Abstract
Previous studies have suggested an important role of retinoic acid (RA) and ascorbic acid (AA) in the stimulation of osteoblastic differentiation; however, the function of RA and AA in the osteogenic differentiation from human dental pulp (hDPSCs) remains unclear.
Objective
This in vitro study investigated the effects of RA and AA on the differentiation of osteoblast from hDPSCs.
Methods
hDPSCs were treated with different doses of RA and AA, separately or in combination (RA + AA). Morphology and cell proliferation were assessed. Osteoblast differentiation was evaluated by alizarin red, alkaline phosphatase staining, and RUNX2 gene expression.
Results
A significant reduction was observed in the number of cells treated with RA (26%) and RA + AA (30%) after 12 days of treatment. AA treatment alone induced a 12% reduction in the number of cells. Morphologically, the cells treated with RA and RA + AA were larger and more elongated than the control cells. A mesh pattern was observed in cells treated with AA. Numerous calcified nodules were present in cells treated with RA, AA, and RA + AA. This coincided with increased expression of RUNX2 and high alkaline phosphatase staining levels.
Conclusions
hDPSCs treated with RA and RA + AA showed significant reduction in proliferation, detectable morphological changes, and expression of the key differentiation gene RUNX2, consistent with an osteoblast phenotype. AA induced morphological changes and early formation of calcified nodules. RA had a predominant effect when AA and RA were used together.
Keywords: Cell differentiation, Mesenchymal stem cells, Osteoblasts, Retinoic acid, Ascorbic acid
1. Introduction
Tooth movement requires a series of events that lead to bone remodeling mediated by the induction of bone resorption by osteoclasts and bone matrix formation by osteoblasts. In vitro, osteogenic differentiation and matrix mineralization have been studied using a variety of stimuli, including vitamin D.1
Other molecules known to affect bone metabolism during tooth movement also include retinoic acid (Vitamin A) and ascorbic acid (Vitamin C).2,3 Retinoic acid (RA) is an important nutrient acquired through dietary intake of retinyl esters and carotenoids. This vitamin participates in various cellular functions, such as differentiation, proliferation, inflammation, and apoptosis.4 Previous studies suggested that RA stimulates osteoclastogenesis by increasing differentiation of mononuclear precursors and RANKL expression.5 Its effect on osteoblasts is more complex: some studies suggested that the RA pathway induces osteogenic differentiation,2,4,6 while other studies reported that RA can inhibit osteoblast differentiation and in vitro mineralization.7, 8, 9, 10 These contradicting results in cultured cells have been explained by differences in doses used, since the low-dose treatment of RA inhibits osteoblastic differentiation, and the opposite occurs at high doses.9,11 Therefore, the role of RA in bone formation is still controversial.
Ascorbic acid (AA) is a potent reducing and antioxidant agent involved in the development and function of several cell types, such as adipocytes, myoblasts, chondroblasts, odontoblasts, and osteoblasts.12 AA must be incorporated through the diet, since humans are unable to synthesize it, and vitamin deficiencies lead to tissue alterations, mainly in tissues rich in collagens, such as bone, cartilage, and skin.13 This vitamin is involved in synthesis of extracellular matrix, cell proliferation, and differentiation. Previous studies have determined that AA stimulates the proliferation and differentiation of bone cells and increases collagen synthesis to promote bone formation.14 In addition, AA induces the expression of osteoblast differentiation and mineralization markers in some osteoblastic cell lines.15,16 In cell culture, AA promotes the proliferation of different cells and stimulation of DNA synthesis; however, very high concentrations can be cytotoxic and lead to the inhibition of cell proliferation and increase of apoptosis.15 Although results regarding the effect of AA on dental movement are contradictory, some studies reported increased proliferation of osteoclasts and their progenitor cells, stimulating faster tooth movement.17
Human dental pulp stem cells (hDPSCs) are mesenchymal stem cells with high self-renewal potential that have the capacity to differentiate into several cell types, including osteoblasts. Bone marrow-derived mesenchymal stem cells (BMMSCs) are considered the gold standard cells for bone biology studies; however, hDPSCs represent an attractive alternative due to ease of isolation and use.18
Little is known about the effect of RA and AA on hDPSC proliferation and differentiation, and it is not clear whether the combined use of RA and AA could affect osteoblast differentiation from this type of cells. Therefore, the main aim of this study was to investigate the effect of RA and AA on the viability, proliferation, and differentiation of hDPSCs.
2. Materials and methods
2.1. Collection and isolation of human dental pulp stem cells (hDPSC)
This in vitro study was approved by the Ethics Institutional Committee (Minutes B.CIEFO-118-18). hDPSCs were obtained from healthy premolars of individuals aged 18–20 who required extraction due to orthodontic treatment. Extracted teeth were decontaminated by immersion in 5% sodium hypochlorite and sectioned using high-speed handpiece to obtain the whole pulp tissue. Explants were placed in Dulbecco’s modified Eagle low glucose (DMEM) culture medium (Hyclone, Thermo Fisher Scientific), supplemented with fetal bovine serum (FBS) (Hyclone, Thermo Fisher Scientific) and antibiotics. For enzymatic dissociation, samples were incubated in a medium containing collagenase (3 mg/mL) (Sigma-Aldrich) and dispase (4 mg/mL) (Gibco, Invitrogen) at 37 °C for 16 h with 5% CO2. Next, the suspension was centrifuged and the cell pellet was resuspended in DMEM. hDPSCs were seeded in 25 cm2 flasks and cultured until ~80% confluence was reached.19, 20, 21
2.2. Characterization of hDPSCs
The isolated cell phenotype was characterized using the mesenchymal cells of human origin typing kit (Miltenyi Biotec, Ref.#130-095-198). The cultured cells were trypsinized and incubated in separate tubes with 10 μL of fluorochrome-conjugated monoclonal antibodies (CD34, CD45, CD73, CD90, and CD105). Analysis was performed using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA). The phenotype of hDPSC was determined considering the criteria of the International Society for Cell Therapy for mesenchymal stem cells.22
2.3. Evaluation of hDPSC proliferation and morphology
Cells were treated with different doses of RA (all−trans−Retinoic acid, Sigma-Aldrich, R2625), and AA (l-ascorbic acid 2-phosphate Sigma-Aldrich, A8960) for 7 days to determine the dose that induces the most evident changes in cell number and morphology. Once the optimal dose was selected, the cells were treated with RA and AA either separately or in combination (RA + AA). hDPSCs proliferation with or without treatment was evaluated using the resazurin fluorometric assay. Resazurin (Sigma-Aldrich) solution (44 μM) was added to cultured cells to obtain a final concentration of 0.44 μM in each well and incubated at 37 °C for 4 h. The fluorescence reading was performed using a Tecan Infinite M2000 Pro reader at an excitation wavelength of 535 nm and emission wavelength of 595 nm.23
2.4. Osteoblast differentiation
hDPSCs were cultured in differentiation medium (DM) containing DMEM supplemented with 10% FBS, antibiotics (10.000 U/mL penicillin, 10.000 μg/mL streptomycin and 25 μg/mL amphotericin B (Hyclone, Thermo Fisher Scientific), 0.1 μM dexamethasone (Sigma-Aldrich), 5 mM β-glycerophosphate (Santa Cruz), and 50 μg/mL AA (Sigma-Aldrich) for 7, 14, or 21 days at 37 °C in a 5% CO2 incubator, and the medium was changed every third day.15,21
2.5. Evaluation of calcium deposition and matrix mineralization
To evaluate calcium deposition, we used the protocol described by Gregory et al.24 After each time point, each group was fixed with paraformaldehyde and stained with 2% Alizarin Red S (Sigma-Aldrich). The excess dye was removed using 1 × phosphate buffered saline (PBS) wash. The formation of calcified nodules was evaluated using an inverted microscope, while the absorbance of the dye was quantified using an absorbance reader Tecan Infinite 200 PRO at 550 nm.24
2.6. Alkaline phosphatase detection
After 7 and 14 days of treatment with RA, AA, and RA + AA, the cytochemical SIGMAFAST™ Fast Red TR/Napthol AS-MX kit (Sigma-Aldrich) was used to detect alkaline phosphatase positive cells.
2.7. RUNX2 gene expression
RUNX2 is one of the key transcription factors involved in osteoblast differentiation.25 Total RNA was extracted 7, 14, and 21 days after the treatment using the Quick-RNA MicroPrep kit (Zymo Research USA R1050) following the manufacturer’s protocol. cDNA was obtained using the Protoscript First Strand cDNA Synthesis kit (BIOHAUS SAS). To determine the expression levels of RUNX2 and GAPDH, RT-PCR was performed using the Luna Universal RT-PCR master mix system (New England Biolabs, USA) and SYBR Green, and the CFX96 Real-Time Thermal Cycler detection system (Bio-Rad; Hercules, CA, USA). The amplification conditions and primers are shown in Table 1. Efficiencies were calculated using LinRegPCR (AMC, The Netherlands), and the relative quantification of expression was performed following Scheffe’s method.26
Table 1.
Primers used in this study. RUNX2: Runt-Related Transcription Factor 2, GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase.
| Gene Name |
Forward Reverse |
Amplification conditions |
|---|---|---|
| RUNX2 | 5′-CATCTAATGACACCACCAGGC-3′ 5′-GCCTACAAAGGTGGGTTTGA-3′ |
3 min at 95 °C 10 s at 95 °C 30 s at 60 °C 20 s at 72 °C 5 s at 65 °C 5 s at 95 °C. |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGTC-3′ 5′-GAAGATGGTGATGGGATTTC-3′ |
2.8. Statistical analysis
Statistical analysis was performed using SPSS software, version 21.0 (SPSS, Chicago, IL, USA). All results were analyzed using one-way ANOVA with post-hoc Tukey test. All experiments were carried out in triplicates using two independent cultures (n = 6).
3. Results
3.1. Characterization of hDPSCs
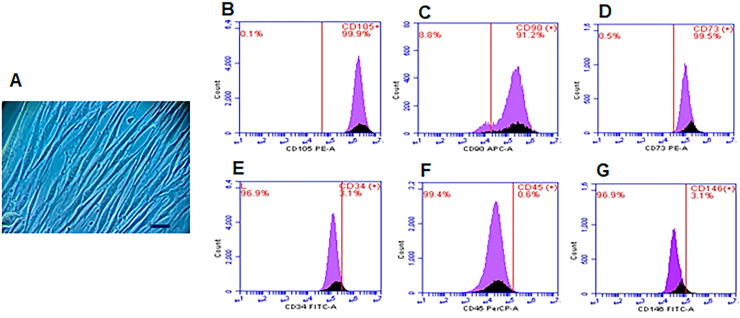
After culturing hDPSCs for 7 days, adherent cells with a polygonal or spindle-shaped appearance were observed. Immunophenotyping of the cells revealed high expression of CD105, CD90, and CD73 cell surface markers and low expression of CD34, CD45, and CD146 (Fig. 1).
Fig. 1.
Isolation and characterization of hDPSC. (A) Photomicrograph of mesenchymal cells with fibroblastoid appearance after 7 days of culture. Flow cytometry histograms with positive surface markers for CD105 (B), CD90 (C), CD73 (D) and negative for CD34 (E), CD45 (F) and CD 146 (G). Bar: 200 μm.
3.2. Changes in hDPSC proliferation and morphology in response to treatment with RA and AA
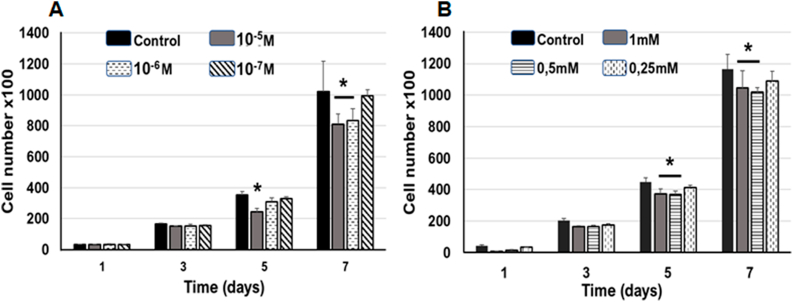
Previous studies evaluating the effect of RA on cell differentiation were performed using doses ranging from 10−2 to 10−8 M.4,27,28 Therefore, we selected three different doses (10−5 M, 10−6 M, and 10−7 M) to determine the concentration that induced changes in proliferation and morphology most effectively. Treatment with 10−5 M RA significantly reduced the number of hDPSCs after 5 days of treatment compared to the untreated group (control), while 10−6 M produced changes only after 7 days, and no significant changes in the number of cells were observed in 10−7 M group at any time point (Fig. 2A).
Fig. 2.
Quantification of cell number in cultures treated with AA and RA. (A) RA produced a decrease in cell number after 5 days of treatment with 10−5 M and at 7 days with 10−5 M and 10−6 M, compared with the control group. (B) AA reduced the number of hDPSC treated with 1 mM and 0.5 mM doses after 5 and 7 days of treatment. The 0.25 mM dose did not show significant differences in comparison with the control group. Asterisks indicate significance (p < 0.05). Data are is expressed as mean ± SD.
Previous studies used doses between 0.25 and 1 mM to assess the effect of AA on proliferation and osteoblastic differentiation.15,16 Therefore, three different doses (1 mM, 0.5 mM, and 0.25 mM) were used for 7 days of treatment (Fig. 2B). A significant decrease in cell number was observed after 1 mM and 0.5 mM AA treatment for 5 and 7 days, while 0.25 mM dose did not have any effect on the number of hDPSCs at any time point (Fig. 2B). Both RA and AA treatments only decreased cell proliferation and did not affect cell viability in any of the groups, as confirmed by trypan blue staining.
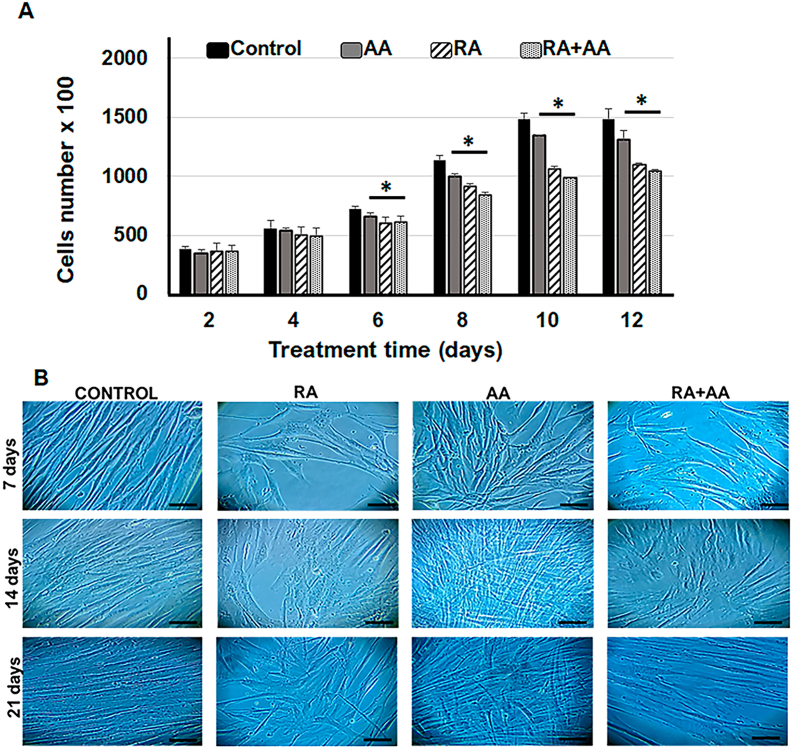
For the combined treatment (RA + AA) of hDPSCs, we selected 0.5 mM AA and 10−5 M RA concentrations. Treatment of hDPSCs with RA, AA, and RA + AA produced a significant reduction in the number of cells starting from day 6 of the treatment compared to control. Treatment with RA + AA induced the highest reduction (30%) of cell number after 12 days, while the RA treatment resulted in a 26% reduction of cell number after 12 days. No significant differences were observed between the groups treated with RA alone or in combination with AA. AA reduced the number of hDPSCs by 12% after 12 days of treatment (Fig. 3A).
Fig. 3.
Cell proliferation in response to RA + AA treatment. (A) Changes in cell number induced by treatment with AA (0.5 mM), RA (10−5 M) and (RA + AA) for 12 days. All three treatments produced a significant reduction in the number of cells from day 6 of treatment relative to the control group. ∗p < 0.05. The data are expressed as mean ± SD. (B) Photomicrograph of hDPSC cultures. The cells were treated with RA (10−5 M), AA (0.5 mM) and RA + AA for 7, 14 and 21 days. Bar: 200 μm.
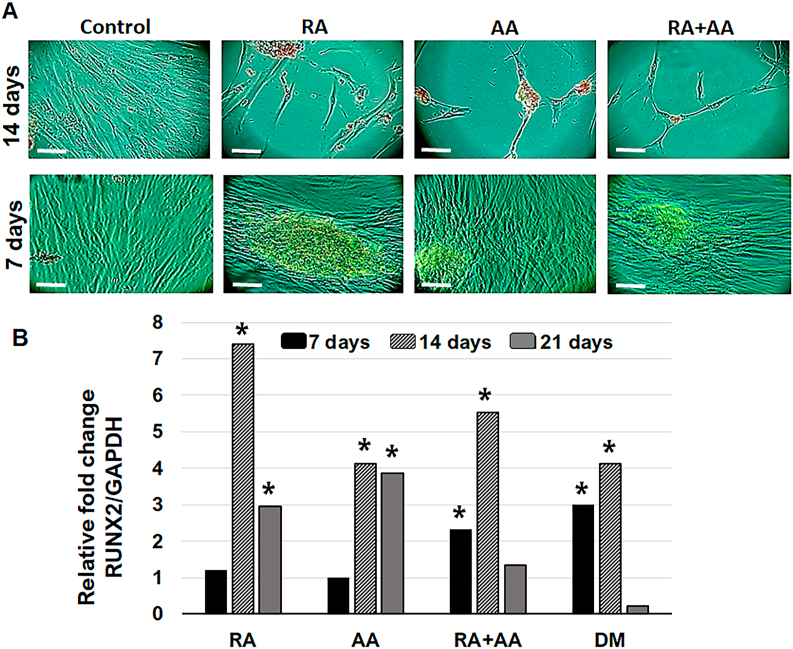
Cells treated with RA and AA in the first 7 days were observed to be larger compared to control cells, with elongated shape and increased cytoplasmic size. In cells treated with RA + AA, the observed morphological changes were consistent with RA only treatment (Figs. 3B–7 days). At day 14, lower cell density was observed in the groups of cells treated with RA and RA + AA compared to control, whereas the AA treated cells had a mesh-like arrangement (Figs. 3B–14 days). At day 21, the cell confluence increased significantly, with a higher cell number observed in the control group and in AA group, compared to RA and RA + AA groups (Figs. 3B–21 days).
3.3. Osteoblastic differentiation of hDPSCs induced by RA and AA treatment
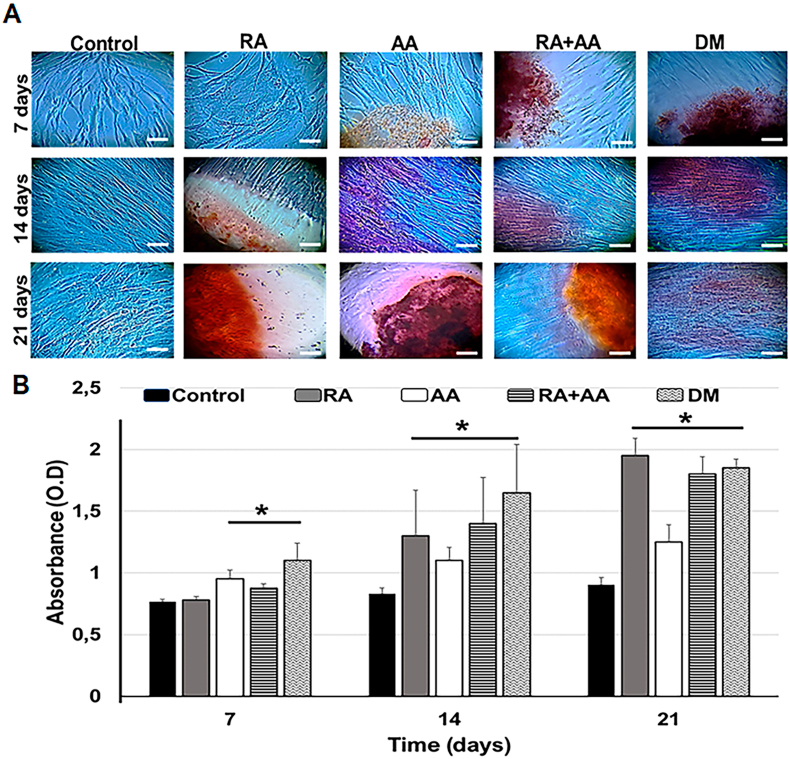
Alizarin Red S staining was used to identify calcified nodule formation in the cells treated with RA, AA, and RA + AA for 7, 14, and 21 days (Fig. 4A). At the day 7 time point, the initial formation of calcified nodules was observed in cells treated with AA and RA + AA; however, there was no evidence of the nodules in RA group (Figs. 4A–7 days). At day 14, a significant increase in calcified nodule formation was observed in RA group compared to AA and RA + AA groups, where a lower number of nodules was observed (Figs. 4A–14 days). At day 21, a slight increase in the number of calcified nodules was observed in all treatment groups (Figs. 4A–21 days). There was no evidence of calcified nodule formation in the control group (Fig. 4A-control). Cells treated with differentiation medium (DM) were used as a positive control (Fig. 4A-DM).
Fig. 4.
Quantification of mineralization. (A) Mineralization determined by Alizarin red S staining. Strong matrix staining was observed in the photomicrographs, which indicated the apparent formation of calcification nodules. Bar: 200 μm. (B) Measurement of absorbance of Alizarin red S stain extracted from cells under different treatments at 7, 14, and 21 days. Differentiation medium (DM). ∗p < 0.05. Data are expressed as mean ± SD.
Quantification of alizarin red extracted from the cells showed significant differences between the cells treated with AA, RA + AA, and DM compared to the control group cells after 7 days of culture. At days 14 and 21, a significant increase in the amount of extracted alizarin red S was observed in all the experimental groups (Fig. 4B).
Alkaline phosphatase activity was clearly observed in the cells treated with RA, AA, and RA + AA after 7 and 14 days of treatment. No alkaline phosphatase staining was observed in untreated hDPSCs (control) (Fig. 5A).
Fig. 5.
Characterization of the osteoblast differentiation. (A) Staining of hDPSC cells for alkaline phosphatase. Cells without treatment (Control), and treated with RA, AA and RA + AA, were immunohistochemically stained for alkaline phosphatase at 7 and 14 days of treatment. Bar: 200 μm. (B) Quantification of RUNX2 relative gene expression. Quantification of RUNX2 expression was performed in cells treated with RA, AA and RA + AA during 7, 14, and 21 days. The data are normalized to GAPDH; cells treated with differentiation medium (DM) were used as a positive control. ∗p < 0.05.
Next, we investigated the expression of an early marker of osteoblastic differentiation using real-time RT-PCR analysis. The results indicated a seven-fold increase in RUNX2 expression associated with RA treatment for 14 days. AA also induced a four-fold increase in RUNX2 expression after 14 days of treatment, similar to DM group. Compared to RA and AA groups, RA + AA increased RUNX2 expression two-fold at day 7 and over five-fold at day 14. RUNX2 expression decreased in all groups after 21 days of treatment (Fig. 5B).
4. Discussion
It has been established that RA is involved in the formation and metabolism of bone and cartilage, although the mechanisms involved are not yet understood. In this study, treatment with RA significantly reduced cell proliferation after 12 days, with a concomitant increase in the number of calcified nodules and an increase of 56% and 115% alizarin red absorbance after 14 and 21 days of treatment, respectively. The detection of alkaline phosphatase was also more evident after 14 days of RA treatment. Furthermore, there was a seven-fold increase in the relative expression of the osteoblastic differentiation factor RUNX2 after RA treatment. This increased expression was three times higher compared to the DM group, the positive control group treated with an osteoinductive medium, suggesting that RA has a positive effect on differentiation of hDPSCs.
Previously, Hisada et al. determined that RA treatment stimulates the expression and activity of alkaline phosphatase, the effect enhanced by the treatment with bone morphogenetic protein BMP2. Hisada et al. and others suggested that RA could stimulate osteoblastic differentiation through the BMP2–Smad–Runx2/Msx2 pathway.27,28
RA effect on the differentiation of osteoblasts in vivo and in vitro remain controversial. Studies performed with different osteoblastic cell lines displayed contradictory results regarding the role of RA in osteoblastic differentiation. For instance, at nanomolar concentrations, RA inhibits the differentiation and function of osteoblasts, while micromolar concentrations stimulate osteoblastogenesis and increase osteoclast formation,5 supporting our findings: RA (10−5 M = 10 μM) induced osteoblastic differentiation. In contrast, the study by Wang et al. showed that in vitro and in vivo activation of RA signaling decreased the osteogenic differentiation potential of DPSCs, while blocking RA signaling enhanced it.10 Different culture conditions and induction times could be responsible this discrepancy. Other studies demonstrated that in preosteoblastic cell lines, RA (400 nM) suppressed in vitro mineralization and reduced RUNX2 and Osterix protein levels, suggesting that it plays a negative regulatory role in osteoblastic mineralization.29 These differences may be due to the use of different cell lines and RA concentrations.
Studies investigating the association between vitamin C and bone metabolism have established that AA plays an important role in the formation of collagen.13 Furthermore, it has been shown that the in vitro proliferation and differentiation of mesenchymal stem cells and skeletal tissues require AA as an essential component of the differentiation medium.15 According to our results, AA treatment, acting as a cofactor in the differentiation medium, induced this phenomenon synergistically with other differentiation medium components. AA alone reduced the proliferation by 13% compared to 40% reduction when differentiation medium was used. The same trend was present in the alizarin red absorbance data, with AA inducing only a third of the value observed in positive control cultures treated with differentiation medium, even though RUNX2 expression and alkaline phosphatase staining results were similar between these two groups. Another study using human osteoblast-like MG-63 cells treated with different AA doses showed reduced proliferation and increased alkaline phosphatase activity, consistent with osteoblastic differentiation.16 At the same time, Choi et al. found that low doses (5–50 μM) of AA increased cell proliferation, while higher doses (250–500 μM) reduced cell proliferation without affecting the potential for differentiation.15 In our study, a dose of 0.5 mM (500 μM) AA reduced proliferation and induced differentiation towards osteoblastic lineage. AA can inhibit or induce the proliferation and differentiation of stem cells, depending on the concentration used, treatment duration, and cell type considered.13 In the present study, 0.5 mM of AA induced changes consistent with cell differentiation, such as reduced proliferation rate, morphological changes, increased formation of calcified nodules, positive alkaline phosphatase staining, and higher relative RUNX2 expression.
The combined treatment of hDPSCs with RA and AA did not appear to have a synergistic effect; rather, the changes in morphology and decreased proliferation were mainly stimulated by the action of RA. Although the relative expression of the RUNX2 gene was increased in the RA + AA treatment group, it did not reach the value observed in the RA treatment group. Few studies have evaluated the combined use of RA and AA. In one of studies, different vitamins were combined and tested on preosteoblastic cell lines.30 The authors found that concurrent RA and AA treatment induced a five-fold and two-fold increase of pro-αl(1) collagen and osteopontin transcripts, respectively. The authors concluded that RA and AA could be considered as important regulators of osteoblastic differentiation; however, the expression of RUNX2 was not analyzed.30 In a another study, it was established that RA competes with AA in regulation of the cell differentiation process in a dose-dependent manner.31 However, further studies are needed to confirm this hypothesis.
In summary, treatment with RA, AA, and RA + AA significantly reduced proliferation, induced morphological changes, and increased RUNX2 expression, indicating stimulation of differentiation of hDPSCs towards the osteoblastic lineage. AA had a lesser effect on proliferation and gene expression; however, it produced early morphological changes and nodule calcification. The combination of two vitamins did not appear to have a synergistic effect and the results were similar to the RA only treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have no conflict of interest to declare.
References
- 1.Almpani K., Kantarci A. Nonsurgical methods for the acceleration of the orthodontic tooth movement. Front Oral Biol. 2016;18:80–91. doi: 10.1159/000382048. [DOI] [PubMed] [Google Scholar]
- 2.Cruz A.C.C., Cardozo F.T.G.S., Magini R.S., Simões C.M.O. Retinoic acid increases the effect of bone morphogenetic protein type 2 on osteogenic differentiation of human adipose-derived stem cells. J Appl Oral Sci. 2019;27 doi: 10.1590/1678-7757-2018-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motoji H., To M., Hidaka K., Matsuo M. Vitamin C and eggshell membrane facilitate orthodontic tooth movement and induce histological changes in the periodontal tissue. J Oral Biosci. 2020;62(1):80–87. doi: 10.1016/j.job.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Weng Z., Wang C., Zhang C. All-trans retinoic acid promotes osteogenic differentiation and bone consolidation in a rat distraction osteogenesis model. Calcif Tissue Int. 2019;104(3):320–330. doi: 10.1007/s00223-018-0501-6. [DOI] [PubMed] [Google Scholar]
- 5.Henning P., Herschel H., Lerner U. Retinoid receptors in bone and their role in bone remodeling. Front Endocrinol. 2015;6:31. doi: 10.3389/fendo.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadipiralla K., Yochim J.M., Bahuleyan B. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. 2010;340(2):323–333. doi: 10.1007/s00441-010-0953-0. [DOI] [PubMed] [Google Scholar]
- 7.Iba K., Chiba H., Yamashita T., Ishii S., Sawada N. Phase-independent inhibition by retinoic acid of mineralization correlated with loss of tetranectin expression in a human osteoblastic cell line. Cell Struct Funct. 2001;26(4):227–333. doi: 10.1247/csf.26.227. [DOI] [PubMed] [Google Scholar]
- 8.Chen M., Huang H.Z., Wang M., Wang A.X. Retinoic acid inhibits osteogenic differentiation of mouse embryonic palate mesenchymal cells. Birth Defects Res A Clin Mol Teratol. 2010;88(11):965–970. doi: 10.1002/bdra.20723. [DOI] [PubMed] [Google Scholar]
- 9.Roa L.A., Bloemen M., Carels C.E.L., Wagener F.A.D.T.G., Von den Hoff J.W. Retinoic acid disrupts osteogenesis in pre-osteoblasts by down-regulating WNT signaling. Int J Biochem Cell Biol. 2019;116:105597. doi: 10.1016/j.biocel.2019.105597. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Li G., Hu L. Retinoic acid signal negatively regulates osteo/odontogenic differentiation of dental pulp stem cells. Stem Cell Int. 2020;2020:5891783. doi: 10.1155/2020/5891783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohishi K., Nishikawa S., Nagata T. Physiological concentrations of retinoic acid suppress the osteoblastic differentiation of fetal rat calvaria cells in vitro. Eur J Endocrinol. 1995;133(3):335–341. doi: 10.1530/eje.0.1330335. [DOI] [PubMed] [Google Scholar]
- 12.Diomede F., Marconi G.D., Serroni M., Pizzicannella G., Trubiani O., Pizzicannella J. Ascorbic acid enhances bone parameter expression in human gingival mesenchymal stem cells. J Biol Regul Homeost Agents. 2019;33(6):1715–1723. doi: 10.23812/19-312-A. [DOI] [PubMed] [Google Scholar]
- 13.D’Aniello C., Cermola F., Patriarca E.J., Minchiotti G. Vitamin C in stem cell biology: impact on extracellular matrix homeostasis and epigenetics. Stem Cell Int. 2017;2017:8936156. doi: 10.1155/2017/8936156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzicannella J., Marconi G.D., Pierdomenico S.D., Cavalcanti M.F.X.B., Diomede F., Trubiani O. Bovine pericardium membrane, gingival stem cells, and ascorbic acid: a novel team in regenerative medicine. Eur J Histochem. 2019;63(3):3064. doi: 10.4081/ejh.2019.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi K.M., Seo Y.K., Yoon H.H. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105(6):586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 16.Takamizawa S., Maehata Y., Imai K., Senoo H., Sato S., Hata R. Effects of ascorbic acid and ascorbic acid2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol. 2004;28(4):255–265. doi: 10.1016/j.cellbi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Miresmaeili A., Mollaei N., Azar R., Farhadian N., Mani Kashani K. Effect of dietary vitamin C on orthodontic tooth movement in rats. J Dent. 2015;12(6):409–413. [PMC free article] [PubMed] [Google Scholar]
- 18.Rodas-Junco B.A., Villicaña C. Dental pulp stem cells: current advances in isolation, expansion and preservation. Tissue Eng Regen Med. 2017;14(4):333–347. doi: 10.1007/s13770-017-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronthos S., Brahim J., Li W. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 20.Baldión P.A., Velandia-Romero M.L., Castellanos J.E. Odontoblast-like cells differentiated from dental pulp stem cells retain their phenotype after subcultivation. Int J Cell Biol. 2018;2018:6853189. doi: 10.1155/2018/6853189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escobar L.M., Bendahan Z., Bayona A., Castellanos J.E., González M.C. Effect of vitamins D and E on the proliferation, viability, and differentiation of human dental pulp stem cells: an in vitro study. Int J Dent. 2020;2020:8860840. doi: 10.1155/2020/8860840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Anoopkumar-Dukie S., Carey J.B., Conere T.O., Sullivan E., Van Pelt F.N., Allshire A. Resazurin assay of radiation response in cultured cells. Br J Radiol. 2005;78(934):945–947. doi: 10.1259/bjr/54004230. [DOI] [PubMed] [Google Scholar]
- 24.Gregory C., Grady W., Peister A., Prockop J. An alizarin red-based assay of mineralization by adherent cells in culture: comparision with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan A., Srinaath N., Rohini M., Selvamurugan N. Regulation of runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019;232:116676. doi: 10.1016/j.lfs.2019.116676. [DOI] [PubMed] [Google Scholar]
- 26.Schefe J.H., Lehmann K.E., Buschmann I.R., Unger T., Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel gene expression’s CT difference formula. J Mol Med. 2006;84(11):901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 27.Hisada K., Hata K., Ichida F. Retinoic acid regulates commitment of undifferentiated mesenchymal stem cells into osteoblasts and adipocytes. J Bone Miner Metabol. 2013;31(1):53–63. doi: 10.1007/s00774-012-0385-x. [DOI] [PubMed] [Google Scholar]
- 28.Cowan C.M., Aalami O.O., Shi Y.Y. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11(3-4):645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 29.Lind T., Sundqvist A., Hu L. Vitamin a is a negative regulator of osteoblast mineralization. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choong P.F., Martin T.J., Ng K.W. Effects of ascorbic acid, calcitriol, and retinoic acid on the differentiation of preosteoblasts. J Orthop Res. 1993;11(5):638–647. doi: 10.1002/jor.1100110505. [DOI] [PubMed] [Google Scholar]
- 31.Rahman F., Bordignon B., Culerrier R. Ascorbic acid drives the differentiation of mesoderm-derived embryonic stem cells. Involvement of p38 MAPK/CREB and SVCT2 transporter. Mol Nutr Food Res. 2017;61(5) doi: 10.1002/mnfr.201600506. [DOI] [PubMed] [Google Scholar]