Abstract
Osteoprotegerin (OPG) is a robust antiresorptive molecule that acts as a decoy receptor for the receptor activator of nuclear factor κB ligand (RANKL), the mediator of osteoclastogenesis. This study was designed to explore the possible role of serum OPG and RANKL in detecting bone metastasis in breast cancer and its interaction with clinicopathologic parameters. Serum levels of RANKL and OPG were estimated in 44 metastatic and 36 nonmetastatic breast cancer patients using ELISA kits. Serum OPG levels were significantly reduced in patients with bone metastasis and correlated negatively with the number of bone lesions and CA 15-3 levels. At concentrations ≤82 pg/ml, OPG showed a high specificity in identifying the presence of bone metastasis (92%), albeit with low sensitivity (59%), which improved after the exclusion of diabetics and patients treated with aromatase inhibitors (AI). Serum RANKL levels were significantly higher in the presence of bone metastasis and hypercalcemia. At concentrations >12.5 pg/ml, RANKL had an associated sensitivity of 86%, albeit with low specificity (53%), in detecting bone metastasis. The RANKL/OPG ratio significantly increased in the presence of bone metastasis with appropriate sensitivity and specificity (73% and 72%, respectively) at a cutoff of ≥0.14 for the detection of bone metastasis. Serum OPG and RANKL/OPG ratios are promising biomarkers for detecting bone metastasis in breast cancer patients.
Key words: Breast cancer, Bone metastasis, Osteoprotegerin (OPG), Receptor activator of nuclear factor κB ligand (RANKL)
INTRODUCTION
Breast cancer is the most common cancer in females worldwide, and it accounts for 29% of all new cancers1. Because of the high affinity of breast cancer cell growth in bones (soil, seed concept), the skeletal system becomes the most common breeding ground for distant metastasis from breast cancer. Approximately 65%–75% of advanced breast cancer patients develop bone metastasis, which leads to pain, fracture, spinal compression, and other skeletal-related events that affect patients’ prognosis and quality of life2. Skeletal homeostasis is maintained by balanced processes of osteolysis and osteogenesis. However, bone metastasis makes an imbalance in these processes by disturbing the RANK/RANKL/OPG pathways with downregulation of osteoprotegerin (OPG) or upregulation of receptor activator of nuclear factor κB ligand (RANKL)3. This results in more osteoclast formation and bone degradation. When osteoblast differentiation is suppressed, new osteoid production is no longer able to keep pace with bone resorption4.
Oncologists have depended on bone scans or bone surveys for the detection of bone metastasis. Unfortunately, these methods are relatively insensitive for the early detection of bone metastasis and lack enough specificity. In addition, they carry the hazards of radiation exposure5. Although improvement of specificity and sensitivity has been reported when merging PET/CT imaging in the diagnosis and confirmation of bone metastasis, these methods are less widely available and more costly6. The recent development of biochemical markers of bone turnover has generated interest for its potential use in the detection of bone metastases7. Therefore, the aim of this study was to explore the possible role of serum OPG and RANKL for the detection of bone metastasis in female breast cancer patients, its relation to the burden of bone metastasis, and other clinicopathologic parameters.
MATERIALS AND METHODS
Patients
From July 2014 to February 2015, 80 female patients with breast cancer, including 44 patients with bone metastasis (as cases) and 36 patients with no clinical evidence of bone metastasis (as control), were recruited from the outpatient clinic of the Oncology Center of Mansoura University, Mansoura, Egypt. Patients with other types of malignancy, advanced medical comorbidity, advanced organ failure, and overt metabolic bone disease, including Paget’s disease and rheumatoid arthritis, were excluded from the study. Diagnosis of metastasis was dependent on clinical examination, plain X-ray, and bone scan. Confirmation of the presence of bone metastasis was accomplished by CT scan, MRI, PET/CT, or biopsy, as appropriate. Informed consent was obtained from all participants. The study has been approved by the local ethical committee.
Measurement of Serum OPG and RANKL
Collection of fasting blood samples from patients in a monovette with no additives was performed. At room temperature, these specimens were allowed to clot for 25 min and then centrifuged at 1,500 rpm for 10 min. The serum was shifted to a polypropylene tube. The samples were frozen and maintained at −80°C until analysis could be performed. Serum OPG and RANKL were measured by the ELISA technique (Human sRANKL ELISA Kit; Boster Bio, Pleasanton, CA, USA), following the recommended protocols of the manufacturer. Serum levels of OPG and RANKL were determined from standard curves by interpolation of sample absorbance. For the RANKL assay, the minimum quantifiable limit was <10 pg/ml. The coefficient of variation in the overall intra-assay was 4.3%, and the coefficient of variation of the overall interassay was 5.4%. The upper limit of normal was defined as 5,000 pg/ml for the OPG assay; the minimum quantifiable limit was <5 pg/ml. The coefficient of variation of the overall intra-assay was 5.26%, and the coefficient of variation of the overall interassay was 7.033%. The upper limit of normal was defined as 6,000 pg/ml.
Statistical Analysis
Data were analyzed with SPSS version 21 (Chicago, IL, USA). The normality of the data was first tested with the one-sample Kolmogorov–Smirnov test. Qualitative data were described using number and percentage. The association between categorical variables was tested using the chi-square test. Continuous variables were presented as means ± standard deviation (SD) for parametric data and the median for nonparametric data. The two groups were compared with Student’s t-test (parametric data) and Mann–Whitney test (nonparametric data). Correlations between variables were determined by Kendall’s tau nonparametric correlation coefficient. A two-tailed value of p ≤ 0.05 was considered statistically significant.
RESULTS
The study was carried out on 44 female patients with breast cancer with bone metastasis (aged 31–74 years, median: 52 years) as cases. In addition, 36 female patients with breast cancer with no bone metastasis (aged 27–91 years, median: 50 years) were included as control. The baseline characteristics of the studied cases show that there was no difference between cases and control except significantly higher serum calcium levels in cases with bone metastasis (Table 1).
Table 1.
Characteristics of Breast Cancer Patients With and Without Bone Metastasis
| Items | Nonmetastatic Breast Cancer (n = 36) | Breast Cancer With Bone Metastasis (n = 44) | Test of Sig. (p Value) |
|---|---|---|---|
| Age | t = 0.534 (p = 0.595) | ||
| Median | 50 | 52 | |
| Min–Max | 27–91 | 31–74 | |
| Menopause | χ2 = 0.235 (p = 0.628) | ||
| Premenopausal | 15 (41.7%) | 16 (36.4%) | |
| Postmenopausal | 21 (58.3%) | 28 (63.6%) | |
| DM | χ2 = 2.2 (p = 0.16) | ||
| Absent | 30 (83.3%) | 29 (65.9%) | |
| Present | 6 (16.7%) | 15 (34.1%) | |
| Grading | χ2 = 0.31 (p = 0.85) | ||
| G1 | 11 (30.6%) | 11 (25%) | |
| G2 | 17 (47.2 %) | 22 (50%) | |
| G3 | 8 (22.2%) | 11 (25%) | |
| ER | χ2 = 0.832 (p = 0.362) | ||
| Positive | 26 (72.2%) | 31 (70.5%) | |
| Negative | 10 (27.8%) | 13 (29.5%) | |
| PR | χ2 = 2.069 (p = 0.150) | ||
| Positive | 22 (61.1%) | 29 (65.9%) | |
| Negative | 14 (38.9%) | 15 (34.1%) | |
| HER2 (2 cases unknown) | χ2 = 0.11 (p = 0.74) | ||
| Positive | 16 (44.4%) | 16 (38.1%) | |
| Negative | 20 (55.6%) | 26 (61.9%) | |
| Serum calcium | χ2 = 4.1 (p = 0.04) | ||
| Hypercalcemia | 2 (5.6%) | 11 (25.0%) | |
| Normocalcemia | 34 (94.4%) | 33 (75.0%) | |
| Subtype (2 cases unknown) | χ2 = 0.22 (p = 0.97) | ||
| Luminal A | 5 (13.9%) | 5 (11.9%) | |
| Luminal B | 16 (44.4%) | 19 (45.2%) | |
| Her2 type | 9 (25%) | 12 (28.6%) | |
| TNBC* | 6 (16.7%) | 6 (14.3%) | |
| Previous therapy with aromatase inhibitors | χ2 = 0.15 (p = 0.69) | ||
| Absent | 30 (83.3%) | 34 (77.3%) | |
| Present | 6 (16.7%) | 10 (22.7%) | |
| Previous therapy with tamoxifen | χ2 = 1.1 (p = 0.29) | ||
| Absent | 24 (66.7%) | 35 (79.5%) | |
| Present | 12 (33.3%) | 9 (20.5%) | |
| Previous chemotherapy | χ2 = 3.3 (p = 0.07) | ||
| Absent | 20 (44.4%) | 34 (77.3%) | |
| Present | 16 (55.6%) | 10 (22.7%) |
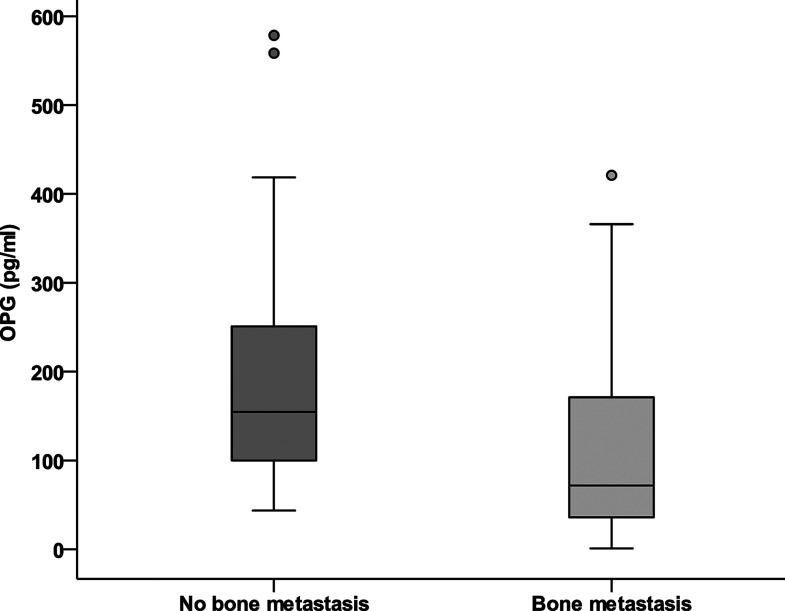
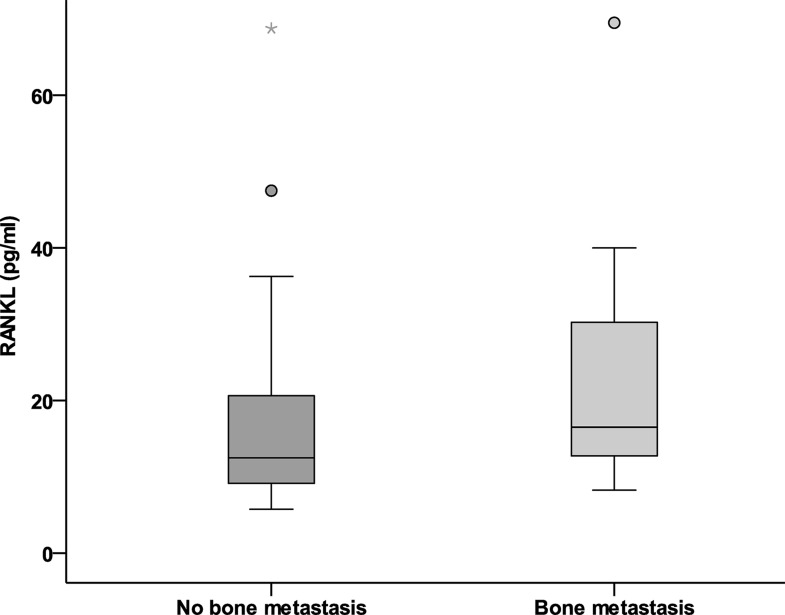
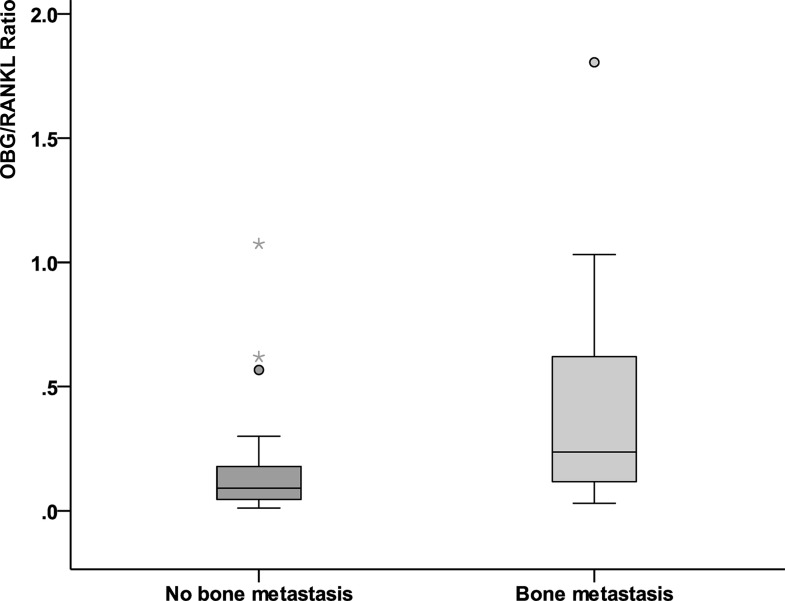
Serum-free OPG levels were significantly reduced in the presence of bone metastases (median 72 vs. 155 pg/ml; p = 0.001) (Fig. 1). Meanwhile, serum RANKL levels were significantly elevated in the presence of bone metastases (median 16.5 vs. 12.5 pg/ml; p = 0.008) (Fig. 2). The ratio (RANKL/OPG) significantly increased in the presence of bone metastasis (median 0.091 vs. 0.236 pg/ml; p = 0.003) (Fig. 3). Serum alkaline phosphatase was not significantly affected by the presence of bone metastases.
Figure 1.
Serum levels of OPG (pg/ml) in breast cancer with bone metastasis versus nonmetastatic cases.
Figure 2.
Serum levels of RANKL (pg/ml) in breast cancer with bone metastasis versus nonmetastatic cases.
Figure 3.
RANKL/OPG ratio in breast cancer with bone metastasis versus nonmetastatic cases.
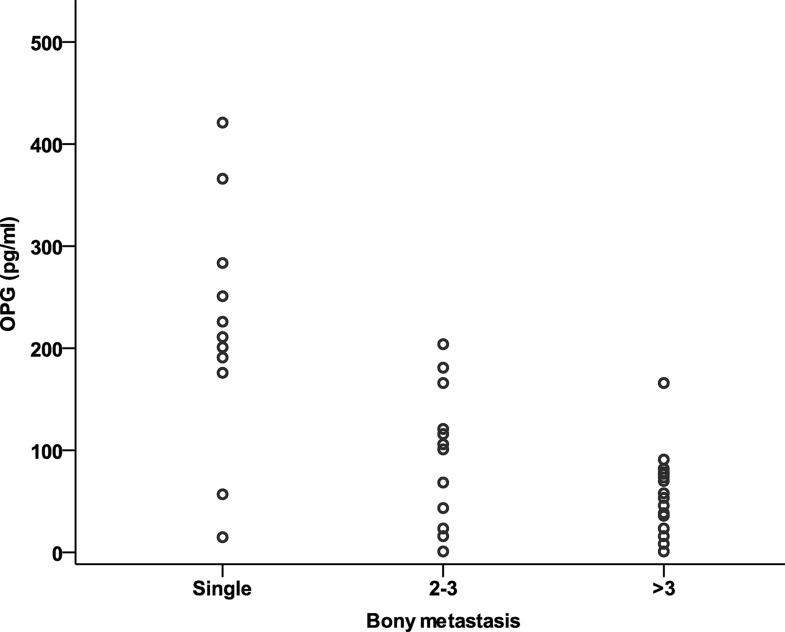
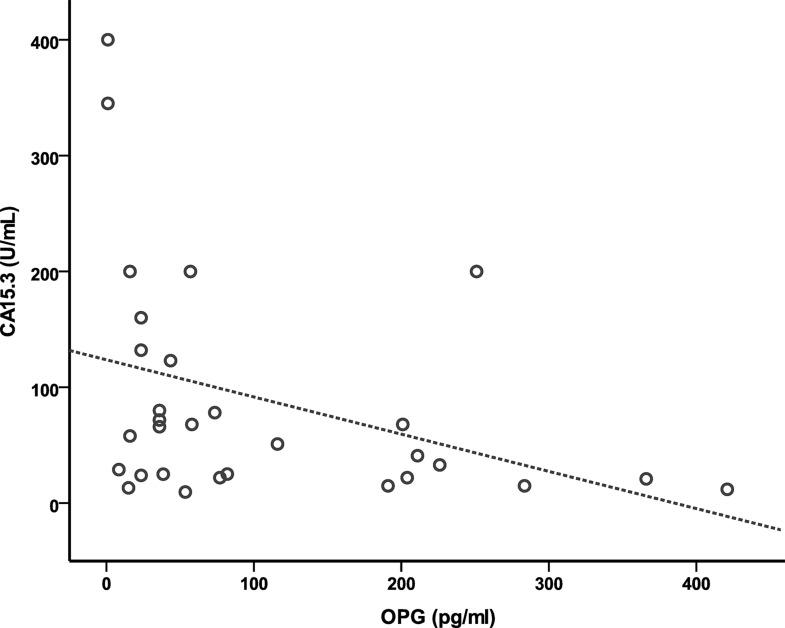
The OPG concentration (pg/ml) correlated negatively with the number of bone metastases (r = −0.65; p = 0.001) (Fig. 4) and with serum levels of CA 15-3 (Fig. 5) (r = −0.35; p = 0.006). Meanwhile, RANKL concentration has neither significant correlation to the number of bone metastases (r = 0.11; p = 0.14) nor with CA 15-3 (r = −0.19; p = 0.157).
Figure 4.
Correlation between the serum levels of OPG and the extent of bone metastases.
Figure 5.
Correlation of serum levels of OPG with serum levels of CA 15-3.
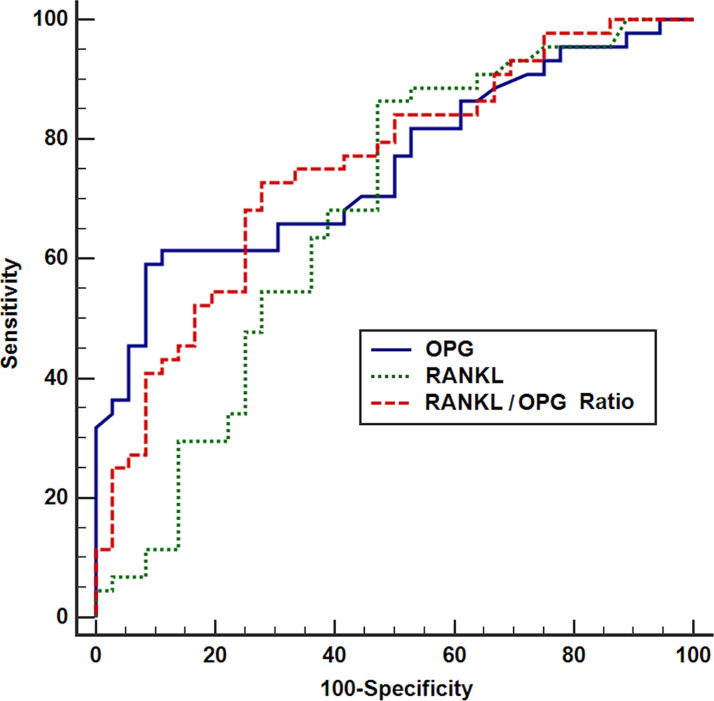
ROC curve analyses of the value of study markers in detecting bone metastasis showed that both markers were associated with a highly significant area under the curve of 0.77 and 0.72 for OPG and RANKL, respectively (p < 0.001) (Fig. 6). At a concentration of ≤82 pg/ml, OPG showed a high specificity in identifying the presence of bone metastasis (92%); however, the sensitivity was low (59%). The positive predictive value was 90%, and the negative predictive value was 65%. At a concentration of >12.5 pg/ml, RANKL showed a high sensitivity (86%) at the expense of low specificity (53%) in identifying the presence of bone metastasis; the positive predictive value was 67%, and the negative predictive value was 83%. Combining both (RANKL/OPG ratio), a cutoff ≥0.14 yielded a good balance between sensitivity and specificity of 73% and 72%, respectively, with a significant AUC of 0.7 (p = 0.001); the positive predictive value was 76.2% and the negative predictive value was 68.4%.
Figure 6.
ROC curve of OPG, RANKL, and RANKL/OPG ratio for the detection of bone metastasis in breast cancer.
The univariate relation between OPG, RANKL, and RANKL/OPG ratios with clinicopathologic parameters of metastatic breast cancer patients is shown in Table 2. Serum OPG levels were significantly increased in diabetics who were previously treated with aromatase inhibitors and in progesterone receptor-negative cases, but neither were significantly affected by the different subtypes of breast cancer, nor by other parameters. Serum RANKL levels were significantly increased in hypercalcemic patients. The RANKL/OPG ratio significantly decreased in diabetic patients but was not significantly affected by other parameters. The multivariate relation between OPG and the clinicopathologic parameters of metastatic breast cancer patients that were significant at the univariate level is shown in Table 3. Only diabetes mellitus and previous therapy with aromatase inhibitors significantly impacted the OPG levels.
Table 2.
Relation of OPG, RANKL, and RANKL/OPG Ratio With Clinicopathologic Parameters of Metastatic Female Breast Cancer Patients
| Clinicopathologic Parameters | No. | OPG (Median) | p | RANKL (Median) | p | RANKL/OPG (Median) | p |
|---|---|---|---|---|---|---|---|
| Menopausal status | 0.14 | 0.21 | 0.45 | ||||
| Pre | 16 (36.4%) | 57 | 15 | 0.43 | |||
| Post | 28 (63.6%) | 62.6 | 17.22 | 0.36 | |||
| Hypertension | 0.076 | 0.63 | 0.094 | ||||
| Present | 19 (43.2%) | 101 | 15.25 | 0.17 | |||
| Absent | 25 (56.8%) | 38.5 | 19 | 0.3793 | |||
| Diabetes mellitus | 0.006 | 0.47 | 0.005 | ||||
| Present | 15 (34.1%) | 106 | 15.25 | 0.16 | |||
| Absent | 29 (65.9%) | 44.75 | 18.37 | 0.34 | |||
| Grading | 0.884 | 0.83 | 0.94 | ||||
| G1 | 11 (25%) | 70 | 24 | 0.43 | |||
| G2 | 22 (50%) | 73.50 | 16.5 | 0.26 | |||
| G3 | 11 (25%) | 73.50 | 16.50 | 0.232 | |||
| ER | 0.124 | 0.54 | 0.19 | ||||
| Positive | 31 (70.5%) | 63.25 | 16.5 | 0.27 | |||
| Negative | 13 (29.5%) | 101.50 | 15.87 | 0.16 | |||
| PR | 0.031 | 0.81 | 0.14 | ||||
| Positive | 29 (65.9%) | 62.75 | 16.5 | 0.27 | |||
| Negative | 15 (34.1%) | 151 | 17.75 | 0.16 | |||
| HER2 (2 cases missing) | 0.890 | 0.52 | 0.49 | ||||
| Positive | 16 (38.1%) | 82 | 15.25 | 0.15 | |||
| Negative | 26 (61.9%) | 73.50 | 17.75 | 0.25 | |||
| Serum calcium | 0.53 | 0.04 | 0.17 | ||||
| Increased | 11 (25.0%) | 69 | 20.6 | 0.17 | |||
| Normal | 33 (75.0%) | 69 | 14.6 | 0.34 | |||
| Subtypes of breast cancer (2 cases unknown) | 0.18 | 0.25 | 0.23 | ||||
| Luminal A | 5 (11.9%) | 57 | 15.3 | 0.30 | |||
| Luminal B | 19 (45.2%) | 75 | 22.1 | 0.25 | |||
| Her2 | 12 (28.6%) | 183 | 12.8 | 0.10 | |||
| TNBC | 6 (14.3%) | 191 | 19 | 0.09 | |||
| Previous aromatase inhibitors | 0.019 | 0.11 | 0.12 | ||||
| Absent | 34 (77.3%) | 36 | 14 | 0.55 | |||
| Present | 10 (22.7%) | 91.50 | 18.37 | 0.20 | |||
| Previous tamoxifen | 0.24 | 0.63 | 0.24 | ||||
| Absent | 35 (79.5%) | 68.15 | 18.4 | 0.22 | |||
| Present | 9 (20.5%) | 93.45 | 14.67 | 0.20 | |||
| Previous chemotherapy | 0.17 | 0.91 | 0.35 | ||||
| Absent | 34 (77.3%) | 98.15 | 14.28 | 0.23 | |||
| Present | 10 (22.7%) | 108.24 | 16.59 | 0.19 |
Table 3.
Multivariate Regression Analysis of the Relation of OPG to Study Parameters
| Clinicopathologic Parameters | OR | 95% CI | p |
|---|---|---|---|
| Diabetes mellitus | 3.75 | 1.88–5.94 | 0.01 |
| Progesterone receptor positivity | 0.62 | 0.15–1.37 | 0.52 |
| Previous aromatase inhibitors | 2.21 | 1.43–3.62 | 0.03 |
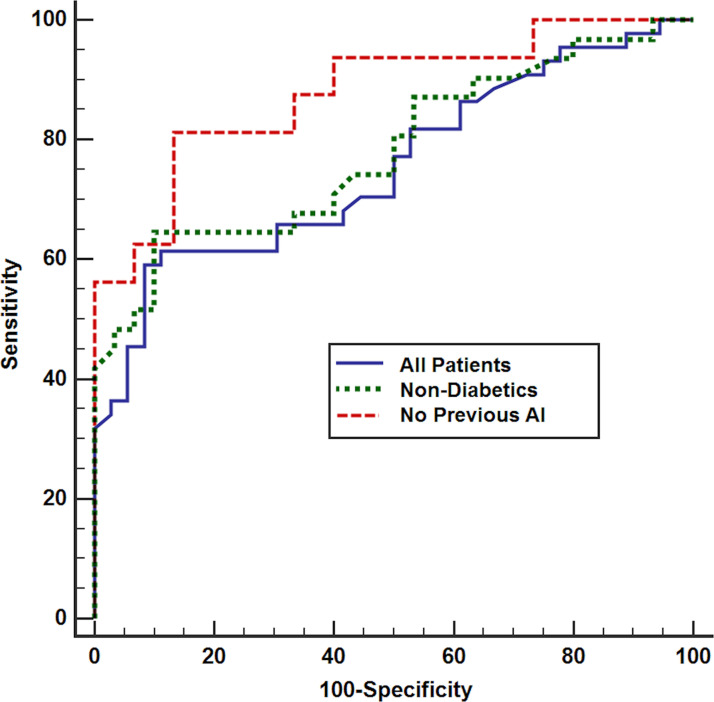
After excluding diabetic patients and those previously treated with aromatase inhibitors, reanalysis of the parameters of serum OPG level test validity found that the sensitivity for identifying bone metastasis increased to 75% and 77.8%, and the specificity was 89% and 93.3%, with positive predictive values of 85.7% and 93.3% and negative predictive values of 80% and 77.8%, respectively (Fig. 7).
Figure 7.
ROC curves of OPG for the detection of bone metastasis in all cases and after excluding diabetic patients and patients previously treated with AI.
DISCUSSION
RANKL is a homotrimeric transmembrane protein expressed by osteoblasts, osteocytes, bone marrow stromal cells, and various tumor cells (e.g., breast cancer). An increase in RANKL/RANK signaling is the leading cause of osteoporosis8. The pathogenesis of bone metastases is a complex process involving a lot of mediators between tumor cells, osteoclasts, and osteoblasts. In bone metastases, cytokines and growth factors secreted by tumor cells [parathyroid hormone-related peptide, interleukin-1 and -6, tumor necrosis factor (TNF), and macrophage colony-stimulating factor] increase the expression of RANKL on both osteoblasts and marrow stromal cells. Following this, RANKL binds to its receptor, RANK, located on the surface of osteoclast precursors and induces its maturation. This excessive RANKL-induced osteoclast activity results in bone resorption and local bone destruction, leading to the release of growth factors from the bone matrix that promotes tumor progression. This relationship constitutes the vicious cycle of bone metastasis9. For all these reasons, our patients with metastatic bone involvement show significantly higher levels of RANKL. This finding reinforces data obtained from previous studies10,11. In addition, serum RANKL levels were significantly increased in hypercalcemic patients because osteoclast differentiation is regulated by RANKL, and osteoclasts have a role in bone resorption leading to elevated serum calcium.
OPG is a secreted homodimeric glycoprotein from the TNF receptor family and lacks a transmembrane domain12. OPG neutralizes RANKL, which leads to a reduced RANK–RANKL interaction, thus inhibiting osteoclastogenesis13,14. In our study, serum levels of OPG are significantly more decreased in breast cancer patients with bone metastasis than in nonmetastatic breast cancer patients. This result is consistent with a previous study by Mercatali et al.15. However, Martinetti et al.16 reported no significant changes in OPG values in breast cancer with bone metastasis, although this may be due to the small sample size of this study. OPG levels correlated negatively with CA 15-3 levels. This may be explained by the positive correlation between CA 15-3 levels and metastasis in breast cancer17. OPG is not only produced in the bone microenvironment by osteoblasts but also produced by breast tumor cells themselves. OPG has a potential role in breast tumorigenesis via the ability of OPG to block the induction of apoptosis by blocking TNF-related apoptosis-inducing ligand3. This finding motivated us to study the relation of OPG to different molecular subtypes and grades of primary breast cancer. In addition, OPG is expressed in endothelial cells acting as an antiapototic factor by binding to the TNF-related apoptosis-inducing ligand to increase endothelial survival18. This finding prompted us to study the relation of OPG to conditions related to vascular dysfunction such as diabetes, hypertension, aging, and obesity19. The relation of OPG to other clinicopathologic parameters revealed that OPG was significantly increased in diabetic patients. Therefore, OPG elevation in diabetic patients can be attributed to the associated endothelial dysfunction20. In addition, OPG was significantly increased in patients with previous therapy with aromatase inhibitors. A similar elevation of serum OPG levels after therapy with aromatase inhibitors was reported in a recent study21. This elevation was limited to patients with bone metastasis in a previous report16. OPG revealed a high specificity (92%) and low sensitivity (59%) in detecting bone metastasis. On the other hand, RANKL showed low specificity (53%) and high sensitivity (86%). The ratio of RANKL/OPG presents appropriate specificity and sensitivity (72% and 73%, respectively), suggesting that the ratio could be a biomarker for the detection of bone metastasis. The sensitivity of serum OPG concentration in the detection of bone metastases was low, maybe due to interaction with other parameters such as diabetes mellitus and previous therapy with aromatase inhibitors. This result was enforced by increased sensitivity of the OPG serum level for the detection of bone metastases after the exclusion of diabetic patients and patients previously treated by aromatase inhibitors (Fig. 7). A negative correlation between the serum level of OPG and the extent of bone metastasis reflects the blocking action of OPG on osteoclastogenesis in a dose-dependent manner (Fig. 4). In addition, OPG appears to block the differentiation of osteoclasts22. This may depend on metastatic breast cancer cell production from a variety of factors that disturb the balance of bone formation and bone destruction. The downregulation of OPG decreases bone deposition; therefore, the stimulation of OPG action is a potential target for the prevention of and treatment for metastatic bone disease.
In conclusion, the present work provides support for the emerging concept that bone turnover markers carry considerable potential for the detection of bone metastases in patients diagnosed with breast cancer. Serum OPG levels, especially in nondiabetics and in the absence of previous AI therapy, and RANKL/OPG ratio are potential cost-effective novel biomarkers for the detection of bone metastases. Confirmation of such findings in other larger studies could open up interesting possibilities for its use as an alternative to radiographic exams or as an aid in the planning of personalized adjuvant bone-targeted therapy.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2. Clemons M, Gelmon KA, Pritchard KI, Paterson AHG. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: The state of the art. Curr Oncol. 2012;19:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weichhaus M, Chung STM, Connelly L. Osteoprotegerin in breast cancer: Beyond bone remodeling. Mol Cancer 2015;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y-C, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bilgin E, Yasasever V, Soydinc HO, Yasasever CT, Ozturk N, Duranyildiz D. Markers of bone metastases in breast and lung cancers. Asian Pac J Cancer Prev. 2012;13:4331–4. [DOI] [PubMed] [Google Scholar]
- 6. Cook GJR, Azad GK, Goh V. Imaging bone metastases in breast cancer: Staging and response assessment. J Nucl Med. 2016;57(Suppl. 1):27S–33S. [DOI] [PubMed] [Google Scholar]
- 7. Kamiya N, Suzuki H, Endo T, Yano M, Naoi M, Nishimi D, Kawamura K, Imamoto T, Ichikawa T. Clinical usefulness of bone markers in prostate cancer with bone metastasis. Int J Urol. 2012;19:968–79. [DOI] [PubMed] [Google Scholar]
- 8. Nagy V, Penninger JM. The RANKL-RANK story. Gerontology 2015;61:534–42. [DOI] [PubMed] [Google Scholar]
- 9. Mantyh P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013;154(Suppl 1):S54–62. [DOI] [PubMed] [Google Scholar]
- 10. Mountzios G, Dimopoulos M-A, Bamias A, Papadopoulos G, Kastritis E, Syrigos K, Pavlakis G, Terpos E. Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor-kappaB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol. 2007;46:221–9. [DOI] [PubMed] [Google Scholar]
- 11. Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–35. [DOI] [PubMed] [Google Scholar]
- 12. Redini F, Heymann D. Bone tumor environment as a potential therapeutic target in Ewing Sarcoma. Front Oncol. 2015;5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin TJ, Sims NA. RANKL/OPG; Critical role in bone physiology. Rev Endocr Metab Disord. 2015;16:131–9. [DOI] [PubMed] [Google Scholar]
- 14. Rachner TD, Rauner M. RANKL/OPG in breast cancer—Extending its territory to BRCA mutation carriers. Ebio Medicine 2015;2:1270–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mercatali L, Ibrahim T, Sacanna E, Flamini E, Scarpi E, Calistri D, Ricci M, Serra P, Ricci R, Zoli W, Kang Y, Amadori D. Bone metastases detection by circulating biomarkers: OPG and RANK-L. Int J Oncol. 2011;39:255–61. [DOI] [PubMed] [Google Scholar]
- 16. Martinetti A, Bajetta E, Ferrari L, Zilembo N, Seregni E, Del Vecchio M, Longarini R, La Torre I, Toffolatti L, Paleari D, Bombardieri E. Osteoprotegerin and osteopontin serum values in postmenopausal advanced breast cancer patients treated with anastrozole. Endocr Relat Cancer 2004;11:771–9. [DOI] [PubMed] [Google Scholar]
- 17. Geng B, Liang M-M, Ye X-B, Zhao W-Y. Association of CA 15-3 and CEA with clinicopathological parameters in patients with metastatic breast cancer. Mol Clin Oncol. 2015;3:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simsek S, Van Den Oever IAM, Raterman HG, Nurmohamed MT. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doupis J, Rahangdale S, Gnardellis C, Pena SE, Malhotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity 2011;19:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin JY, Shin YG, Chung CH. Elevated serum osteoprotegerin levels are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 2006;29:1664–6. [DOI] [PubMed] [Google Scholar]
- 21. Kyvernitakis I, Rachner TD, Urbschat A, Hars O, Hofbauer LC, Hadji P. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J Cancer Res Clin Oncol. 2014;140:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Xu C, Zhao H, Xia P, Song R, Gu J, Liu X, Bian J, Yuan Y, Liu Z. Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the Fas/Fas ligand pathway. PLoS One 2015;10:e0142519. [DOI] [PMC free article] [PubMed] [Google Scholar]