Abstract
Forkhead box K1 (FOXK1) is a member of the FOX transcription factor family and plays an important role in the development of several tumors. However, the role of FOXK1 in the progression of prostate cancer remains unknown. Thus, the objectives of this study were to detect the expression of FOXK1 in prostate cancer and to examine its role in prostate cancer cells. We found that the expression of FOXK1 at both the mRNA and protein levels was significantly upregulated in human prostate cancer cell lines. In addition, the downregulation of FOXK1 obviously inhibited the cell proliferation of prostate cancer cells in vitro and attenuated tumor growth in a xenograft model in vivo. Furthermore, knockdown of FOXK1 suppressed the migration and invasion of prostate cancer cells, and prevented the EMT phenotype through upregulating the expression of E-cadherin, as well as downregulating the expression of N-cadherin in prostate cancer cells. Mechanistically, knockdown of FOXK1 efficiently downregulated the expression levels of β-catenin, c-myc, and cyclin D1 in PC-3 cells. Overall, our results demonstrated that knockdown of FOXK1 inhibited the proliferation and metastasis of prostate cancer, at least in part, through suppressing the Wnt/β-catenin signaling pathway. Therefore, these results suggest that FOXK1 may be a potential therapeutic target for human prostate cancer.
Key words: Forkhead box K1 (FOXK1), Prostate cancer, Proliferation, Invasion
INTRODUCTION
Prostate cancer is the most common cancer diagnosed among men in developed countries and is the leading cause of cancer deaths in the world1. The incidence of prostate cancer has been rising steadily in the past decades. Despite recent diagnostic and therapeutic advances, the overall 5-year survival rate is very low2–4. Many patients with advanced-stage prostate cancer will still die of this disease due to metastasis and recurrence5,6. Thus, understanding of the molecular mechanisms of prostate cancer metastasis is imperative for developing novel and effective therapies to treat prostate cancer.
Forkhead box (FOX) proteins are a family of transcription factors characterized by the presence of an evolutionary conserved “forkhead” or “winged-helix” DNA-binding domain. Increasing evidence has reported that FOX protein members play a critical role in embryogenesis, organogenesis, cell proliferation, migration, and apoptosis7–9. Forkhead box K1 (FOXK1) is a member of the FOX transcription factor family. It plays an important role in cell proliferation, cell growth, and metabolism10. FOXK1 has also been implicated in the development of several tumors11–13. A recent study showed that ectopic expression of FOXK1 could inhibit the migratory and invasive abilities of colorectal cancer cells14. However, the role of FOXK1 in the progression of prostate cancer remains unknown. Thus, the objective of this study was to detect the expression of FOXK1 in prostate cancer and examine its role in prostate cancer cells. In this study, we showed that FOXK1 was highly expressed in prostate cancer cell lines, and knockdown of FOXK1 inhibited the proliferation, migration, and invasion in prostate cancer cells, at least in part, through inactivation of the Wnt/β-catenin signaling pathway.
MATERIALS AND METHODS
Cell Culture
Human prostate cancer cell lines (DU145, PC-3, and LNCaP) and normal prostate cell line (RWPE1) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All of the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 100 U/ml penicillin/streptomycin and 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA) at 37°C in a humidified atmosphere with 5% CO2.
Quantitative Real-Time (qRT)-PCR Analysis
Total RNA was extracted from prostate cancer cells using TRIzol reagent (Invitrogen), and the synthesis of cDNA was completed by reverse transcription. Subsequently, real-time PCR was performed using SsoFast EvaGreen Supermix on a CFX96 Real-Time PCR Detection System (Bio-Rad, Richmond, CA, USA). The following primers were used: FOXK1, 5′-ACACGTCTGGAGGAGACAGC-3′ (forward) and 5′-GAGAGGTTGTGCCGGATAGA-3′ (reverse); GAPDH, 5′-AAGGCTGGGGCTCATTTGC-3′ (forward) and 5′-GCTGATGATCTTGA-GGCTGTTG-3′ (reverse). Relative levels of gene expression were expressed relative to GAPDH and calculated using the 2−ΔΔCT method.
Western Blot
PC-3 cells were harvested and lysed with RIPA buffer (Takara Biotechnology, Dalian, P.R. China) supplemented with protease inhibitors. Equal amounts of protein (40 μg of protein per lane) were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). After blocking with 5% milk, the membranes were incubated with primary antibodies against FOXK1, E-cadherin, N-cadherin, β-catenin, c-myc, cyclin D1, and GAPDH (Abcam, Cambridge, UK) at 4°C overnight. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. The blots were developed using an enhanced chemiluminescence Western blotting detection system (Amersham Bioscience, UK).
shRNA Transfection
The short hairpin RNA against FOXK1 (shFOXK1) and negative control shRNA (shNC) were obtained from Genechem Company (Shanghai, P.R. China). Twenty-four hours prior to transfection, cells were plated onto a 96-well plate (Invitrogen) at 60% confluence. PC-3 cells were then transfected with shFOXK1 or shNC using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s protocols. The knockdown efficiency was confirmed by RT-PCR and Western blot analysis.
Cell Proliferation Assay
Cell proliferation was determined using a cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s instructions. In brief, PC-3 cells (1.0 × 105 cells/well) transfected with shFOXK1 or shNC were seeded onto a 96-well plate and then cultured for 24, 48, 72, or 96 h, respectively. Ten microliters of CCK-8 solution was then added into each plate for 3 h. The absorbance at 490 nm was measured with a microplate reader (Bio-Rad).
Cell Migration and Invasion Assays
The invasive potential of cells was detected using 24-well 8-μm pore size Transwell inserts (Corning Costar, Corning, NY, USA) precoated with 100 μl of Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, PC-3 cells (1.0 × 105 cells/well) transfected with shFOXK1 or shNC were suspended in 200 μl of serum-free DMEM and seeded onto the upper chamber of the Transwell. Five hundred microliters of DMEM containing 10% FBS was added to the lower chamber. After incubating for 24 h at 37°C with 5% CO2, cells that invaded to the bottom surface of the membrane were fixed in formaldehyde, stained with 0.05% crystal violet in PBS for 15 min, and counted under a microscope (Olympus, Tokyo, Japan). For the migration assay, the same procedures described above were used, except that the filters were not precoated with Matrigel.
Tumor Implantation
All animal procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital (P.R. China). Briefly, 1 × 106 PC-3 cells infected with shFOXK1 or scramble shRNA were subcutaneously injected into the right flanks of 4- to 6-week-old female BALB/c-nu/nu mice (n = 6 per group). The tumor volumes were measured every 7 days by a caliper and calculated as follows: total tumor volume (mm3) = L × W 2/2, where L is the length, and W is the width. After 28 days, the mice were sacrificed, and the tumors were dissected out and individually weighed.
Statistical Analysis
Statistical analysis was performed using the SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The data were expressed as mean ± SD. Statistical significance was analyzed with one-way factorial ANOVA or Student’s two-tailed t-test. A value of p < 0.05 was considered as statistically significant.
RESULTS
FOXK1 Was Highly Expressed in Human Prostate Cancer Cell Lines
First, we studied the expression of FOXK1 mRNA in human prostate normal and cancer cell lines by qRT-PCR. The results demonstrated that the expression levels of FOXK1 mRNA were significantly upregulated in all tested prostate cancer cell lines, including DU145, PC-3, and LNCaP, compared to that in the normal prostate RWPE1 cells (Fig. 1A). Similarly, Western blotting results showed that the protein expression of FOXK1 was also significantly higher in prostate cancer cell lines than that in RWPE1 cells (Fig. 1B).
Figure 1.
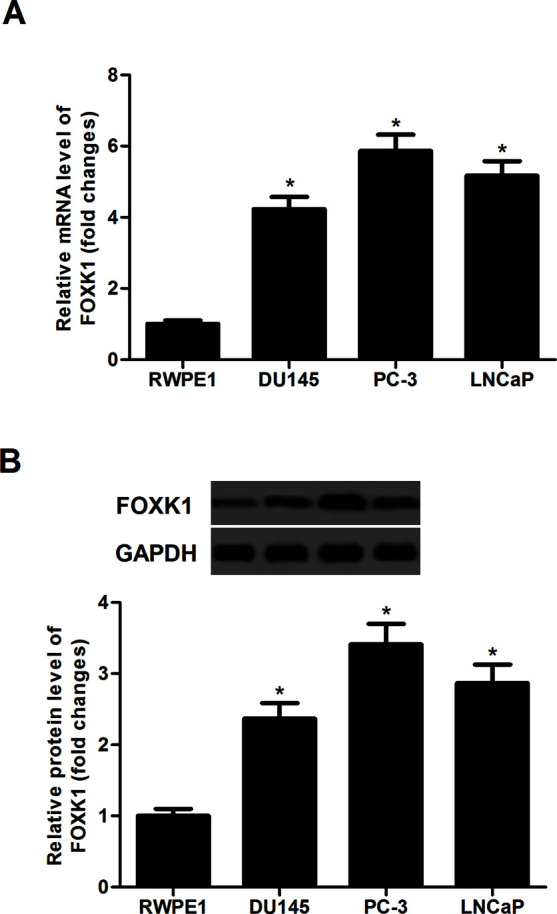
FOXK1 was highly expressed in human prostate cancer cell lines. (A) The mRNA expression of FOXK1 on prostate cancer cell lines (DU145, PC-3, and LNCaP) and normal prostate cell line (RWPE1) was determined by quantitative real-time (qRT)-PCR. (B) The protein expression of FOXK1 was analyzed by Western blot in a panel of prostate cancer cell lines. GAPDH served as loading control. Data are representative of three independent experiments performed under identical experimental conditions. *p < 0.05.
Knockdown of FOXK1 Inhibited Prostate Cancer Cell Proliferation In Vitro
To investigate the impact of FOXK1 on prostate cancer proliferation, we stably knocked down FOXK1 in PC-3 cells. After transfection, the expression of FOXK1 at both mRNA (Fig. 2A) and protein levels (Fig. 2B) was obviously downregulated in PC-3 cells, respectively. We then determined the effect of reducing FOXK1 expression on PC-3 cell proliferation using the CCK-8 assay. Knockdown of FOXK1 greatly inhibited cell proliferation in PC-3 cells, compared with the shNC group (Fig. 2C).
Figure 2.
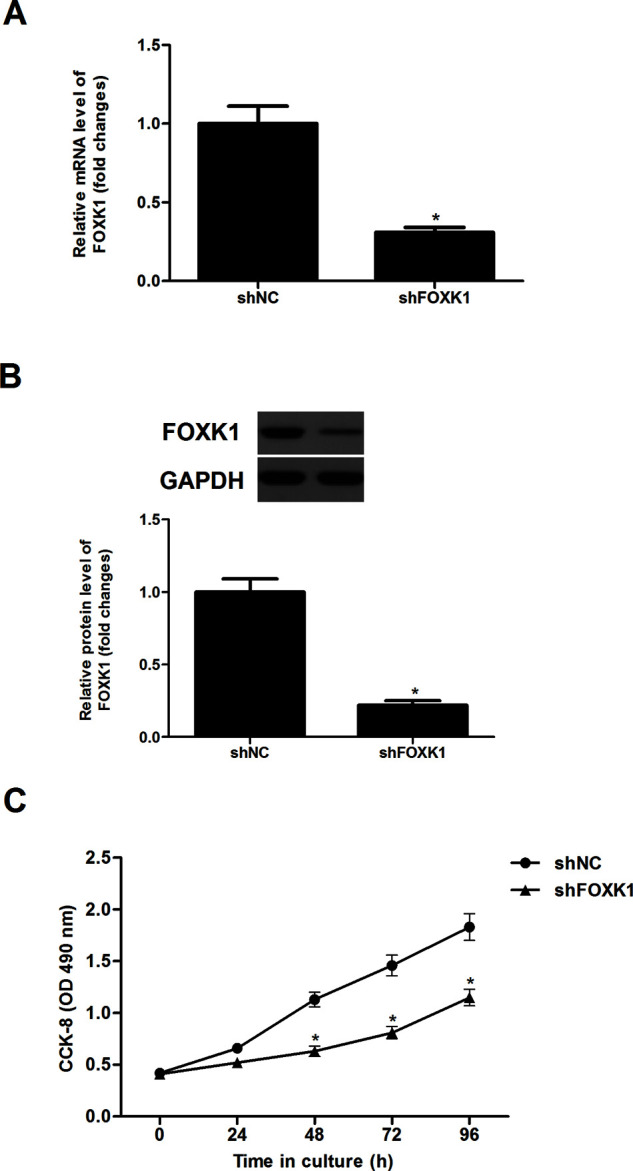
Knockdown of FOXK1 inhibited prostate cancer cell proliferation in vitro. PC-3 cells were transfected with shFOXK1 or shNC for 48 h, respectively. (A) The mRNA expression of FOXK1 was determined by qRT-PCR. (B) The protein expression of FOXK1 was analyzed by Western blot. GAPDH served as loading control. (C) Cell proliferation was evaluated using the CCK-8 assay. Data are representative of three independent experiments performed under identical experimental conditions. *p < 0.05.
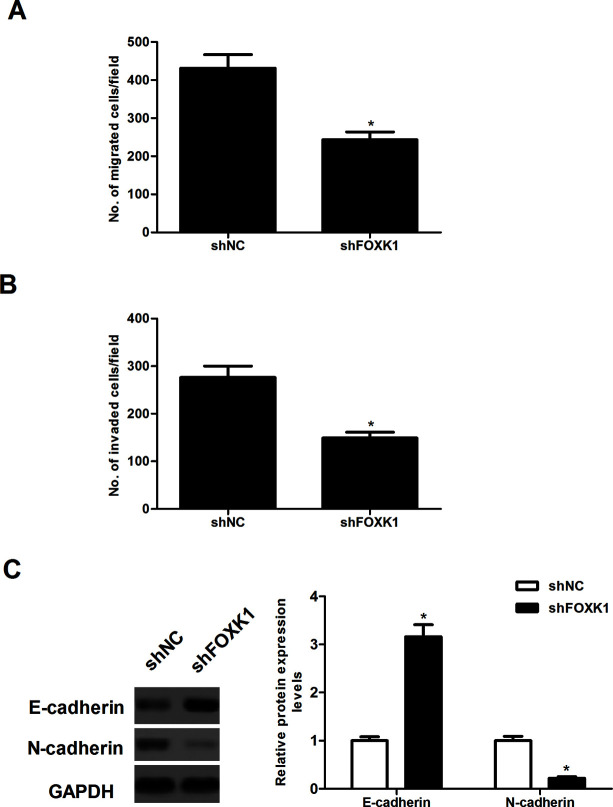
Knockdown of FOXK1 Inhibited Prostate Cancer Cell Migration and Invasion
We investigated the effect of FOXK1 expression on the migration of prostate cancer cells in a Transwell migration assay. The migration capacity of PC-3 cells was significantly suppressed by shFOXK1 (Fig. 3A). In addition, the Matrigel invasion assay demonstrated that PC-3 cells transfected with shFOXK1 had a lower invasive activity than the control cells (Fig. 3B). Moreover, we observed that decreased FOXK1 expression reversed acquisition of the EMT phenotype by upregulating epithelial cell markers E-cadherin, as well as downregulating the expression of the mesenchymal cell markers N-cadherin in PC-3 cells (Fig. 3C).
Figure 3.
Knockdown of FOXK1 inhibited prostate cancer cell migration and invasion. PC-3 cells were transfected with shFOXK1 or shNC for 48 h, respectively. (A) Transwell migration assay was used to examine cell migration. (B) Cell invasion was assessed by the Matrigel invasion assay. (C) The protein expression of E-cadherin and N-cadherin was analyzed by Western blot. GAPDH served as loading control. Data are representative of three independent experiments performed under identical experimental conditions. *p < 0.05.
Knockdown of FOXK1 Attenuated Tumor Growth In Vivo
To investigate whether silencing of FOXK1 was associated with in vivo tumor growth, 1 × 106 PC-3 cells transfected with shFOXK1 or shNC were subcutaneously injected into the right flanks of 4- to 6-week-old female BALB/c-nu/nu mice. The results showed that the tumor volumes that developed from shFOXK1-transfected PC-3 cells were significantly smaller than the tumors derived from the control cells (Fig. 4A). In addition, the tumor weights in mice significantly declined in PC-3 cells transfected with shFOXK1 compared with the controls (Fig. 4B).
Figure 4.
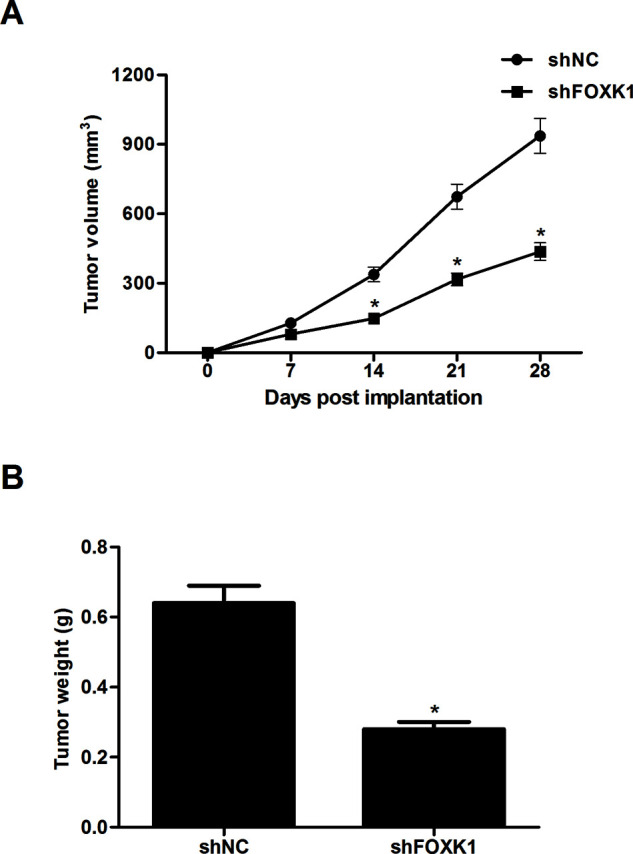
Knockdown of FOXK1 attenuated tumor growth in vivo. PC-3 cells (1 × 106) transfected with shFOXK1 or shNC were subcutaneously injected into the right flanks of 4- to 6-week-old female BALB/c-nu/nu mice. (A) The tumor volume was measured every 7 days. (B) After 28 days, mice were killed and the tumors were weighed. Data are representative of three independent experiments performed under identical experimental conditions. *p < 0.05.
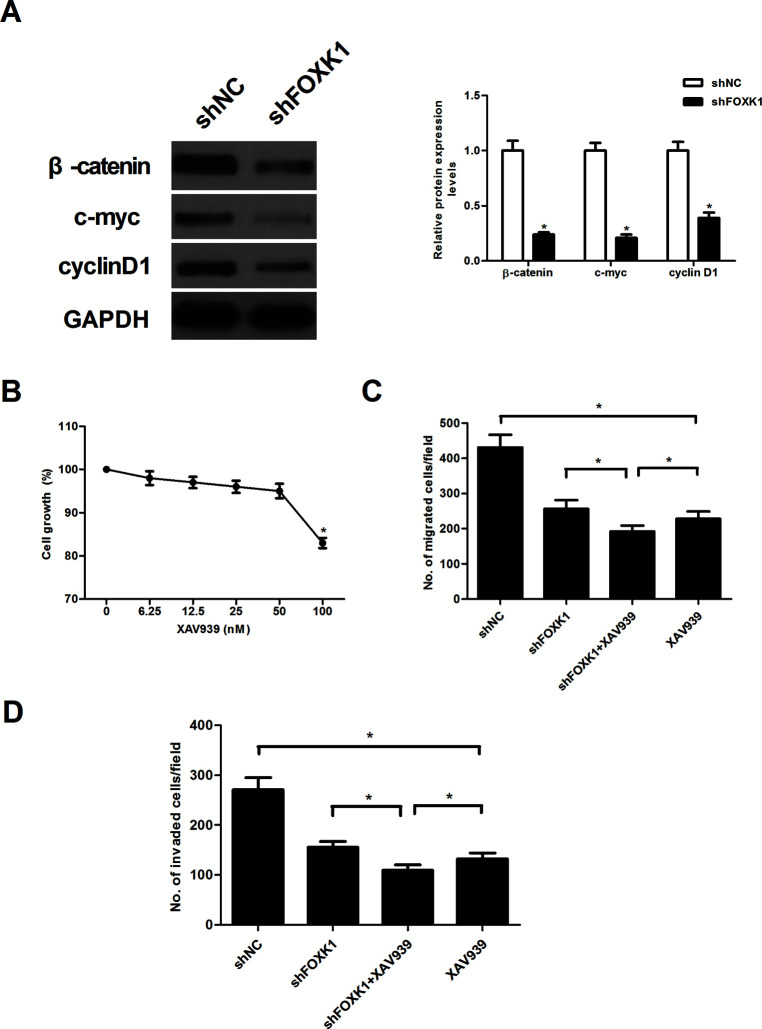
Knockdown of FOXK1 Prevented Activation of the Wnt/β-Catenin Pathway in Prostate Cancer Cells
To explore the underlying biological mechanisms of FOXK1 function in prostate cancer progression, we detected the effect of FOXK1 on the activation of the Wnt/β-catenin pathway in PC-3 cells using Western blot. Knockdown of FOXK1 expression sharply decreased the expression protein levels of β-catenin, c-myc, and cyclin D1 in PC-3 cells, compared with the shNC group (Fig. 5A). We next examined whether an inhibitor of the β-catenin pathway (XAV939) could inhibit the cell migration and invasion capabilities in FOXK1 knockdown PC-3 cells. Because administration of 50 nM XAV939 had no significant effect on cell viability, 50 nM XAV939 was chosen for additional experiments (Fig. 5B). Furthermore, we observed that XAV939 was able to significantly enhance the inhibitory effects of shFOXK1 on PC-3 cell migration (Fig. 5C) and invasion (Fig. 5D).
Figure 5.
Knockdown of FOXK1 prevented the activation of the Wnt/β-catenin pathway in prostate cancer cells. (A) PC-3 cells were transfected with shFOXK1 or shNC for 48 h, respectively. Thereafter, total protein lysates were analyzed by Western blot using the indicated antibodies. GAPDH served as loading control. (B) PC-3 cells were treated with different concentrations of the inhibitor of β-catenin pathway (XAV939) for 24 h, and cell viability was assessed. PC-3 cells were transfected with shFOXK1 or shNC in the presence or absence of XAV939 (50 nM) for 24 h. (C) Transwell migration assay was used to examine cell migration. (D) Cell invasion was assessed by the Matrigel invasion assay. Data are representative of three independent experiments performed under identical experimental conditions. *p < 0.05.
DISCUSSION
In this study, we found that the expression of FOXK1 at both the mRNA and protein levels was significantly upregulated in human prostate cancer cell lines. In addition, the downregulation of FOXK1 obviously inhibited the cell proliferation of prostate cancer cells in vitro and attenuated tumor growth in a xenograft model in vivo. Furthermore, knockdown of FOXK1 suppressed the migration and invasion of prostate cancer cells and prevented the EMT phenotype through upregulating the expression of E-cadherin, as well as downregulating the expression of N-cadherin in prostate cancer cells. Mechanistically, knockdown of FOXK1 efficiently downregulated the expression levels of β-catenin, c-myc, and cyclin D1 in PC-3 cells.
Emerging evidence has shown that deregulation of FOXK1 is involved in tumorigenesis and cancer progression. A recent study revealed that FOXK1 protein expression was higher in human gastric cancer tissues compared with that in adjacent normal tissues, and FOXK1 overexpression significantly promoted the proliferation of gastric cancer cells11. Wu et al. reported that FOXK1 expression was upregulated in colorectal cancer tissues compared with matched normal tissues, and the elevated expression of FOXK1 was correlated with tumor progression14. In accordance with these findings, we found that FOXK1 was highly expressed in human prostate cancer cell lines, and the downregulation of FOXK1 obviously inhibited the cell proliferation of prostate cancer cells in vitro and attenuated tumor growth in a xenograft model in vivo. These data imply that FOXK1 may be an oncogene in the development of prostate cancer.
Tumor invasion and metastasis are the main causes of mortality in patients with prostate cancer. EMT is considered to be a crucial early step in cancer progression and metastasis. During EMT, stationary epithelial cells lose polarity with cell–cell junctions and acquire a mesenchymal morphology and the ability to migrate and invade15,16. It has been reported that downregulation of E-cadherin greatly decreased tumor cell adhesion ability and induced cell mobility and invasion in prostate cancer cells17. Similarly, herein we observed that knockdown of FOXK1 suppressed the migration and invasion of prostate cancer cells and prevented the EMT phenotype through upregulating the expression of E-cadherin, as well as downregulating the expression of N-cadherin in prostate cancer cells. Our findings identified FOXK1 as a novel inducer of EMT and a potential enhancer of the metastatic potential in prostate cancer.
Previous studies suggest that the Wnt/β-catenin signaling pathway is implicated in prostate cancer development and progression18–20. The activation of the Wnt/β-catenin signaling pathway induced the EMT process and invasion in prostate cancer cells21,22. β-Catenin is a core component of the canonical Wnt signaling pathway that regulates the transcription of several Wnt downstream target genes, such as cyclin D1, c-myc, and matrix metalloprotease, leading to uncontrolled cell proliferation and invasion23. In addition, FOXK1 was found to positively regulate Wnt/β-catenin signaling by translocating disheveled (DVL) proteins into the nucleus24. In line with the results of the prior study, in this study we observed that knockdown of FOXK1 efficiently downregulated the expression levels of β-catenin, c-myc, and cyclin D1 in PC-3 cells. Furthermore, we observed that XAV939 was able to significantly enhance the inhibitory effects of shFOXK1 on PC-3 cell migration and invasion. These data suggest that knockdown of FOXK1 inhibited the metastasis and tumorigenesis in prostate cancer cells, at least in part, through suppressing the Wnt/β-catenin signaling pathway.
In conclusion, our results demonstrated that knockdown of FOXK1 inhibited the proliferation and metastasis of prostate cancer, at least in part, through suppressing the Wnt/β-catenin signaling pathway. Therefore, these results suggest that FOXK1 may be a potential therapeutic target for human prostate cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Plonis J, Bokums K, Cauce V, Miklasevics E, Vaganovs P, Irmejs A, Gardovskis J, Vjaters E. Prostate cancer trends in Latvia during 1990–2012: Incidence, prevalence, mortality, and survival rates. Medicina 2014;50:313–7. [DOI] [PubMed] [Google Scholar]
- 3. Orecchia R, Jereczek-Fossa BA, Ciocca M, Vavassori A, Cambria R, Cattani F, Zerini D, Matei DV, Rocco B, Verweij F, Scardino E, De Cobelli O. Intraoperative radiotherapy for locally advanced prostate cancer: Treatment technique and ultrasound-based analysis of dose distribution. Anticancer Res. 2007;27:3471–6. [PubMed] [Google Scholar]
- 4. Lowrance WT, Chang SS. Advancing prostate cancer: Treatment options for the urologist. Urol Clin North Am. 2006;33:211–7. [DOI] [PubMed] [Google Scholar]
- 5. Antonarakis ES, Chen Y, Elsamanoudi SI, Brassell SA, Rocha MVD, Eisenberger MA, McLeod DG. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: Analysis of the Center for Prostate Disease Research National Database. BJU Int. 2010;108:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, Vickers A, Scardino PT. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: A comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu H. Forkhead box transcription factors in embryonic heart development and congenital heart disease. Life Sci. 2016;144:194–201. [DOI] [PubMed] [Google Scholar]
- 8. Golson ML, Kaestner KH. Fox transcription factors: From development to disease. Development 2016;143:4558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: Key players in health and disease. Trends Genet. 2011;27:224–32. [DOI] [PubMed] [Google Scholar]
- 10. Shi X, Wallis AM, Gerard RD, Voelker KA, Grange RW, Depinho RA, Garry MG, Garry DJ. Foxk1 promotes cell proliferation and represses myogenic differentiation by regulating Foxo4 and Mef2. J Cell Sci. 2012;125:5329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng Y, Zhang P, Huang X, Yan Q, Wu M, Xie R, Wu Y, Zhang M, Nan Q, Zhao J, Li A, Xiong J, Ren Y, Bai Y, Chen Y, Liu S, Wang J. Direct regulation of FOXK1 by C-jun promotes proliferation, invasion and metastasis in gastric cancer cells. Cell Death Disease 2016;7:e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Y, Xie R, Liu X, Wang J, Peng Y, Tang W, Wu M, Zhang P, Ba Y, Zhao J, Li A, Nan Q, Chen Y, Liu S, Wang J. Knockdown of FOXK1 alone or in combination with apoptosis-inducing 5-FU inhibits cell growth in colorectal cancer. Oncol Rep. 2016;36:2151–9. [DOI] [PubMed] [Google Scholar]
- 13. Sun T, Wang H, Qiang L, Qian Z, Shen C. Forkhead box protein k1 recruits TET1 to act as a tumor suppressor and is associated with MRI detection. Jpn J Clin Oncol. 2016;46:209–21. [DOI] [PubMed] [Google Scholar]
- 14. Wu Y, Peng Y, Wu M, Zhang W, Zhang M, Xie R, Zhang P, Bai Y, Zhao J, Li A, Nan Q, Chen Y, Ren Y, Liu S, Wang J. Oncogene FOXK1 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Oncotarget 2016;7:51150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 16. De CB, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- 17. Fan L, Wang H, Xia X, Rao Y, Ma X, Ma D, Wu P, Chen G. Loss of E-cadherin promotes prostate cancer metastasis via upregulation of metastasis-associated gene 1 expression. Oncol Lett. 2012;4:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Suppression of Wnt/β-catenin signaling inhibits prostate cancer cell proliferation. Eur J Pharmacol. 2009;602:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. [DOI] [PubMed] [Google Scholar]
- 20. Tycko B, Li CM, Buttyan R. The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr Mol Med. 2007;7:479–89. [DOI] [PubMed] [Google Scholar]
- 21. Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH. Role of Wnt/-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1. Int J Urol. 2007;14:1034–39. [DOI] [PubMed] [Google Scholar]
- 22. Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol. 2012;9:418–28. [DOI] [PubMed] [Google Scholar]
- 23. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–80. [DOI] [PubMed] [Google Scholar]
- 24. Wang W, Li X, Lee M, Jun S, Aziz KE, Feng L, Tran MK, Li N, McCrea PD, Park JI, Chen J. FOXKs promote Wnt/β-catenin signaling by translocating DVL into the nucleus. Dev Cell 2015;32:707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]