this is the first study that evaluated the performance and cutoff values of TyG-BMI and TyG-WC in a non-Asian population. However, considering that this is a cross-sectional study, further prospective studies are required to validate the relationship between each surrogate measure and cardiovascular risk factors.
Conclusion
The present study supports the clinical relevance of novel lipid indices in identifying IR in the general US population. Considering that lipid indices can be easily calculated with routine laboratory tests, they can be useful markers of insulin resistance risk assessments in clinical settings. Moreover, the cutoff values presented in our study may be useful in interpreting the results of lipid indices for IR.
Methods
Study population
The NHANES is a cross-sectional study that uses a representative sample of the population living in the United States. The NHANES, administered by the Centers for Disease Control and Prevention every two years, consists of health and nutrition surveys, physical examinations, and laboratory tests. Of the 92,062 individuals who participated in the NHANES between 1999 and 2016, 11,378 adult participants were included in this study after excluding those who were aged < 20 years (n = 42,550), those with missing or incomplete anthropometric and fasting laboratory data (n = 28,694), those on medication for dyslipidemia and hypertension (n = 6598), those with DM, CVD, stroke, cancer, chronic liver disease, and estimated glomerular filtration rate < 60 ml/min/1.73 m2 (n = 2842) (Fig. 1).
Anthropometric and laboratory measurements
Waist circumference was measured using a flexible tape between the uppermost lateral border of the right ilium and that of the left ilium. BMI was defined as the weight in kilograms divided by the height in meters squared (kg/m2). Blood pressure was measured 3 times in the sitting position, with at least 5 min of rest in between each reading. The mean value of the three recorded blood pressure readings was used in this study. Fasting blood glucose and lipid levels were measured using the enzymatic method, and fasting insulin was measured using an immune-enzymometric assay. Detailed sample collection and processing instructions are described in the NHANES Laboratory Procedures Manual57.
Calculation of parameters for insulin resistance
Parameters for insulin resistance were calculated as follows21–25,29,58:
We define IR as a HOMA-IR value above the 75th percentile for each race/ethnicity and sex (Hispanics: Men > 3.62, Women > 3.44; Non-Hispanic Whites: Men > 3.07, Women > 2.53; Non-Hispanic Blacks: Men > 3.18, Women > 3.59; other race: Men > 2.96, Women > 2.53)12,15.
Statistical analysis
Data were presented as mean with standard deviation, or number with prevalence (%) of IR status. Between groups, the differences were determined using t-tests and a Pearson chi-square test. The values of each lipid index for IR were presented as median and interquartile range. To compare the relative diagnostic strength of each lipid index for insulin resistance, AUC was compared using the ROC curve; de Long’s test was used to identify the surrogate measures that were significantly superior for insulin resistance. The cutoff value of each lipid index was determined as the value with the highest Youden index score. Considering the heterogeneity of demographic characteristics such as sex and race/ethnicities, subgroup analyses for IR were performed. Further analysis was performed by 1:1 PSM with age, sex, and race/ethnicities. This was performed using the “MatchIt” package with nearest-neighbor 1-to-1 matching59. Furthermore, OR of HOMA-IR defined IR was checked using the multivariate logistic regression models based on the estimated cutoff values. Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA) and R ver. 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). The results were considered statistically significant if the p-value was less than 0.05.
Ethics statement
This study was approved by the institutional review board of Kangnam Sacred Heart Hospital (IRB No. HKS 2017-07-007) and the NHANES was approved by the Research Ethics Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention (NHANES 1999–2004, Protocol #98-12; NHANES 2005–2010, Protocol #2005–06; NHANES 2011–2016, Protocol #2011-17). All participants volunteered and provided written informed consent before enrolment. All participants’ records were anonymized before being accessed by the authors. All methods were carried out in accordance with the principles contained in the Declaration of Helsinki.
Author contributions
S.M. and J.G.K. contributed to the research design; W.K., C.A., H.Y.C., J.G.K., J.K. and H.S. participated in the design and performance of the research and data analysis; S.M., B.K., J.G.K. and J.L. wrote the main manuscript text and S.M. prepared Figs. 1 and 2. All authors reviewed the manuscript. Correspondence and requests for materials should be addressed to J.G.K. or S.M.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Juncheol Lee and Bongyoung Kim.
Contributor Information
Jun Goo Kang, Email: kjg0804@empas.com.
Shinje Moon, Email: sinjei1129@gmail.com.
References
- 1.Ascaso JF, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 2.Hanefeld M. The metabolic syndrome: Roots, myths, and facts. In: Hanefeld M, Leonhardt W, editors. The Metabolic Syndrome. Portland: Gustav Fischer; 1997. pp. 13–24. [Google Scholar]
- 3.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Després JP, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 5.Kuusisto J, Mykkanen L, Pyorala K, Laakso M. Hyperinsulinemic microalbuminuria. A new risk indicator for coronary heart disease. Circulation. 1995;91:831–837. doi: 10.1161/01.CIR.91.3.831. [DOI] [PubMed] [Google Scholar]
- 6.Shinozaki K, et al. Role of insulin resistance associated with compensatory hyperinsulinemia in ischemic stroke. Stroke. 1996;27:37–43. doi: 10.1161/01.STR.27.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Goodarzi MO, et al. Relative impact of insulin resistance and obesity on cardiovascular risk factors in polycystic ovary syndrome. Metabolism. 2003;52:713–719. doi: 10.1016/S0026-0495(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J. Clin. Investig. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenfield MS, et al. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 12.Du T, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Miller WG, et al. Toward standardization of insulin immunoassays. Clin. Chem. 2009;55:1011–1018. doi: 10.1373/clinchem.2008.118380. [DOI] [PubMed] [Google Scholar]
- 15.Radikova Z, et al. Insulin sensitivity indices: A proposal of cut-off points for simple identification of insulin-resistant subjects. Exp. Clin. Endocrinol. Diabetes. 2006;114:249–256. doi: 10.1055/s-2006-924233. [DOI] [PubMed] [Google Scholar]
- 16.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet. Med. 2000;17:299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 17.Marques-Vidal P, et al. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25:1371–1377. doi: 10.2337/diacare.25.8.1371. [DOI] [PubMed] [Google Scholar]
- 18.Gayoso-Diz P, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013;13:47. doi: 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon S, et al. The cut-off values of surrogate measures for insulin sensitivity in a healthy population in Korea according to the Korean National Health and nutrition examination survey (KNHANES) 2007–2010. J. Korean Med. Sci. 2018;33:e197. doi: 10.3346/jkms.2018.33.e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh WC, et al. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: A cross-sectional study. Lipids Health Dis. 2019;18:176. doi: 10.1186/s12944-019-1123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amato MC, et al. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am. J. Clin. Nutr. 2003;78:928–934. doi: 10.1093/ajcn/78.5.928. [DOI] [PubMed] [Google Scholar]
- 23.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: A population-based comparison. Diabetes Care. 2006;29:151–153. doi: 10.2337/diacare.29.01.06.dc05-1805. [DOI] [PubMed] [Google Scholar]
- 24.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero-Romero F, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 26.Uruska A, et al. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J. Clin. Lipidol. 2018;12:734–740. doi: 10.1016/j.jacl.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Long J, et al. Screening for a simple and effective indicator of insulin resistance in Chinese reproductive-aged women, with the insulin clamp technique as a reference. Metab. Syndr. Relat. Disord. 2019;17:423–429. doi: 10.1089/met.2019.0019. [DOI] [PubMed] [Google Scholar]
- 28.Vasques AC, et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Er LK, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng S, et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 2016;14:260. doi: 10.1186/s12967-016-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and nutrition examination survey. PLoS ONE. 2019;14:e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bickerton AS, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 33.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dresner A, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Investig. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidyli nositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 36.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 37.Szendroedi J, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JK, et al. Glucose toxicity and the development of diabetes in mice with muscle specific inactivation of GLUT4. J. Clin. Investig. 2001;108:153–160. doi: 10.1172/JCI10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen KF, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Investig. 2002;109:1345–1350. doi: 10.1172/JCI0215001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry RJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry RJ, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Med. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Previs SF, Cline GW, Shulman GI. A critical evaluation of mass isotopomer distribution analysis of gluconeogenesis in vivo. Am. J. Physiol. 1999;277:E154–E160. doi: 10.1152/ajpendo.1999.277.1.E154. [DOI] [PubMed] [Google Scholar]
- 44.Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring) 2013;21:1690–1694. doi: 10.1002/oby.20096. [DOI] [PubMed] [Google Scholar]
- 45.Ramezani Tehrani F, Minooee S, Azizi F. Comparison of various adiposity indexes in women with polycystic ovary syndrome and normo-ovulatory non-hirsute women: A population-based study. Eur. J. Endocrinol. 2014;171:199–207. doi: 10.1530/EJE-14-0094. [DOI] [PubMed] [Google Scholar]
- 46.Huang X, et al. Body fat indices as effective predictors of insulin resistance in obese/non-obese polycystic ovary syndrome women in the Southwest of China. Endocrine. 2019;65:81–85. doi: 10.1007/s12020-019-01912-1. [DOI] [PubMed] [Google Scholar]
- 47.Abruzzese GA, et al. Lipid accumulation product (LAP) and visceral adiposity index (VAI) as markers of insulin resistance and metabolic associated disturbances in young argentine women with polycystic ovary syndrome. Horm. Metab. Res. 2017;49:23–29. doi: 10.1055/s-0042-113463. [DOI] [PubMed] [Google Scholar]
- 48.Li S, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: A retrospective cohort analysis. Sci. Rep. 2019;9:7320. doi: 10.1038/s41598-019-43776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim B, et al. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean genome and epidemiology study (KOGES) PLoS ONE. 2018;13:e0206994. doi: 10.1371/journal.pone.0206994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SH, et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju metabolic disease cohort (CMC) study. PLoS ONE. 2014;9:e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013;7:e330–341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, et al. Triglyceride glucose-body mass index is effective in identifying nonalcoholic fatty liver disease in nonobese subjects. Medicine (Baltimore) 2017;96:e7041. doi: 10.1097/MD.0000000000007041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu Q, et al. Associations of triglyceride-glucose index and its derivatives with hyperuricemia risk: A cohort study in Chinese general population. Int. J. Endocrinol. 2020;2020:3214716. doi: 10.1155/2020/3214716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramdas Nayak VK, Nayak KR, Vidyasagar S, Rekha P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndr. 2020;14:1265–1272. doi: 10.1016/j.dsx.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey (NHANES) MEC Laboratory Procedures Manual (2016). https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_mec_laboratory_procedures_manual.pdf. Accessed 18 January 2021.
- 58.Morales-Gurrola G, et al. The triglycerides and glucose index is associated with cardiovascular risk factors in metabolically obese normal-weight subjects. J. Endocrinol. Investig. 2020;43:995–1000. doi: 10.1007/s40618-020-01184-x. [DOI] [PubMed] [Google Scholar]
- 59.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]


