Abstract
The forkhead box A2 (FOXA2) is the key transcriptional factor that plays an important role in tumorigenesis. However, until now the expression pattern and role of FOXA2 in glioma have yet to be elucidated. Therefore, the aim of this study was to evaluate the expression of FOXA2 in glioma and investigate its role in glioma cells. Our data showed that FOXA2 was significantly downregulated in human glioma cell lines. Forced expression of FOXA2 suppressed the ability of glioma cells to proliferate, migrate, and invade and influenced the expression level of EMT-associated proteins. In addition, forced expression of FOXA2 attenuated tumor growth of glioma in a nude mouse xenograft model. Mechanistically, we disclosed that forced expression of FOXA2 greatly downregulated the expression of β-catenin, cyclin D1, and c-Myc in glioma cells. Taken together, these results show that FOXA2 may play an important role in proliferation, invasion, and tumorigenesis in glioma cells. Thus, FOXA2 may be a potential therapeutic target for the treatment of glioma.
Key words: Glioma, Forkhead box A2 (FOXA2), Migration, Invasion
INTRODUCTION
Glioma is the most devastating and aggressive tumor of the brain, with an increasing incidence across the world in the past decade1. The prognosis of patients diagnosed with glioma remains poor despite progress with surgical and nonsurgical therapies2–4. The majority of glioma deaths are due to metastasis. Therefore, it is important to disclose the molecular mechanism underlying the initiation and development of glioma for additional insight into the treatment of glioma.
The forkhead box A (FOXA) family of transcription factors regulates chromatin structure and gene expression during embryonic development. A growing body of evidence suggests that FOXA proteins are involved in cell cycle progression, proliferation, and migration, as well as in metabolism, senescence, and apoptosis5–7. FOXA2 is a member of the FOXA family that plays an important role in regulating embryo development and body homeostasis8,9. In addition, it has been reported that FOXA2 acts as a suppressor in various solid tumors, including breast, lung, liver, and pancreatic carcinomas10–13. Zhu et al. reported that the expression of FOXA2 was significantly decreased in human gastric cancer tissues and cell lines, and overexpression of FOXA2 markedly inhibited cell tumorigenesis in vitro and in vivo14. Another study confirmed that the expression of FOXA2 was downregulated in patients with glioblastoma15. However, until now the expression pattern and role of FOXA2 in glioma have yet to be elucidated. Therefore, the aim of this study was to evaluate the expression of FOXA2 in glioma and investigate its role in glioma cells. Our data showed that FOXA2 may function as a tumor suppressor and inhibit invasion and tumorigenesis, at least partially, through inactivation of the Wnt/β-catenin signaling pathway in glioma cells.
MATERIALS AND METHODS
Cell Culture
Human glioma cell lines (LN229, U-373MG, and U-87MG) and the normal human astrocyte cell line 1800 were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Rockville, MD, USA), penicillin (100 U/ml; Sigma-Aldrich, St. Louis, MO, USA), and streptomycin (100 mg/ml; Sigma-Aldrich). All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from glioma cells using TRIzol reagent (Invitrogen) and reverse transcribed using PrimeScript RT Reagent kit (Takara, Inc., Otsu, Japan). qPCR was then conducted using the Takara SYBR Premix Ex Taq II (Takara) and the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). FOXA2, 5′-TTCTTTCCCGTTTTCCTCCTT-3′ (sense primer) and 5′-GAGAAGAAATCCATAACACCCCC-3′ (antisense primer); GAPDH, 5′-CAAGCTCATTTCCTGGTATGAC-3′ (sense primer) and 5′-CAGTGAGGGTCTCTCTCTTCCT-3′ (antisense primer). All samples were normalized to the control GAPDH, and fold changes were calculated on the basis of relative quantification using the 2−ΔΔCt method16.
Western Blotting
Total proteins were extracted using radioimmunoprecipitation assay lysis buffer (Beyotime, Nantong, P.R. China), and protein concentrations were measured using the Quick Start Bradford protein assay (Bio-Rad, Hercules, CA, USA). Total protein (30 μg) was loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. Membranes were blocked with 5% skim milk, 1% Tween 20 in PBS (Sigma-Aldrich) for 1 h, and incubated with primary antibodies overnight at 4°C in the same buffer. Antibodies against FOXA2, E-cadherin, N-cadherin, vimentin, β-catenin, cyclin D1, c-Myc, and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G antibody was used as the secondary antibody (1:5,000; Santa Cruz Biotechnology). The blots were developed using chemiluminescence (ECL) and quantified by the Quantity ONE (Bio-Rad) software.
Establishment of a Stable FOXA2-Expressing Cell Line
Full-length FOXA2 cDNA was cloned into pcDNA3.1 vector. For in vitro transfection, U-87MG cells (1 × 105 cells/well) were seeded in each cell of a 24-well microplate. After the cells were 70% confluent, the recombinant plasmid FOXA2 and vector plasmid were transferred into U-87MG cells using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions.
Cell Proliferation Assay
Cell proliferation was detected by a cell counting kit-8 (CCK-8) assay according to the manufacturer’s instructions (Dojindo, Kumamoto, Japan). Briefly, infected U-87MG cells (1 × 104 cells/well) were seeded onto a 96-well plate and incubated for 24, 48, 72, or 96 h, respectively. At the indicated time points, 10 μl of the CCK-8 solution was added to each well and incubated for another 4 h. The absorbance at 490 nm was measured on a microplate reader.
Cell Migration and Invasion Assays
Cell migration assays were performed using Transwell migration chambers (8-μm pore size; Costar). Briefly, infected U-87MG cells (1 × 104 cells/well) in 500 μl of serum-free medium were added to the upper chambers, while 500 μl of DMEM supplemented with 10% FBS was added to the lower chamber. After 24 h of incubation, the nonmigrating cells on the upper sides of the filters were detached using cotton swabs, and the cells that passed through the filter were fixed with 4% paraformaldehyde for 15 min, stained with Giemsa for 20 min, and counted in five randomly chosen fields (magnification: 100×) per well under a microscope. For the cell invasion assay, inserts were precoated with Matrigel, and the other steps were the same as for the migration assay.
In Vivo Xenograft Tumor Assay
Four- to six-week-old BALB/c athymic nude mice were purchased from Experimental Animal Center of Henan University (P.R. China) and housed under pathogen-free conditions. All animal work was done in accordance with a protocol approved by the Animal Care Commission of Huaihe Hospital (P.R. China). U-87MG cells expressing stable FOXA2 were injected subcutaneously into the flank of nude mice (n = 6 per group), while U-87MG cells containing an empty vector were used as a control. Tumor size was measured every 5 days using a caliper and calculated using the formula: volume = length × width2 × π/6. After 20 days, mice were euthanized by subcutaneous injection with sodium pentobarbital (40 mg/kg), and the tumors were weighed.
Statistical Analysis
The experimental data were presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA. A value of p < 0.05 was considered statistically significant.
RESULTS
FOXA2 Had a Low Expression in Human Glioma Cell Lines
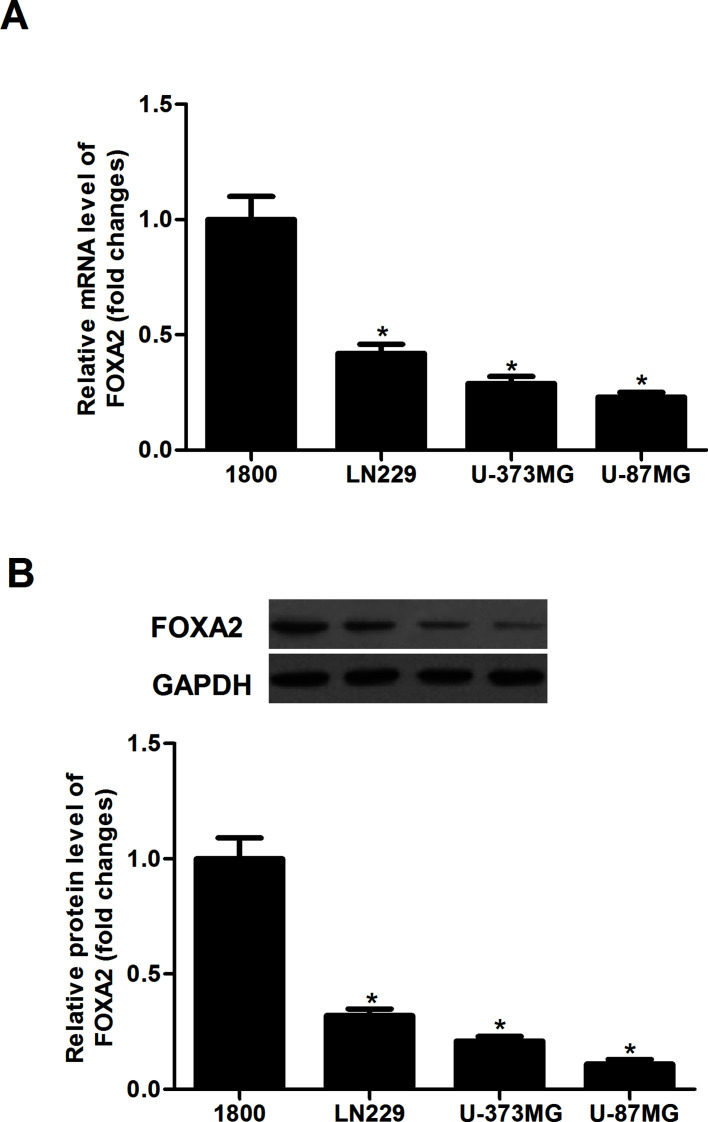
We analyzed the expression levels of FOXA2 in glioma cell lines using qRT-PCR and Western blotting. The result of the qRT-PCR analysis indicated that the mRNA expression levels of FOXA2 were lower in all of the tested glioma cell lines than that of the normal human astrocyte cell line 1800 (Fig. 1A). Consistently, the protein expression levels of FOXA2 were significantly reduced in human glioma cell lines (Fig. 1B). Thus, we selected the U-87MG cell line, which expresses a relatively low level of FOXA2, for the following study.
Figure 1.
FOXA2 was lowly expressed in human glioma cell lines. (A) The expression levels of FOXA2 mRNA in human glioma cell lines were analyzed by qRT-PCR. (B) The expression levels of FOXA2 protein in human glioma cell lines were evaluated by Western blotting. Data are shown as mean ± SD (n = 3). *p < 0.05 versus human astrocyte cell line 1800.
Forced Expression of FOXA2 Inhibited Glioma Cell Proliferation In Vitro
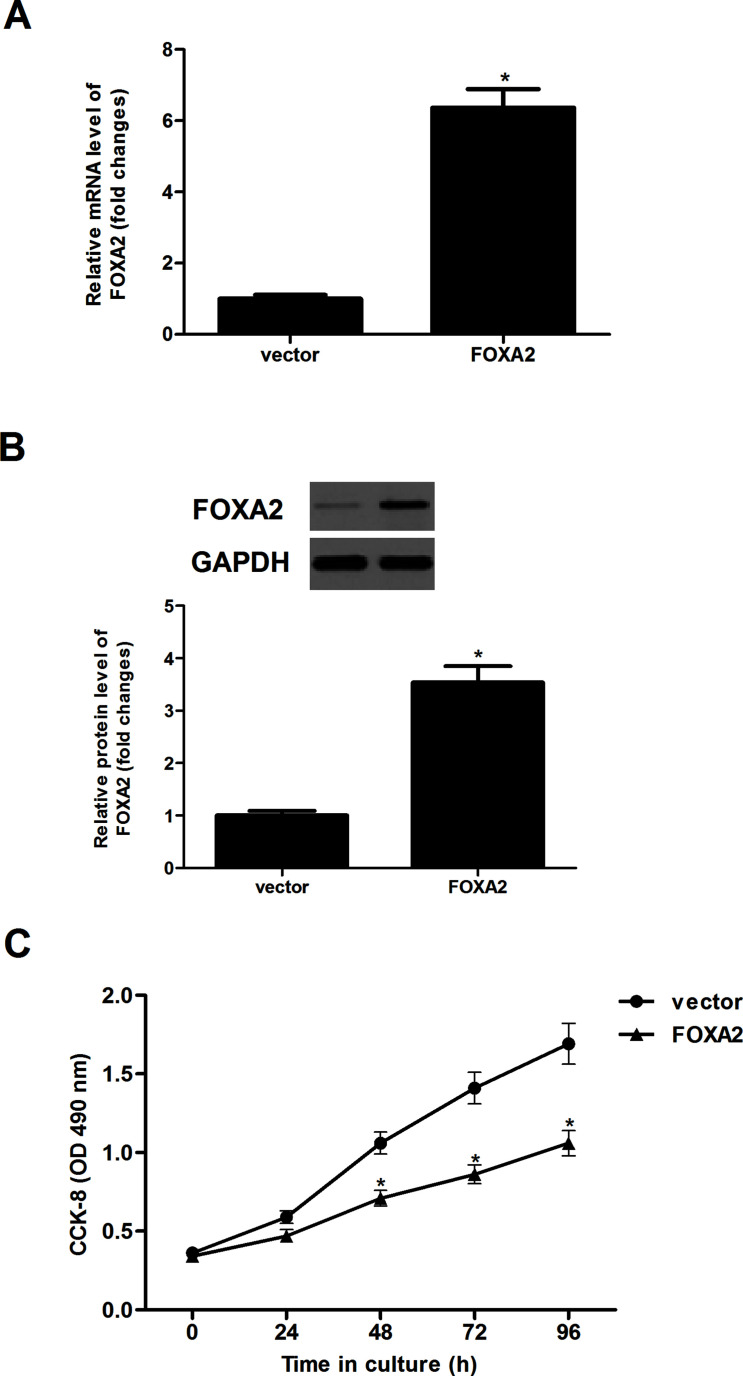
To examine the effect of FOXA2 on glioma cell proliferation, we restored FOXA2 levels by ectopically expressing FOXA2 in U-87MG cells. The results showed that the expression levels of FOXA2 in both mRNA (Fig. 2A) and protein (Fig. 2B) were greatly upregulated in U-87MG cells, as evidenced by qRT-PCR and Western blotting analysis. A CCK-8 assay was then performed to assess the role of FOXA2 in U-87MG cell proliferation. Overexpression of FOXA2 significantly reduced cellular proliferation of U-87MG cells in a time-dependent manner, compared with controls (Fig. 2C).
Figure 2.
Forced expression of FOXA2 inhibited glioma cell proliferation in vitro. U-87MG cells were transfected with FOXA2 or vector for 24 h, and the efficiency of transfection was determined by qRT-PCR (A) and Western blotting (B) assays. (C) Cell proliferation was evaluated using the CCK-8 assay. Data are shown as mean ± SD (n = 3). *p < 0.05 versus the vector group.
Forced Expression of FOXA2 Inhibited Glioma Cell Migration and Invasion In Vitro
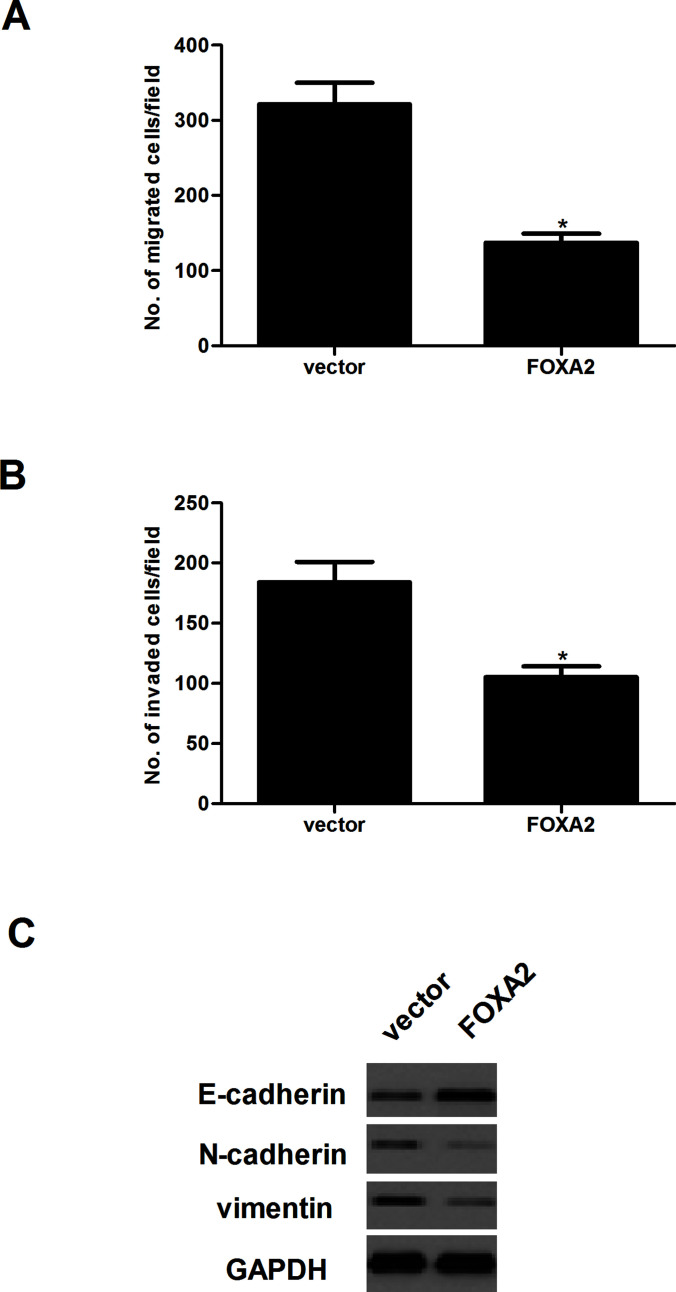
We examined the effect of FOXA2 on the invasion and migration of glioma cells using Transwell chambers with and without Matrigel. U-87MG cells transfected with FOXA2 had lower migratory activity than control cells (Fig. 3A). Similarly, the number of invading cells was markedly reduced in U-87MG cells transfected with FOXA2, compared with the vector group (Fig. 3B). We then examined the expression of EMT-related markers in U-87MG cells after the overexpression of FOXA2 using Western blotting assay. The results demonstrated that U-87MG cells transfected with FOXA2 resulted in the upregulation of E-cadherin and the downregulation of N-cadherin and vimentin (Fig. 3C).
Figure 3.
Forced expression of FOXA2 inhibited glioma cell migration and invasion in vitro. U-87MG cells were transfected with FOXA2 or vector for 24 h. (A) The migratory potential was detected by Transwell assay. (B) The invasive ability was detected by Transwell assay with Matrigel. (C) The protein expression levels of E-cadherin, N-cadherin, and vimentin were detected by Western blotting. Data are shown as mean ± SD (n = 3). *p < 0.05 versus the vector group.
Forced Expression of FOXA2 Inhibited the Activation of the Wnt/β-Catenin Pathway in Glioma Cells
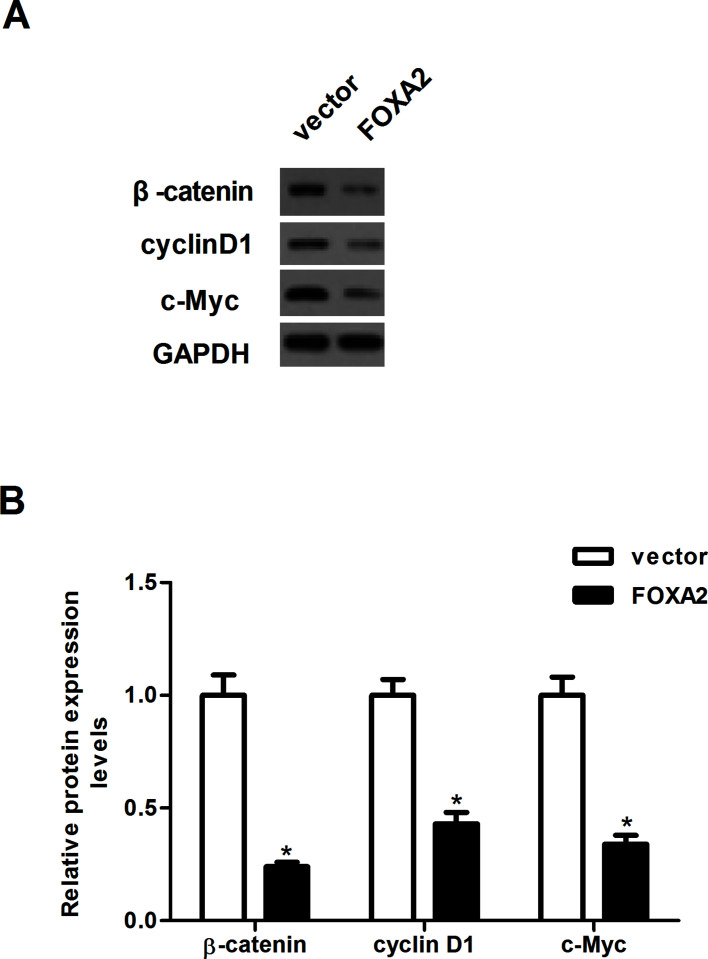
To further explore the molecular mechanism responsible for the function of FOXA2 in glioma, we examined the expression levels of β-catenin, cyclin D1, and c-Myc in U-87MG cells after the overexpression of FOXA2 using Western blotting assay. Compared with the vector group, overexpression of FOXA2 sharply downregulated the protein expression levels of β-catenin, cyclin D1, and c-myc in U-87MG cells (Fig. 4).
Figure 4.
Forced expression of FOXA2 inhibited the activation of Wnt/β-catenin pathway in glioma cells. U87MG cells were transfected with FOXA2 or vector for 24 h. (A) The protein expression levels of β-catenin, cyclin D1, and c-Myc were detected by Western blotting. (B) The optical density of each protein was quantified by GAPDH optical density. Data are shown as mean ± SD (n = 3). *p < 0.05 versus the vector group.
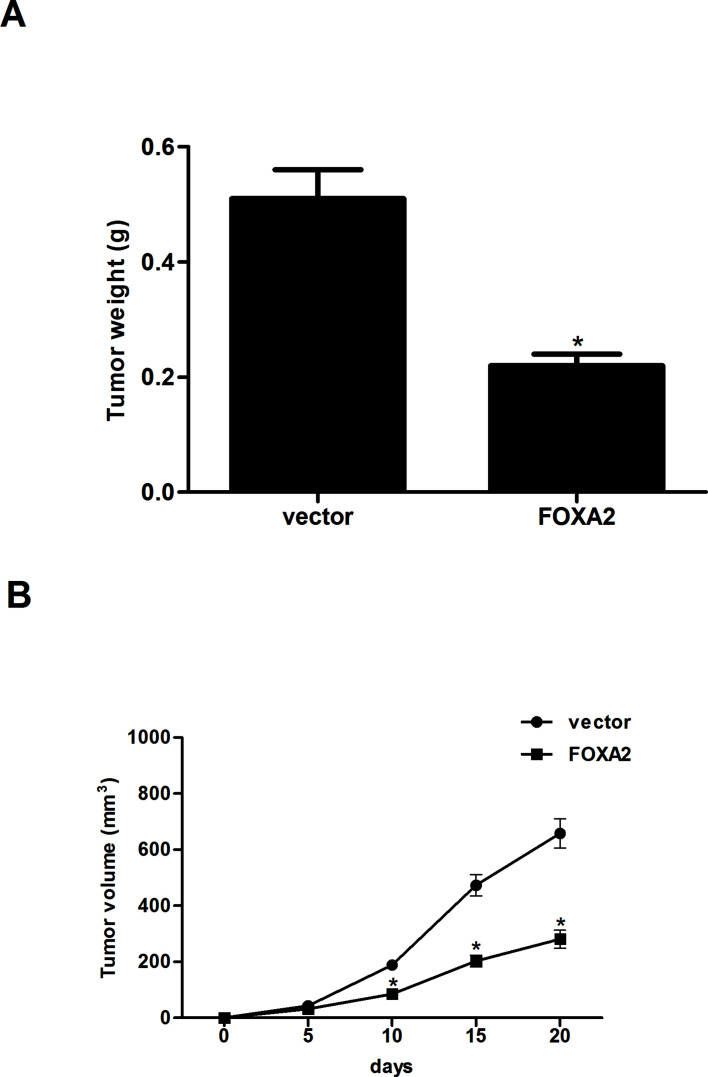
Forced Expression of FOXA2 Inhibited the Tumorigenicity of Glioma Cells In Vivo
Finally, a xenograft model was established to explore FOXA2-mediated tumorigenesis in vivo. The results demonstrated that tumor weight was remarkably decreased in the FOXA2-expressing tumor-bearing mice in comparison with the control group (Fig. 5A). In addition, forced expression of FOXA2 significantly suppressed the volume of tumors compared with control mice (Fig. 5B).
Figure 5.
Forced expression of FOXA2 inhibited tumorigenicity of glioma cells in vivo. U-87MG cells expressing stable FOXA2 or empty vector were injected subcutaneously into the flank of nude mice. (A) At day 20, the mice were euthanized, and the tumors were excised and weighed. (B) The tumor volumes were calculated in each group every 5 days. Data are shown as mean ± SD (n = 6). *p < 0.05 versus the vector group.
DISCUSSION
To our knowledge, our data provide the first evidence that FOXA2 plays a critical role in the development of glioma. FOXA2 was found to be downregulated in human glioma cell lines. Forced expression of FOXA2 suppressed the proliferation, migration, and invasion abilities of glioma cells and influenced the expression level of EMT-associated proteins. In addition, forced expression of FOXA2 attenuated tumor growth of glioma in a nude mouse xenograft model. Mechanistically, we disclosed that forced expression of FOXA2 greatly downregulated the expression of β-catenin, cyclin D1, and c-Myc in glioma cells.
FOXA2 has recently been shown to inhibit tumor progression and development. Kondratyeva et al. reported that the expression of FOXA2 was significantly downregulated in pancreatic cancer cells13. Basseres et al. confirmed that FOXA2 is frequently downregulated in lung cancer as a result of epigenetic silencing17. Consistent with these findings, herein we found that FOXA2 was lowly expressed in human glioma cell lines, and forced expression of FOXA2 suppressed the proliferation of glioma cells in vitro and attenuated tumor growth of glioma in a nude mouse xenograft model. These results suggest that FOXA2 may function as a tumor suppressor in the development and progression of glioma.
Glioma is characterized by its invasive growth, and it is difficult to treat with surgery18. EMT plays a critical role in driving tumor invasion and metastasis. During the EMT process, epithelial cells downregulate the expression of cell adhesion molecules including E-cadherin, dissolve cell–cell junctions, lose their apical–basal polarity, and acquire migratory and invasive properties19,20. It was reported that overexpression of FOXA2 reduced EMT and invasion of lung cancer cells21. A study by Zhang et al. confirmed that stable overexpression of FOXA2 attenuated EMT in breast cancer cells22. Similarly, herein we found that forced expression of FOXA2 suppressed the migration and invasion abilities of glioma cells and influenced the expression level of EMT-associated proteins. These data suggest that FOXA2 suppressed glioma cell migration and invasion by blocking the EMT phenotype.
Accumulating evidence has shown that the Wnt/β-catenin signaling pathway plays a critical role in tumor progression, and this pathway is activated in a wide variety of cancer types, including glioma23–25. β-Catenin is a main downstream effector of the canonical Wnt signaling pathway that regulates the expression of cyclin D1, c-Myc, and matrix metalloprotease, leading to uncontrolled cell proliferation and invasion26. It was reported that the protein expression levels of Wnt1, β-catenin, and cyclin D1 were all positively related to the grades of patients with gliomas27. Therefore, inhibition of the Wnt/β-catenin signaling pathway could be a useful approach for the treatment of glioma. In this study, we found that forced expression of FOXA2 greatly downregulated the expression of β-catenin, cyclin D1, and c-Myc in U-87MG cells. These results suggest that FOXA2 inhibits invasion and tumorigenesis, at least partially, through inactivation of the Wnt/β-catenin signaling pathway in glioma cells.
In conclusion, our data revealed that FOXA2 may function as a tumor suppressor and inhibit invasion and tumorigenesis, at least partially, through inactivation of the Wnt/β-catenin signaling pathway in glioma cells, implying that FOXA2 might be a novel therapeutic target for the treatment of glioma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. [DOI] [PubMed] [Google Scholar]
- 2. Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: Diagnostic, prognostic, and predictive markers. Lancet Neurol. 2010;9:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamza MA, Kamiyamatsuoka C, Liu D, Yuan Y, Puduvalli VK. Outcome of patients with malignant glioma and synchronous or metachronous non-central. J Neuro-Oncol. 2016;126:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng J, Liu B, Zhang X, Lin W, Zhang Y, Liu W, Zhang J, Lin H, Wang R, Yin H. Health-related quality of life in glioma patients in China. BMC Cancer 2010;10:305–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman JR, Kaestner KH. The FOXA family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakshatri H, Badve S. FOXA1 as a therapeutic target for breast cancer. Expert Opin Ther Targets 2007;11:507–14. [DOI] [PubMed] [Google Scholar]
- 7. Ionescu A, Kozhemyakina E, Nicolae C, Kaestner K, Olsen B, Lassar A. FOXA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev Cell 2012;22:927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katoh M. Human FOX gene family (review). Int J Oncol. 2004;25:1495–500. [PubMed] [Google Scholar]
- 9. Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of FOXA2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perezbalaguer A, Ortizmartínez F, Garcíamartínez A, Pomaresnavarro C, Lerma E, Peiró G. FOXA2 mRNA expression is associated with relapse in patients with triple-negative/basal-like breast carcinoma. Breast Cancer Res Treat. 2015;153:465–74. [DOI] [PubMed] [Google Scholar]
- 11. Jang SM, An JH, Kim CH, Kim JW, Choi KH. Transcription factor FOXA2-centered transcriptional regulation network in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;463:961–7. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Zhu CP, Hu PF, Qian H, Ning BF, Zhang Q, Chen F, Liu J, Shi B, Zhang X. FOXA2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition. Carcinogenesis 2014;35:2576–83. [DOI] [PubMed] [Google Scholar]
- 13. Kondratyeva LG, Sveshnikova AA, Grankina EV, Chernov IP, Kopantseva MR, Kopantzev EP, Sverdlov ED. Downregulation of expression of mater genes SOX9, FOXA2, and GATA4 in pancreatic cancer cells stimulated with TGFβ1 epithelial-mesenchymal transition. Dokl Biochem Biophys. 2016;469:257–9. [DOI] [PubMed] [Google Scholar]
- 14. Zhu CP, Wang J, Shi B, Hu PF, Ning BF, Zhang Q, Chen F, Chen WS, Zhang X, Xie WF. The transcription factor FOXA2 suppresses gastric tumorigenesis in vitro and in vivo. Digest Dis Sci. 2015;60:109–17. [DOI] [PubMed] [Google Scholar]
- 15. Robertson E, Perry C, Doherty R, Madhusudan S. Transcriptomic profiling of Forkhead box transcription factors in adult glioblastoma multiforme. Cancer Genomics Proteomics 2015;12:103–12. [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-T quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 17. Basseres DS, D’Alò F, Yeap BY, Löwenberg EC, Gonzalez DA, Yasuda H, Dayaram T, Kocher ON, Godleski JJ, Richards WG. Frequent downregulation of the transcription factor FOXA2 in lung cancer through epigenetic silencing. Lung Cancer 2012;77:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González AM. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;21:33–8. [DOI] [PubMed] [Google Scholar]
- 19. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 20. Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–94. [DOI] [PubMed] [Google Scholar]
- 21. Tang Y, Shu G, Yuan X, Jing N, Song J. FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. 2011;21:316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z, Yang C, Gao W, Chen T, Qian T, Hu J, Tan Y. FOXA2 attenuates the epithelial to mesenchymal transition by regulating the transcription of E-cadherin and ZEB2 in human breast cancer. Cancer Lett. 2015;361:240–50. [DOI] [PubMed] [Google Scholar]
- 23. Zhang K, Zhang J, Han L, Pu P, Kang C. Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharm. 2012;7:740–9. [DOI] [PubMed] [Google Scholar]
- 24. Denysenko T, Annovazzi L, Cassoni P, Melcarne A, Mellai M, Schiffer D. WNT/β-catenin signaling pathway and downstream modulators in low- and high-grade glioma. Cancer Genomics Proteomics 2015;13:31–45. [PubMed] [Google Scholar]
- 25. Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, Jiang H. Downregulation of Wnt2 and |[beta]|-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2008;16:351–61. [DOI] [PubMed] [Google Scholar]
- 26. Li YJ, Wei ZM, Meng YX, Ji XR. β-Catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: Relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005;11:2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu C, Tu Y, Sun X, Jian J, Jin X, Bo X, Li Z, Bian A, Wang X, Dai L. Wnt/beta-catenin pathway in human glioma: Expression pattern and clinical/prognostic correlations. Clin Exp Med. 2011;11:105–12. [DOI] [PubMed] [Google Scholar]