Abstract
We analyzed the results of previously treated patients with metastatic colorectal cancer (mCRC) who received regorafenib plus FOLFIRI with the irinotecan dose escalation on the basis of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping. Thirteen patients with previously treated mCRC were subjected to UGT1A1 genotyping between October 2013 and June 2015 and were administered regorafenib plus FOLFIRI with irinotecan dose escalation. Patients with UGT1A1*1/*1 and *1/*28 genotypes were administered 180 mg/m2 of irinotecan, whereas those with the UGT1A1*28/*28 genotype were administered 120 mg/m2 of irinotecan. For all patients, the irinotecan dose was increased by 30 mg/m2 every two cycles until grade ≥3 adverse events or severe adverse events developed, following which the dose was reverted to and maintained at the previously tolerated level. The oral regorafenib dose was adjusted to 120 mg/day daily. The median follow-up period was 10.0 months (1.0–21.0 months). The disease control rate was 69.2%, whereas the median progression-free survival and overall survival were 9.5 and 13.0 months, respectively. Our findings indicate that regorafenib plus FOLFIRI with irinotecan dose escalation based on UGT1A1 genotyping in previously treated patients with mCRC and with UGT1A1*1/*1 and UGT1A1*1/*28 genotypes is clinically effective and yields improved oncological outcomes.
Key words: UGT1A1, Regorafenib, FORFIRI, Dose escalation, Metastatic colorectal cancer (mCRC)
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths worldwide1. Approximately one fourth of patients with CRC present with metastases at the time of diagnosis. Furthermore, in almost 40% of the remaining patients, the initially limited diseases progress to metastases during treatment, such that up to 60% of these patients are expected to eventually succumb to metastatic CRC (mCRC)2. Moreover, the present chemotherapy for mCRC, 5-fluorouracil (5-FU)/leucovorin (LV) combined with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI), yields a median overall survival (OS) of approximately 20 months, with the 5-year survival not exceeding 10%3. The recent introduction of biological therapies combined with chemotherapies has yielded improved oncological outcomes, with a median OS of approximately 30 months4.
Regorafenib, a novel oral multikinase inhibitor, targets stromal, angiogenic, and oncogenic receptor tyrosine kinases (RTKs). Regorafenib inhibits intracellular and membrane-bound RTKs involved in angiogenesis, oncogenesis, and tumor proliferation signaling5. The CORRECT trial demonstrated that oral regorafenib monotherapy yielded significant differences in the disease control rate, progression-free survival (PFS), and OS for previously treated mCRC (41% vs. 15%, p < 0.001; 1.9 vs. 1.7 months, p < 0.001; 6.4 vs. 5.0 months, p = 0.0052, respectively)6.
The cytotoxicity of irinotecan is derived from its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), which acts on topoisomerase I in vivo to interrupt DNA replication in cancer cells, consequently causing cell death. SN-38 is further metabolized by uridine diphosphate glucuronosyl transferase (UGT) in the liver, mainly by the UGT1A1 isoenzyme, to the inactive metabolite SN-38G7. SN-38 glucuronidation is the rate-limiting step in irinotecan metabolism and detoxification. Genetic polymorphisms of UGT1A1 alter the degree of SN-38 glucuronidation and the consequent effect on the pharmacokinetics and toxicities of irinotecan8. The number of repeats in the TATA box UGT1A1 promoter causes such differences, with six TA repeats representing the most common UGT1A1 allele (UGT1A1*1, wild type) and seven TA repeats representing a variant allele (UGT1A1*28, mutant type)9. Patients with UGT1A1*28 exhibit a reduced UGT1A1 transcription and expression and consequently reduced SN-38 glucuronidation and increased irinotecan-related toxicities8.
Various clinical outcomes and adverse events (AEs) have been observed in patients treated with the recommended irinotecan dose of 180 mg/m2 biweekly in combination with the 5-FU and LV (FOLFIRI) regimen. In patients with UGT1A1*1, active SN-38 is more efficiently metabolized, and AEs are favorably tolerated; however, the consequent cytotoxicity is less effective and thus may yield poorer oncological outcomes. By contrast, patients with homozygous UGT1A1*28 may experience severe AEs, requiring dose reduction or even complete withdrawal of irinotecan10–13. Therefore, the irinotecan dose should be adjusted according to UGT1A1 genotyping for obtaining minimal AEs and optimal oncological results. Recently, we successfully treated a case of mCRC with escalated irinotecan doses (FOLFIRI regimen) combined with regorafenib in the fourth-line treatment after UGT1A1 genotyping14. Herein we report an analysis in which regorafenib combined with FOLFIRI adjusted according to UGT1A1 genotyping was used for treating previously treated patients with mCRC.
MATERIALS AND METHODS
Patient Population
From a single institution, this prospective study recruited 13 patients with progressing mCRC who were previously treated with FOLFOX, FOLFIRI, monoclonal anti-vascular epithelial growth factor receptor (VEGFR), and monocolonal anti-epidermal growth factor receptor (EGFR) if KRAS wild-type tumors were identified, between October 2013 and June 2015. The genomic DNA of these patients was extracted from the peripheral blood and subjected to polymerase chain reaction sequencing for genotyping of the promoter region of UGT1A1 as described elsewhere15. The protocol was approved by the institutional ethics committee and was conducted in accordance with the 1964 Declaration of Helsinki (2008 revision). Written informed consent was obtained from all patients.
Treatment With Regorafenib Plus FOLFIRI With Irinotecan Dose Escalation
For each patient, regorafenib plus FOLFIRI with an irinotecan dose adjusted according to UGT1A1 genotyping was administered. Because grade ≥3 hand–foot syndrome developed frequently in patients receiving oral regorafenib at 160 mg/day (21 days at a 7-day interval), the dose was adjusted to 120 mg/day daily. If grade ≥3 regorafenib-induced AEs, such as hand–foot syndrome still developed, regorafenib was discontinued until the AEs subsided. Furthermore, according to our previous clinical results15, patients with UGT1A1*1/*1 and UGT1A1*1/*28 genotypes were initially administered a standard dose of 180 mg/m2 irinotecan, and those with the UGT1A1*28/*28 genotype were administered 120 mg/m2 of irinotecan. Irinotecan was administered for over 2 h on day 1 followed by 5-FU (2,800 mg/m2 intravenously infused for over 46 h in a 2-week cycle). For all patients, the irinotecan dose was increased by 30 mg/m2 every two cycles until grade ≥3 AEs or severe AEs (SAEs) of irinotecan developed (mainly diarrhea and neutropenia), following which the dose was reverted to and maintained at the previously tolerated level.
The treatment response was radiologically assessed every 2 months through computed tomography, magnetic resonance imaging, or positron emission tomography. Objective responses were classified according to the Response Evaluation Criteria in Solid Tumors, and optimal treatment responses were recorded. Common Terminology Criteria for Adverse Events version 3.0 was used for evaluating the treatment-associated AEs. Treatment of regorafenib plus irinotecan dose escalation was stopped if progressive disease occurred.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences Version 18.0 (SPSS Inc., Chicago, IL, USA). PFS and OS rates were calculated using the Kaplan–Meier method. PFS was defined as the time from the initiation of treatment until the first radiological evidence of progression, whereas OS was defined as the time from the beginning of treatment until death from any cause.
RESULTS
Demographic patient data are summarized in Table 1. The study included 13 patients (8 men and 5 women) with a median age of 63.0 years (33–75 years). Two patients had liver metastasis, three patients had lung metastasis, one patient had peritoneal metastasis, and seven patients had at least two metastatic sites. Moreover, four and nine patients had KRAS-mutated and KRAS wild-type CRCs, respectively. Twelve patients had the UGT1A1*1/*1 genotype, for whom the highest prescribed irinotecan dose was 290 mg/m2 (180–290 mg/m2); the corresponding dose was 120 mg/m2 for the remaining patient with the UGT1A1*28/*28 genotype. Among these patients, four, seven, and two patients were receiving third-, fourth-, and fifth-line treatments, respectively. Patients with KRAS-mutated-type CRCs were first treated with bevacizumab and FOLFIRI (irinotecan dose was 180 mg/m2) followed by FOLFOX6 if the disease progressed and were administrated with regorafenib plus FOLFIRI with irinotecan escalation as third-line treatment if FOLFOX6 failed. On the other hand, first-line treatment for those with KRAS wild-type CRCs was cetuximab plus FOLFORI with no dose escalation, and second-line treatment was FOLFOX6 and bevacizumab plus FOLFORI (irinotecan dose was 180 mg/m2). FOLFOXIRI (no irinotecan dose escalation) was administered in addition for two of nine of these patients before the reimbursement of regorafenib in Taiwan as fourth-line treatment. All treatments were substituted only when the diseases progressed. Occurrence of previously encountered neutropenia was 15%–20% and of diarrhea was 18–22% with irinotecan dose of 180 mg/m2. Median length of previous salvage treatments altogether was 13.4 months. The most commonly encountered grade ≥3 AE was hand–foot syndrome (n = 8, 61.5%), followed by mucositis (n = 5, 38.5%), neutropenia (n = 4, 30.8%), diarrhea (n = 4, 30.8%), and fatigue (n = 3, 23.1%).
Table 1.
Demographic Data in the Studied Patients
| Clinical Characteristics | No. of Cases (%) |
|---|---|
| Gender | |
| Male | 8 (61.5) |
| Female | 5 (38.5) |
| Median age (range) | 63 (33–75) |
| Site of metastasis | |
| Liver | 9 (69.2) |
| Lung | 7 (53.8) |
| Peritoneum | 4 (30.8) |
| Brain | 1 (7.7) |
| Number of sites of metastasis | |
| 1 | 6 (46.2) |
| 2 | 6 (46.2) |
| 3 | 1 (7.7) |
| KRAS status | |
| Wild type | 9 (69.2) |
| Mutation | 4 (30.8) |
| UGT1A1 status | |
| *1/*1 | 12 (92.3) |
| *1/*28 | 0 (0.0) |
| *28/*28 | 1 (7.7) |
| Irinotecan dose (mg/m2) | |
| 290 | 2 (15.4) |
| 260 | 2 (15.4) |
| 240 | 3 (23.1) |
| 210 | 1 (7.7) |
| 180 | 4 (30.8) |
| 120 | 1 (7.7) |
| Lines of systemic therapy | |
| Third | 4 (30.8) |
| Fourth | 7 (53.8) |
| Fifth | 2 (15.4) |
| Grade ≥3 adverse events | |
| Hand–foot syndrome | 8 (61.5) |
| Mucositis | 5 (38.5) |
| Neutropenia | 4 (30.8) |
| Diarrhea | 4 (30.8) |
| Fatigue | 3 (23.1) |
| Best objective response | |
| Partial response | 2 (15.4) |
| Stable disease | 7 (53.8) |
| Progressive disease | 4 (30.8) |
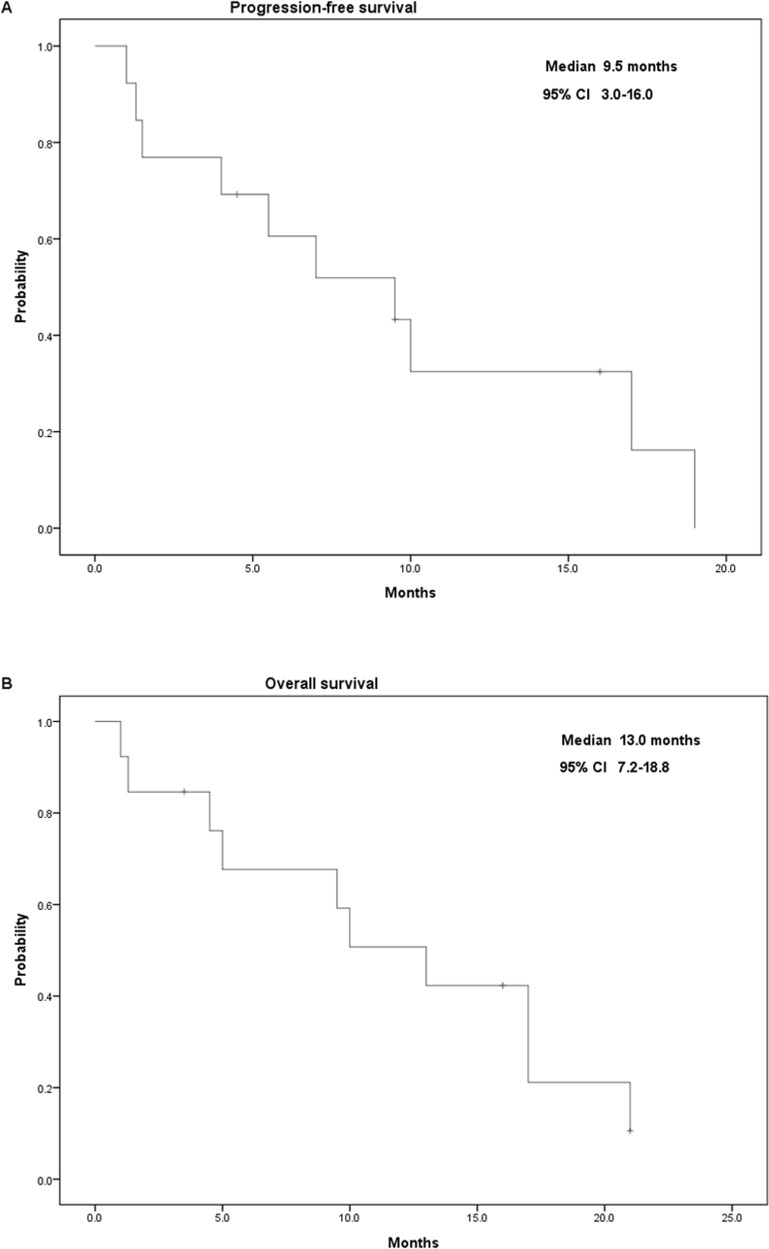
The median follow-up period was 10.0 months (1.0–21.0 months) and 16.0 months (3.5–21.0 months) for all patients and for surviving patients, respectively. All patients were followed until February 2016 or their death. The only UGT1A1*28/*28 patient received only one cycle of treatment with regorafenib plus initial irinotecan dose of 120 mg/m2; this patient developed grade 3 mucositis and died a month later. Two patients (15.4%) had a partial response, seven (53.8%) had a stable disease, and four (30.8%) had a progressive disease, yielding an overall disease control rate of 69.2%. Moreover, the median PFS and OS were 9.5 months [95% confidence interval (CI): 3.0–16.0] and 13.0 months (95% CI: 7.2–18.8), respectively (Fig. 1).
Figure 1.
Kaplan–Meier survival analysis of 13 patients with metastatic colorectal cancer. (A) Progression-free survival and (B) overall survival.
DISCUSSION
A carboxylesterase converts irinotecan to SN-38, which is cytotoxic to cancer cells and is detoxified by UGT, predominantly by the UGT1A1 isoenzyme. Marcuello et al.13 conducted a genotype-directed dose-finding study on irinotecan in FOLFIRI administered as the first-line treatment for advanced CRC and reported that patients with homozygous UGT1A1*28/*28 more frequently developed irinotecan-associated SAEs. Consequently, individuals homozygous for UGT1A1*28/*28 are more vulnerable to irinotecan, and a low initial irinotecan dose should be considered. In our study, an initial dose of 120 mg/m2 was tolerated by patients. Theoretically, patients with a heterozygous polymorphism of the UGT1A1 promoter (i.e., UGT1A1*1/*28) have an intermediate UGT1A1 activity and may be at an increased risk for toxicity. However, clinical presentations are variable, and patients with UGT1A1*1/*28 can typically tolerate the recommended initial irinotecan dose of 180 mg/m2( 16 ). By contrast, patients homozygous for UGT1A1*1/*1 tolerate higher doses of irinotecan owing to an active UGT1A1; in accordance with this observation, we observed that such patients can tolerate an irinotecan dose as high as 290 mg/m2( 14 ). For safety reasons, in the aforementioned study, we administered an initial dose of 180 mg/m2, subsequently increasing it by 30 mg/m2 every two cycles and observing for the development of any grade ≥3 AEs or SAEs, reverting to the previously tolerated dosage upon observation of any grade ≥3 AEs or SAEs. Increasing the irinotecan dose based on UGT1A1 genotyping was well tolerated and yielded satisfactory clinical outcomes15,17.
Theoretically, AEs correlate with the pharmacokinetic properties of irinotecan, and the higher area under the concentration curve (AUC) ratio of SN-38G to SN-38 (AUCSN-38G/AUCSN-38) accompanies a lower incidence of AEs. Hoskins et al.18 revealed that the risk of severe hematologic toxicity is higher in patients with UGT1A1*28/*28 than those with UGT1A1*1/*28 or UGT1A1*1/*1 at higher doses (>250–350 mg/m2) and medium doses (150–250 mg/m2) instead of at lower doses (100–125 mg/m2). Similarly, a dose-finding study of irinotecan in gastrointestinal cancer patients with a maximum dose of 150 mg/m2 demonstrated the significant highest AUCSN-38G/AUCSN-38 ratio in the wild-type group, intermediate in the heterozygous, and lowest in the homozygous mutant-type group. Severe (grade >3) hematologic toxicity was also associated with genotype during the first cycle, but no severe diarrhea in the three groups19. In contrast, Li et al.20 treated mCRC with a fixed irinotecan dose of 180 mg/m2 as the first-line treatment and showed a higher incidence of severe diarrhea in patients with two alleles or single-allele variants of UGT1A1*28/*6, but no differences of severe neutropenia in patients with different alleles. In the present study, severe diarrhea and neutropenia were both 30.8%, and the differences between studies might be because of heterogeneity in the irinotecan dose, therapeutic line, ethnicity, and cancer type. In addition to UGT1A1*28 alleles, the UGT1A1*6 genotype, which is more frequently identified in Asian populations and included in the analyses of the studies mentioned above, might play an important role.
UGT1A1*6 is a single-nucleotide polymorphism in exon 1 of the UGT1A1 gene (211G>A) and is contributory to the decreased catalytic activity of the UGT1A1 isoenzyme for SN-38 glucuronidation21–23. Contrary to about 10% prevalence in Caucasians24, UGT1A1*28 homozygotes were less than 5%20,25 whereas UGT1A1*6 homozygotes counted for about 5% in Asian populations26, but extremely low in Caucasians and African-Americans27. Moreover, the frequency of homozygous and heterozygous defected alleles, including *28/*28, *6/*6, and *6/*28, reached 10.1%26. Minami et al.23 discovered a gene–dose effect wherein AUCSN-38G/AUCSN-38 ratios reduced with the number of UGT1A1*28 or *6, and homozygous *28/*28, *6/*6, and heterozygous *28/*6 were significantly associated with severe neutropenia28. Compared with the Japanese population, UGT1A1*28 and *6 contributed to delayed diarrhea toxicity more significantly in Chinese patients20,29. The association of other UGT1A members and toxicities as well as the genetic racial differences require further investigation. When we conducted the present study, there was no relevant information regarding UGT1A1*6 genotyping that could be used for directing dose escalation of irinotecan in Taiwanese mCRC patients. In fact, one recent dose-finding study published by Kim et al. in 201530, in which patients with mCRC receiving FOLFIRI were genotyped for UGT1A1*28 and *6 and stratified according to the number of defective alleles (DA), showed that the recommended irinotecan doses were 300 (0 DA), 270 (1 DA), and 150 (0 DA) mg/m2. Lack of analysis of UGT1A1*6 is the limitation of the present study, and UGT1A1*6 genotyping may be helpful in the treatment with irinotecan as more evidence accumulates.
Regorafenib targets RTKs involved in various pathways of oncogenesis, angiogenesis, metastasis, and cancer microenvironments. Regorafenib was approved by the US Food and Drug Administration in September 2012 for treating mCRCs unresponsive to FOLFOX, FOLFIRI, and VEGF and EGFR monoclonal antibodies. The CORRECT trial was a worldwide, randomized, placebo-controlled, phase III study that was conducted for patients with mCRC who had received standard therapies, including treatment with fluoropyrimidine, oxaliplatin, irinotecan, and bevacizumab, as well as for patients with KRAS wild-type tumors who had received cetuximab or panitumumab5. Patients in this study were previously treated with a standard dose of irinotecan in FOLFIRI plus bevacizumab, and the disease progressed, though reintroduction of FOLFIRI with irinotecan dose escalation based on UGT1A1 polymorphism plus regorafenib still demonstrated satisfactory clinical results. Furthermore, compared with the CORRECT trial in which regorafenib was administered as a monotherapy, our results were remarkably encouraging, with a markedly high disease control rate (69.2%) and a longer PFS (9.5 months) and OS (13.0 months), suggesting potential synergistic effects between chemotherapy and biological therapy in refractory CRC.
Hand–foot syndrome (n = 8, 61.5%) was the most common regorafenib-induced grade ≥3 AE in this study, and treatment was generally discontinued in response to the AE. Adjusting the regorafenib dose to 120 mg/day for a continuous use reduced the occurrence of hand–foot syndrome, enabling its inclusion in the FOLFIRI regimen. Furthermore, UGT1A1 genotyping-based irinotecan escalation facilitated the tolerability of a combination of cytotoxic agents and optimized the oncological outcomes of all patients. To the best of our knowledge, the research from Schultheis et al. in Germany is the only clinical study investigating the FOLFIRI plus regorafenib combination for mCRC published in the literature to date31. In this phase Ib study, patients were treated with regorafenib in combination with either FOLFIRI or FOLFOX as first- or second-line treatment, and 33 out of 45 patients (73.3%) achieved disease control for a median of 126 (42–281) days. AEs grade ≥3 occurred in 32 patients (71.1%), mostly neutropenia (37.8%) and leucopenia, hand–foot syndrome, and hypophosphatemia (8.9% each). Our results show relatively similar findings; however, this is only an observational study with a small sample size. Therefore, a prospective, randomized large-scale study is necessary for confirming the present findings.
Treatment with regorafenib plus FOLFIRI with irinotecan dose escalation according to UGT1A1 genotyping is clinically effective and yields favorable oncological results with acceptable toxicities in previously heavily treated patients with mCRC.
ACKNOWLEDGMENTS
This work was supported by grants from Pfizer (WI186515), Roche (ML29373), and the Excellence for Cancer Research Center Grant (MOST104-2325-B-037-001); the Taiwan Ministry of Health and Welfare (MOHW106-TDU-B-212-144007); and Health and Welfare Surcharge of Tobacco Products, in addition to grants from Kaohsiung Medical University Hospital (KMUH99-9M09, KMUH100-0M13, KMUH-10323, KMUH-S10418, and KMUH-S10455), the Center for Biomarkers and Biotech Drugs of Kaohsiung Medical University (KMU-TP104A11, KMU-PT104002, and KMU-DK105001), and the Grant of Biosignature in Colorectal Cancers from the Academia Sinica of Taiwan.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: Potential place in therapy. Drugs 2013;73:883–91. [DOI] [PubMed] [Google Scholar]
- 3. O’Neil BH, Goldberg RM. Innovations in chemotherapy for metastatic colorectal cancer: An update of recent clinical trials. Oncologist 2008;13:1074–83. [DOI] [PubMed] [Google Scholar]
- 4. Peeters M, Price T. Biologic therapies in the metastatic colorectal cancer treatment continuum—Applying current evidence to clinical practice. Cancer Treat Rev. 2012;38:397–406. [DOI] [PubMed] [Google Scholar]
- 5. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- 6. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- 7. Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7:2182–94. [PubMed] [Google Scholar]
- 8. Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 1996;347:578–81. [DOI] [PubMed] [Google Scholar]
- 10. Hebbar M, Ychou M, Ducreux M. Current place of high-dose irinotecan chemotherapy in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2009;135:749–52. [DOI] [PubMed] [Google Scholar]
- 11. Palomaki GE, Bradley LA, Douglas MP, Kolor K, Dotson WD. Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? An evidence-based review. Genet Med. 1009;11:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toffoli G, Cecchin E, Gasparini G, D’Andrea M, Azzarello G, Basso U, Mini E, Pessa S, De Mattia E, Lo Re G, Buonadonna A, Nobili S, De Paoli P, Innocenti F. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcuello E, Paez D, Pare L, Salazar J, Sebio A, del Rio E, Baiget M. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 2011;105:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu CY, Yeh YS, Huang CW, Ma CJ, Yu FJ, Wang JY. FOLFIRI and regorafenib combination therapy with dose escalation of irinotecan as fourth-line treatment for patients with metastatic colon cancer according to UGT1A1 genotyping. Onco Targets Ther. 2014;7:2143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu CY, Huang CW, Hu HM, Tsai HL, Huang CM, Yu FJ, Huang MY, Chang SF, Huang ML, Wang JY. Prognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line setting. Transl Res. 2014;164:169–76. [DOI] [PubMed] [Google Scholar]
- 16. Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C Study. J Clin Oncol. 2007;25:4779–86. [DOI] [PubMed] [Google Scholar]
- 17. Lu CY, Huang CW, Wu IC, Tsai HL, Ma CJ, Yeh YS, Chang SF, Huang ML, Wang JY. Clinical implication of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab combined with FOLFIRI in the first-line setting. Transl Oncol. 2015;8:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoskins JM, Marcuello E, Altes A, Marsh S, Maxwell T, Van Booven DJ, Pare L, Culverhouse R, McLeod HL, Baiget M. Irinotecan pharmacogenetics: Influence of pharmacodynamic genes. Clin Cancer Res. 1008;14:1788–96. [DOI] [PubMed] [Google Scholar]
- 19. Satoh T, Ura T, Yamada Y, Yamazaki K, Tsujinaka T, Munakata M, Nishina T, Okamura S, Esaki T, Sasaki Y, Koizumi W, Kakeji Y, Ishizuka N, Hyodo I, Sakata Y. Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer Sci. 2011;102:1868–73. [DOI] [PubMed] [Google Scholar]
- 20. Li M, Wang Z, Guo J, Liu J, Li C, Liu L, Shi H, Liu L, Li H, Xie C, Zhang X, Sun W, Fang S, Bi X. Clinical significance of UGT1A1 gene polymorphisms on irinotecan-based regimens as the treatment in metastatic colorectal cancer. Onco Targets Ther. 2014;7:1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagne JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). Mol Pharmacol. 2002;62:608–17. [DOI] [PubMed] [Google Scholar]
- 22. Fujita K, Ando Y, Nagashima F, Yamamoto W, Eodo H, Araki K, Kodama K, Miya T, Narabayashi M, Sasaki Y. Genetic linkage of UGT1A7 and UGT1A9 polymorphisms to UGT1A1*6 is associated with reduced activity for SN-38 in Japanese patients with cancer. Cancer Chemother Pharmacol. 2007;60:515–22. [DOI] [PubMed] [Google Scholar]
- 23. Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, Kaniwa N, Sawada J, Hamaguchi T, Yamamoto N, Shirao K, Yamada Y, Ohmatsu H, Kubota K, Yoshida T, Ohtsu A, Saijo N. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: Roles of UGT1A1*6 and *28. Pharmacogenet Genomics 2007;17:497–504. [DOI] [PubMed] [Google Scholar]
- 24. Shulman K, Cohen I, Barnett-Griness O, Kuten A, Gruber SB, Lejbkowicz F, Rennert G. Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer 2001;117:3156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai X, Cao W, Ding H, Liu T, Zhou X, Wang M, Zhong M, Zhao Z, Xu Q, Wang L. Analysis of UGT1A1*28 genotype and SN-38 pharmacokinetics for irinotecan-based chemotherapy in patients with advanced colorectal cancer: Results from a multicenter, retrospective study in Shanghai. J Cancer Res Clin Oncol. 2013;139:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akiyama Y, Fujita K, Nagashima F, Yamamoto W, Endo H, Sunakawa Y, Yamashita K, Ishida H, Mizuno K, Araki K, Ichikawa W, Miya T, Narabayashi M, Kawara K, Sugiyama M, Hirose T, Ando Y, Sasaki Y. Genetic testing for UGT1A1*28 and *6 in Japanese patients who receive irinotecan chemotherapy. Ann Oncol. 2008;19:2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaniwa N, Kurose K, Jinno H, Tanaka-Kagawa T, Saito Y, Saeki M, Sawada J, Tohkin M, Hasegawa R. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C T (P229L) found in an African-American. Drug Metab Dispos. 2005;33:458–65. [DOI] [PubMed] [Google Scholar]
- 28. Okuyama Y, Hazama S, Nozawa H, Kobayashi M, Takahashi K, Fujikawa K, Kato T, Nagata N, Kimura H, Oba K, Sakamoto J, Mishima H. Prospective phase II study of FOLFIRI for mCRC in Japan, including the analysis of UGT1A1 28/6 polymorphisms. Jpn J Clin Oncol. 2011;41:477–82. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Shen L, Xu N, Wang JW, Jiao SC, Liu ZY, Xu JM. UGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracil. World J Gastroenterol. 2012;18:6635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim KP, Hong YS, Lee JL, Bae KS, Kim HS, Shin JG, Lee JS, Kim TW. A phase I study of UGT1A1 *28/*6 genotype-directed dosing of irinotecan (CPT-11) in Korean patients with metastatic colorectal cancer receiving FOLFIRI. Oncology 2015;88:164–72. [DOI] [PubMed] [Google Scholar]
- 31. Schultheis B, Folprecht G, Kuhlmann J, Ehrenberg R, Hacker UT, Kohne CH, Kornacker M, Boix O, Lettieri J, Krauss J, Fischer R, Hamann S, Strumberg D, Mross KB. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: Results of a multicenter, phase Ib study. Ann Oncol. 2013;24:1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]