Abstract
Increasing studies have suggested that microRNAs (miRNAs) are involved in the development of gliomas. MicroRNA-216a has been reported to be a tumor-associated miRNA in many types of cancer, either as an oncogene or as a tumor suppressor. However, little is known about the function of miR-216a in gliomas. The present study was designed to explore the potential role of miR-216a in gliomas. We found that miR-216a was significantly decreased in glioma tissues and cell lines. Overexpression of miR-216a significantly suppressed the proliferation, migration, and invasion of glioma cells. Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) was identified as a target gene of miR-216a in glioma cells by bioinformatics analysis, dual-luciferase reporter assay, real-time quantitative polymerase chain reaction, and Western blot analysis. Moreover, miR-216a overexpression inhibited the Wnt/β-catenin signaling pathway. The restoration of LGR5 expression markedly reversed the antitumor effect of miR-216a in glioma cells. Taken together, these findings suggest a tumor suppressor role for miR-216a in gliomas, which inhibits glioma cell proliferation, migration, and invasion by targeting LGR5. Our study suggests that miR-216a may serve as a potential therapeutic target for future glioma treatment.
Key words: Glioma, Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), Proliferation, Migration, Invasion
INTRODUCTION
Gliomas are the most prevalent and malignant brain tumor with an increasing incidence worldwide1. The diagnosis and prognosis of glioma remain poor2. Although treatment approaches have made certain progress, overall survival has not effectively improved3. Glioma remains a refractory disease because of the unrestricted proliferation and extensive metastasis of tumors4. Therefore, an improved understanding of the molecular pathogenesis of glioma is essential for the development of effective treatment.
MicroRNAs (miRNAs), a group of small, endogenous, and noncoding RNAs (∼22 nucleotides in length), play a vital role in various life activities and diseases5. miRNAs negatively modulate gene expression on a transcription level by targeting the 3′-untranslated region (3′-UTR) of mRNA6,7. Many studies have shown that miRNAs regulate various cellular processes, including cell proliferation, differentiation, migration, invasion, and apoptosis8. Aberrantly expressed miRNAs in cancer development and progression have been suggested as oncogenes or tumor suppressors9,10. Emerging evidence has suggested that miRNAs are promising targets for cancer treatment11. In recent years, increasing studies have reported that miRNAs participate in glioma tumorigenesis12. Targeting specific miRNAs inhibits the tumorigenesis of glioma cells13,14. Thus, a better understanding of miRNAs in glioma may provide novel insights for treatment development.
The leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), also known as G-protein coupled receptor 49, has been recognized as an oncogene in several types of cancers15,16. LGR5 is identified as an orphan G protein-coupled receptor encompassing a seven-transmembrane domain and an extracellular domain with 17 leucine-rich repeats17. LGR5 is a somatic stem cell marker and plays an important role during development18,19. LGR5 is highly expressed during embryonic development, but its expression is restricted in stem cells in postnatal tissues20,21. However, aberrantly expressed LGR5 is associated with tumorigenesis15,16. LGR5 is correlated with the clinical grade of glioma, and depletion of LGR5 promotes apoptosis of brain cancer stem-like cells22. Silencing of LGR5 inhibits the proliferation of glioma cells23. Several studies have shown that LGR5 is an important regulator of the Wnt/β-catenin signaling pathway, a critical oncogenic signaling pathway in cancers24. LGR5 may therefore represent a potential therapeutic target for glioma.
Several studies have shown that miR-216a is involved in tumorigenesis in some types of human cancers such as pancreatic cancer25, oral squamous cell carcinoma26, and liver cancer27. However, little is known about the function of miR-216a in glioma. In the present study, we demonstrated an important role for miR-216a in glioma. We found that miR-216a was significantly decreased in glioma tissues and cell lines. Overexpression of miR-216a significantly suppressed the proliferation, migration, and invasion of glioma cells. Interestingly, LGR5 was identified as a target gene of miR-216a in glioma cells. Moreover, miR-216a overexpression inhibited the Wnt/β-catenin signaling pathway in glioma cells. In addition, the restoration of LGR5 expression markedly reverses the antitumor effect of miR-216a in glioma cells. Taken together, these findings suggest a tumor suppressor role for miR-216a in glioma, which inhibits glioma cell proliferation, migration, and invasion by targeting LGR5. Our study suggests that miR-216a/LGR5 may play an important role in the progression of glioma, serving as a potential therapeutic target for future glioma treatment.
MATERIALS AND METHODS
Human Tissue Samples
Human glioma tissue samples were collected from 15 patients who underwent surgical resection in The No.1 Hospital of Xi’an. Normal brain tissue samples were collected from five patients who underwent traumatic brain injuries. All patients who participated in this study signed an informed consent prior to sample collection. The experiment was approved by the Institutional Human Experiment and Ethics Committee of The No.1 Hospital of Xi’an and was performed in accordance with the Helsinki Declaration.
Cell Lines
Human glioma cell lines (U251MG, U87MG, U118, and A172) and normal human astrocytes (NHA) were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin mix (Sigma-Aldrich, St. Louis, MO, USA) and were incubated in 5% CO2 at 37°C.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Dusseldorf, Germany) and reverse transcribed into cDNA using M-MLV reverse transcriptase (TaKaRa, Dalian, P.R. China) and the miRcute miRNA cDNA kit (TIANGEN, Beijing, P.R. China) according to the manufacturers’ instructions. RT-qPCR was carried out with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and the appropriate primers: miR-216a, 5′-ATCCAGTGCGTGTCGTG-3′ (forward) and 5′-TGCTTAATCTCAGCTGGCA-3′ (reverse); U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse); LGR5, 5′-GAGGATCTGGTGAGCCTGAGAA-3′ (forward) and 5′-CATAAGTGATGCTGGAGCTGGTAA-3′ (reverse); cyclin D1, 5′-AACTACCTGGACCGCTTCCT-3′ (forward) and 5′-CCACTTGAGCTTGTTCACCA-3′ (reverse); c-myc, 5′-TCAAGAGGTGCCACGTCTCC-3′ (forward) and 5′-TCTTGGCAGCAGGATAGTCCTT-3′ (reverse); GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse). U6 and GAPDH served as internal controls. Relative gene expression was calculated using the 2−ΔΔCt method.
Cell Transfection
The miR-216a mimics and negative control (NC) were purchased from GenePharma (Shanghai, P.R. China). The cDNA of LGR5 without 3′-UTR was cloned into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to generate the pcDNA3.1-LGR5 vector. Cells were allowed to grow to 80% cell confluence before transfection. Cell transfection of miRNAs or the vector was conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. The transfection efficacy was measured by RT-qPCR or Western blot after 48 h of transfection.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
Cells were plated into 96-well plates at a density of 1 × 104 cells/well. After transfection of miR-216a mimics for 48 h, cells were incubated with 5 mg/ml MTT (Sigma-Aldrich; 20 μl/well) for 4 h. Thereafter, the medium was discarded from the wells by aspiration, and the formazan crystals in living cells were dissolved by 200 μl/well dimethyl sulfoxide (DMSO; Sigma-Aldrich). The absorbance value at 490 nm was measured with an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Rad, Hercules, CA, USA).
Colony Formation Assay
Cells were transfected with miR-216a mimics for 48 h and then seeded into six-well plates (1,000 cells/well) in 0.4% agar medium for 2 weeks. Colonies were stained with 0.1% crystal violet (Sigma-Aldrich) and counted under a microscope (Olympus, Tokyo, Japan).
Cell Cycle Assay
The cell cycle was synchronized by serum starvation for 24 h and then transfected with miR-216a mimics for 48 h. Cells were collected with trypsin, washed with ice-cold phosphate-buffered saline, and fixed with 70% ethanol at 4°C overnight. Afterward, cells were treated with 50 μg/ml RNase A for 30 min at room temperature and then treated with 100 μg/ml of propidium iodide (Sigma-Aldrich) for 30 min in the dark. Cell cycle distribution was detected by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed by CellQuest software.
Cell Migration and Invasion Assays
For the migration assay, transfected cells (1 × 104 cells) were suspended in 0.5 ml of serum-free medium and seeded into the upper chambers of 24-well Transwell plates (Corning Inc., Corning, NY, USA). The lower chambers were filled with 0.5 ml of growth medium containing 10% FBS. Cells were cultured at 37°C and allowed to migrate for 24 h. After migration, cells in the top chambers were removed by a cotton swab, and the cells in the bottom chambers were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet (Sigma-Aldrich). The stained cells were counted under a microscope (Olympus). For the invasion assay, a similar protocol was followed except that the top chamber of the Transwell plate was precoated with Matrigel (BD Biosciences).
Dual-Luciferase Reporter Assay
The cDNA fragment of LGR5 3′-UTR harboring the seed-matched sequences or mutated sequences of miR-216a was inserted into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA). Cells were cotransfected with miR-216a mimics and pmirGLO luciferase reporter for 48 h. Then cell lysates were harvested, and luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega).
Western Blot Analysis
Equivalent amounts of protein (50 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gels and transferred to a nitrocellulose membrane (Millipore, Boston, MA, USA). The membrane was blocked with 3% nonfat milk and then probed with primary antibodies (anti-LGR5, anti-β-catenin, and anti-GAPDH; Abcam, Cambridge, UK) with the recommended concentrations at 4°C overnight. Horseradish peroxidase-conjugated secondary antibody (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was then used to probe the membrane for 1 h at room temperature. Blots were developed with the Pierce ECL Western Blotting Kit (Pierce, Rockford, IL, USA). The intensity of the membrane was analyzed by Image-Pro Plus 6.0 software.
Data Analysis
Data were expressed in the form of means ± standard deviation. SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis. Comparisons between the two groups (n = 2) were performed using the Student’s t-test. Comparisons among multiple groups (n ≥ 3) were performed using one-way analysis of variance followed by the Bonferroni test. Values of p < 0.05 were regarded as statistically significant.
RESULTS
Expression of miR-216a Is Decreased in Glioma Tissues and Cell Lines
To investigate the potential role of miR-216a in glioma, we first detected its expression profile in glioma tissue specimens by RT-qPCR. The data showed that miR-216a expression in glioma tissues was much lower than that in normal brain tissues (Fig. 1A). We then examined the expression of miR-216a in human glioma cell lines. We found that miR-216a expression was significantly downregulated in glioma cell lines including U251MG, U87MG, U118, and A172 compared with NHA (Fig. 1B). These results suggest a tumor-suppressive role for miR-216a in glioma.
Figure 1.
Decreased expression of miR-216a in glioma. (A) Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of miR-216a expression in human glioma specimens and normal brain tissues. *p < 0.05. (B) RT-qPCR analysis of miR-216a expression in human glioma cell lines (U251MG, U87MG, U118, and A172) and normal human astrocytes (NHA). *p < 0.05 versus NHA.
Overexpression of miR-216a Suppresses the Proliferation of Glioma Cells
To investigate the exact biological role of miR-216a in glioma cells, we overexpressed miR-216a in U251MG and U87MG cells by transfection of miR-216a mimics and detected its effect on glioma cell proliferation. The expression of miR-216a was significantly upregulated by transfection of miR-216a mimics (Fig. 2A). The MTT assay showed that overexpression of miR-216a significantly inhibited glioma cell proliferation (Fig. 2B). The colony-forming capacity of glioma cells was also markedly repressed by miR-216a overexpression, as detected by the colony formation assay (Fig. 2C). Moreover, overexpression of miR-216a induced cell cycle arrest in the G0/G1 phase (Fig. 2D). Taken together, these results suggest that miR-216a functions as a tumor suppressor by inhibiting glioma cell proliferation.
Figure 2.
miR-216a suppresses glioma cell proliferation. U251MG and U87MG cells were transfected with miR-216a mimics or miR-NC for 48 h and then subjected to the following detections: (A) the expression of miR-216a in U251MG and U87MG cells was examined by RT-qPCR analysis; (B) the cell proliferation of U251MG and U87MG cells was assessed by the MTT assay; (C) the colony-forming capacity of U251MG and U87MG cells was evaluated by the colony formation assay; (D) the cell cycle in the G0/G1 phase of U251MG and U87MG cells was detected by flow cytometry. *p < 0.05; **p < 0.01.
Overexpression of miR-216a Inhibits the Migration and Invasion of Glioma Cells
To further investigate the antitumor effect of miR-216a in glioma cells, we detected the effect of miR-216a overexpression of cell migration and invasion by Transwell assays. The results showed that both the migration (Fig. 3A) and invasion (Fig. 3B) abilities of glioma cells were significantly reduced by miR-216a overexpression. These data indicate that miR-216a suppresses glioma cell migration and invasion.
Figure 3.
miR-216a suppresses glioma cell migration and invasion. The migration (A) and invasion (B) of U251MG and U87MG cells were determined by Transwell assays. U251MG and U87MG cells transfected with miR-216a mimics or miR-NC for 48 h were subjected to Transwell assays. After being cultured for 24 h in Transwell plates, the migrated or invaded cells were stained and counted under a microscope. *p < 0.05.
miR-216a Directly Targets LGR5 in Glioma Cells
To investigate the underlying mechanism of miR-216a in regulating glioma cell proliferation, migration, and invasion, we adopted bioinformatics analysis to predict the potential targets of miR-216a in glioma cells. We found that the 3′-UTR of LGR5, an oncogene of glioma23, contains binding sites of miR-216a (Fig. 4A). To investigate whether miR-216a directly binds to the 3′-UTR of LGR5, we constructed a luciferase reporter vector containing the 3′-UTR of LGR5. We found that the luciferase activity in the luciferase reporter vector containing the 3′-UTR of LGR5 was significantly decreased by miR-216a overexpression (Fig. 4B and C). However, the luciferase activity in the luciferase reporter vector containing the mutant 3′-UTR of LGR5 was not obviously affected by miR-216a overexpression (Fig. 4B and C). Furthermore, both mRNA (Fig. 5A) and protein (Fig. 5B) levels were significantly decreased upon miR-216a overexpression. Overall, these results suggest that LGR5 is a direct target gene of miR-216a in glioma cells.
Figure 4.
miR-216a targets the LGR5 3′-untranslated region (3′-UTR). (A) Predicted miR-216a target sequences in 3′-UTR of LGR5. Dual-luciferase reporter assay in U251MG (B) and U87MG (C) cells cotransfected with miR-216a mimics and luciferase reporters containing either the predicted miR-216a target sites in LGR5 3′-UTR or its corresponding mutant form. *p < 0.05.
Figure 5.
miR-216a inhibits LGR5 expression. U251MG and U87MG cells were transfected with miR-216a mimics or miR-NC for 48 h and then subjected to detection. (A) The mRNA expression of LGR5 was detected by RT-qPCR. (B) The protein expression of LGR5 was examined by Western blot analysis. *p < 0.05.
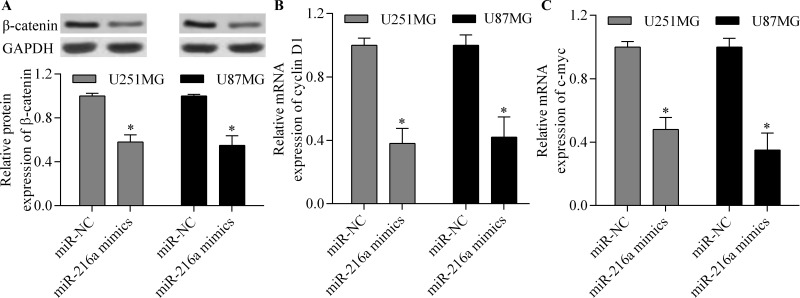
Overexpression of miR-216a Inhibits the Wnt/β-Catenin Signaling Pathway
To further elucidate the molecular basis of miR-216a in regulating glioma cell proliferation, migration, and invasion, we detected the effect of miR-216a on the Wnt/β-catenin signaling pathway. The results showed that overexpression of miR-216a significantly inhibited the protein expression of β-catenin (Fig. 6A). Moreover, the transcription of target genes of the Wnt/β-catenin signaling pathway, including cyclin D1 (Fig. 6B) and c-myc (Fig. 6C), was also significantly reduced by miR-216a overexpression. Overall, these results suggest that the antitumor effect of miR-216a is associated with suppression of the Wnt/β-catenin signaling pathway.
Figure 6.
miR-216a inhibits the Wnt/β-catenin signaling pathway. U251MG and U87MG cells were transfected with miR-216a mimics or miR-NC for 48 h and then subjected to analysis. (A) Protein expression of β-catenin was detected by Western blot assay. The mRNA expression of cyclin D1 (B) and c-myc (C) was detected by RT-qPCR. *p < 0.05.
Overexpression of LGR5 Abolishes the Antitumor Effects of miR-216a
To confirm that LGR5 contributes to miR-216a-induced antitumor effects, we performed a rescue assay. U251MG and U87MG cells were cotransfected with miR-216a mimics and LGR5-expressing vector. The results showed that transfection with the LGR5-expressing vector significantly restored LGR5 expression in U251MG- and U87MG-miR-216a-transfected cells (Fig. 7A and B). The restoration of LGR5 expression significantly reversed the inhibitor effects of miR-216a overexpression on glioma cell proliferation (Fig. 8A), migration (Fig. 8B), and invasion (Fig. 8C). Overall, these results suggest that miR-216a inhibits glioma cell proliferation, migration, and invasion through suppression of LGR5.
Figure 7.
Restoration of LGR5 in glioma cells. U251MG and U87MG cells were cotransfected with miR-216a mimics and the pcDNA3.1-LGR5 vector and incubated for 48 h. (A) The mRNA expression of LGR5 was detected by RT-qPCR. (B) The protein expression of LGR5 was examined by Western blot analysis. *p < 0.05 versus miR-NC. &p < 0.05 versus miR-216a mimics + vector.
Figure 8.
Restoration of LGR5 abolishes the antitumor effects of miR-216a. U251MG and U87MG cells were cotransfected with miR-216a mimics and the pcDNA3.1-LGR5 vector for 48 h and then subjected to the following detection. (A) Cell proliferation was detected by MTT assay. Cell migration (B) and invasion (C) were determined by Transwell assays. *p < 0.05 versus miR-NC. &p < 0.05 versus miR-216a mimics + vector.
DISCUSSION
A growing body of evidence suggests the importance of miRNAs in glioma28,29. However, the precise function of dysregulated miRNAs in glioma progression and development is still poorly understood. In this study, we report a tumor-suppressive role for miR-216a in glioma cells. We found that miR-216a was decreased in glioma tissues and cell lines and that overexpression of miR-216a inhibited glioma cell proliferation and invasion. Our study suggests that dysregulated miR-216a expression contributes to glioma tumorigenesis.
miR-216a, a member of the miR-216 family, is widely expressed in many species30. It has been reported to play a critical role in the pathogenesis of kidney disorders31, pancreatitis32, diabetic nephropathy33, and endothelial dysfunction34. Interestingly, the role of miR-216a in tumorigenesis has also been extensively studied. The expression of miR-216a is decreased in pancreatic cancer35,36, and decreased expression of miR-261a in feces may serve as a biomarker for pancreatic cancer37. Overexpression of miR-216a inhibits the tumor growth of pancreatic cancer through targeting Janus kinase 225,38. Moreover, miR-261a promotes the radiosensitivity of pancreatic cancer cells through suppression of Beclin-1-mediated autophagy39. Li et al. reported that miR-216a suppressed the growth, migration, and invasion of oral squamous cell carcinoma cells by targeting eukaryotic translation initiation factor 4B26. miR-216a may inhibit the tumorigenesis and angiogenesis of breast cancer cells by targeting the 3′-UTR of CD4430. All of these reports demonstrate a tumor suppressive role for miR-216a. However, an oncogenic role for miR-216a has also been found in other cancers. The increased expression of miR-216a induced by the androgen pathway inhibits the tumor suppression in the lung cancer-1 gene in early hepatocarcinogenesis40. miR-216a induces the epithelial–mesenchymal transition of hepatocellular carcinoma cells through inhibiting the phosphatase and tensin homolog and mothers against decapentaplegic homolog 727. Overexpression of miR-216a suppresses bicalutamide-mediated growth suppression in prostate cancer cells41. In our study, we found that miR-216a was decreased in glioma, and overexpression of miR-216a inhibits glioma cell proliferation and invasion, supporting a tumor-suppressive role for miR-216a. However, the precise role of miR-216a in tumorigenesis needs to be further studied.
LGR5, an orphan G protein-coupled receptor, has been suggested as a molecular marker for self-renewing stem cells18,19. The dysregulation of LGR5 has been found in numerous cancers, including gastric cancer, hepatocellular carcinoma, and colorectal cancer15,16,42,43. Increased expression of LGR5 is correlated with lymphatic invasion and poor patient survival15,44,45. LGR5 promotes tumor formation and cell proliferation in basal cell carcinoma cells46. LGR5 also plays a role in glioma. High expression of LGR5 is correlated with the clinical grade of glioma, and depletion of LGR5 promotes apoptosis of brain cancer stem-like cells22. LGR5 is regulated by the proneural factor, which maintains the stem-like cells in glioma47. LGR5 is highly expressed in glioma tissues and cell lines, and silencing of LGR5 inhibits the proliferation of glioma cells in vivo and in vitro23,48. LGR5 induced by SOX9 contributes to the proliferation and tumorigenicity of glioma cells49. All of these findings suggest that LGR5 is a novel and potential therapeutic target for glioma. In this study, we demonstrated that LGR5 is a target gene of miR-216a. The decreased expression of miR-216a may contribute to the high expression of LGR5 in glioma cells. We also showed that inhibition of LGR5 by miR-216a overexpression significantly suppressed the proliferation and invasion of glioma cells. Our study indicates that inhibition of LGR5 by miR-216a may be a promising strategy for the treatment of glioma.
LGR5 has been suggested to be an important regulator of the Wnt/β-catenin signaling pathway, a critical oncogenic signaling pathway in cancers24. R-spondins have been identified as ligands for LGR5, and their engagement can activate the Wnt/β-catenin signaling pathway19,50. LGR5 promotes cancer cell progression by activation of the Wnt/β-catenin signaling pathway in neuroblastoma51, hepatocellular carcinoma43, colon cancer52, and breast cancer53. In this study, we showed that inhibition of LGR5 by miR-216a reduced β-catenin protein levels as well as the transcription of cyclin D1 and c-myc. In line with our findings, a recent study reported that suppression of LGR5 by trichosanthin inhibits the proliferation of glioma cells by suppression of the Wnt/β-catenin signaling pathway54. These findings confirm that LGR5 promotes the tumorigenicity of glioma cells associated with the activation of the Wnt/β-catenin signaling pathway.
Evidence has reported that LGR5 is targeted by miR-142-3p and miR-100 in colon cancer cells55,56, indicating that an epigenetic regulation of LGR5 contributes to tumorigenesis. In this study, we identified that miR-216a was a novel miRNA that targeted and inhibited LGR5 expression. LGR5 epigenetically regulated by miR-216a may contribute to the pathogenesis of glioma. Targeting LGR5 by miR-216a may represent a novel strategy for glioma treatment.
In conclusion, our study reports a tumor suppressive role for miR-216a in glioma. We showed that miR-216a inhibited glioma cell proliferation and invasion by targeting LGR5. miR-216a/LGR5 may play an important role in the pathogenesis of glioma and may serve as potential therapeutic targets for glioma treatment.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Nos. 81301041, 31371501, and 81400382), the Scientific Research Program Funded by the Department of Science and Technology of Shaanxi Province (No. 2015KJXX-43), the Leading Disciplines Development Government Foundation of Shaanxi, China [No. (2014)3-1001], and the Supporting Program Funded by Xi’an Medical University (No. 2016PT06).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: Diagnostic, prognostic, and predictive markers. Lancet Neurol. 2010;9:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Pollack IF. Neuro-oncology: Therapeutic benefits of reirradiation for recurrent brain tumors. Nat Rev Neurol. 2010;6:533–5. [DOI] [PubMed] [Google Scholar]
- 4. Mrugala MM. Advances and challenges in the treatment of glioblastoma: A clinician’s perspective. Discov Med. 2013;15:221–30. [PubMed] [Google Scholar]
- 5. Burnet NG, Lynch AG, Jefferies SJ, Price SJ, Jones PH, Antoun NM, Xuereb JH, Pohl U. High grade glioma: Imaging combined with pathological grade defines management and predicts prognosis. Radiother Oncol. 2007;85:371–8. [DOI] [PubMed] [Google Scholar]
- 6. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 7. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. [DOI] [PubMed] [Google Scholar]
- 8. Manikandan J, Aarthi JJ, Kumar SD, Pushparaj PN. Oncomirs: The potential role of non-coding microRNAs in understanding cancer. Bioinformation 2008;2:330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X, Li Y, Lu H. MiR-1193 suppresses proliferation and invasion of human breast cancer cells through directly targeting IGF2BP2. Oncol Res. 2017;25(4):579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ji S, Zhang B, Kong Y, Ma F, Hua Y. MiR-326 inhibits gastric cancer cell growth through down regulating NOB1. Oncol Res. 2017;25(6):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gentilin E, Degli Uberti E, Zatelli MC. Strategies to use microRNAs as therapeutic targets. Best Pract Res Clin Endocrinol Metab. 2016;30:629–39. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Dutta A, Abounader R. The role of microRNAs in glioma initiation and progression. Front Biosci. (Landmark Ed) 2012;17:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Wang H, Zhu Z, Ye Y, Mao H, Zhang S. MicroRNA-105 targets SOX9 and inhibits human glioma cell progression. FEBS Lett. 2016;590(23):4329–42. [DOI] [PubMed] [Google Scholar]
- 14. Shan ZN, Tian R, Zhang M, Gui ZH, Wu J, Ding M, Zhou XF, He J. miR128-1 inhibits the growth of glioblastoma multiforme and glioma stem-like cells via targeting BMI1 and E2F3. Oncotarget 2016;7(48):78813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon E, Petke D, Boger C, Behrens HM, Warneke V, Ebert M, Rocken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One 2012;7:e35486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuma M, Tanese K, Effendi K, Yamazaki K, Masugi Y, Suda M, Sakamoto M. Leucine-rich repeat-containing G protein-coupled receptor 5 regulates epithelial cell phenotype and survival of hepatocellular carcinoma cells. Exp Cell Res. 2013;319:113–21. [DOI] [PubMed] [Google Scholar]
- 17. Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuijers J, Clevers H. Adult mammalian stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011;476:293–7. [DOI] [PubMed] [Google Scholar]
- 20. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- 21. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- 22. Nakata S, Campos B, Bageritz J, Bermejo JL, Becker N, Engel F, Acker T, Momma S, Herold-Mende C, Lichter P, Radlwimmer B, Goidts V. LGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cells. Brain Pathol. 2013;23:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Zhou J, Fan C, Jiao F, Liu B, Sun P, Miao J, Zhang Q. Knockdown of LGR5 suppresses the proliferation of glioma cells in vitro and in vivo. Oncol Rep. 2014;31:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/beta-catenin signaling. Mol Cell Biol. 2012;32:2054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Chen X, Tang M. MicroRNA-216a inhibits pancreatic cancer by directly targeting Janus kinase 2. Oncol Rep. 2014;32:2824–30. [DOI] [PubMed] [Google Scholar]
- 26. Li L, Ma HQ. MicroRNA-216a inhibits the growth and metastasis of oral squamous cell carcinoma by targeting eukaryotic translation initiation factor 4B. Mol Med Rep. 2015;12:3156–62. [DOI] [PubMed] [Google Scholar]
- 27. Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology 2013;58:629–41. [DOI] [PubMed] [Google Scholar]
- 28. Hummel R, Maurer J, Haier J. MicroRNAs in brain tumors: A new diagnostic and therapeutic perspective? Mol Neurobiol. 2011;44:223–34. [DOI] [PubMed] [Google Scholar]
- 29. Palumbo S, Miracco C, Pirtoli L, Comincini S. Emerging roles of microRNA in modulating cell-death processes in malignant glioma. J Cell Physiol. 2014;229:277–86. [DOI] [PubMed] [Google Scholar]
- 30. Jeyapalan Z, Deng Z, Shatseva T, Fang L, He C, Yang BB. Expression of CD44 3′-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res. 2011;39:3026–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Usborne AL, Smith AT, Engle SK, Watson DE, Sullivan JM, Walgren JL. Biomarkers of exocrine pancreatic injury in 2 rat acute pancreatitis models. Toxicol Pathol. 2014;42:195–203. [DOI] [PubMed] [Google Scholar]
- 33. Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J Biol Chem. 2010;285:34004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A, Ippoliti A, Martelli F, Lauro R, Federici M. MiR-216a: A link between endothelial dysfunction and autophagy. Cell Death Dis. 2014;5:e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azevedo-Pouly AC, Sutaria DS, Jiang J, Elgamal OA, Amari F, Allard D, Grippo PJ, Coppola V, Schmittgen TD. miR-216 and miR-217 expression is reduced in transgenic mouse models of pancreatic adenocarcinoma, knockout of miR-216/miR-217 host gene is embryonic lethal. Funct Integr Genomics 2017;17(2–3):203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Link A, Becker V, Goel A, Wex T, Malfertheiner P. Feasibility of fecal microRNAs as novel biomarkers for pancreatic cancer. PLoS One 2012;7:e42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ, Ou JR. miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Lett. 2015;589:2224–32. [DOI] [PubMed] [Google Scholar]
- 39. Zhang X, Shi H, Lin S, Ba M, Cui S. MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy. Oncol Rep. 2015;34:1557–64. [DOI] [PubMed] [Google Scholar]
- 40. Chen PJ, Yeh SH, Liu WH, Lin CC, Huang HC, Chen CL, Chen DS. Androgen pathway stimulates microRNA-216a transcription to suppress the tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis. Hepatology 2012;56:632–43. [DOI] [PubMed] [Google Scholar]
- 41. Miyazaki T, Ikeda K, Sato W, Horie-Inoue K, Okamoto K, Inoue S. MicroRNA library-based functional screening identified androgen-sensitive miR-216a as a player in bicalutamide resistance in prostate cancer. J Clin Med. 2015;4:1853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–26. [DOI] [PubMed] [Google Scholar]
- 43. Effendi K, Yamazaki K, Fukuma M, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) represents a typical Wnt/beta-catenin pathway-activated hepatocellular carcinoma. Liver Cancer 2014;3:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uchida H, Yamazaki K, Fukuma M, Yamada T, Hayashida T, Hasegawa H, Kitajima M, Kitagawa Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in colorectal cancer. Cancer Sci. 2010;101:1731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamanoi K, Fukuma M, Uchida H, Kushima R, Yamazaki K, Katai H, Kanai Y, Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in gastric cancer. Pathol Int. 2013;63:13–9. [DOI] [PubMed] [Google Scholar]
- 46. Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol. 2008;173:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mao XG, Song SJ, Xue XY, Yan M, Wang L, Lin W, Guo G, Zhang X. LGR5 is a proneural factor and is regulated by OLIG2 in glioma stem-like cells. Cell Mol Neurobiol. 2013;33:851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parry PV, Engh JA. Knockdown of LGR5 suppresses the proliferation of glioma cells in vitro and in vivo. Neurosurgery 2014;74:N14–5. [DOI] [PubMed] [Google Scholar]
- 49. Hiraoka K, Hayashi T, Kaneko R, Nasu-Nishimura Y, Koyama-Nasu R, Kawasaki Y, Akiyama T. SOX9-mediated upregulation of LGR5 is important for glioblastoma tumorigenicity. Biochem Biophys Res Commun. 2015;460:216–21. [DOI] [PubMed] [Google Scholar]
- 50. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 2011;108:11452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vieira GC, Chockalingam S, Melegh Z, Greenhough A, Malik S, Szemes M, Park JH, Kaidi A, Zhou L, Catchpoole D, Morgan R, Bates DO, Gabb PD, Malik K. LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/beta-catenin signalling in neuroblastoma. Oncotarget 2015;6:40053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M, Li J. LGR5 promotes the proliferation of colorectal cancer cells via the Wnt/beta-catenin signaling pathway. Oncol Lett. 2015;9:2859–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang L, Tang H, Kong Y, Xie X, Chen J, Song C, Liu X, Ye F, Li N, Wang N. LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/beta-catenin signaling. Stem Cells 2015;33:2913–24. [DOI] [PubMed] [Google Scholar]
- 54. Miao J, Jiang Y, Wang D, Zhou J, Fan C, Jiao F, Liu B, Zhang J, Wang Y, Zhang Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/beta-catenin signaling pathway. Oncol Rep. 2015;34:2845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou MK, Liu XJ, Zhao ZG, Cheng YM. MicroRNA-100 functions as a tumor suppressor by inhibiting Lgr5 expression in colon cancer cells. Mol Med Rep. 2015;11:2947–52. [DOI] [PubMed] [Google Scholar]
- 56. Shen WW, Zeng Z, Zhu WX, Fu GH. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med. (Berl) 2013;91:989–1000. [DOI] [PubMed] [Google Scholar]