Abstract
Sorafenib has been globally approved as the standard treatment for patients with advanced hepatocellular carcinoma (HCC). However, the response rate of HCC patients to sorafenib is limited because of tumor recurrence and metastasis. Therefore, seeking combined therapeutic strategies with sorafenib is necessary to improve the antitumor efficiency. Here we demonstrated that expression of MMP-2 is positively correlated with the migration ability of HCC cells. Cells with a higher MMP-2 expression (SK-HEP-1 cells) were less sensitive to sorafenib than those with lower MMP-2 expression (HepG2 cells). Cotreatment of cells with SB-3CT and sorafenib more strongly inhibited migration ability than with sorafenib treatment alone in both HCC cells with high and low expression of MMP-2. In vivo cell metastasis experiments confirmed the synergistic effects of sorafenib and SB-3CT in reducing lung metastasis of SK-HEP-1 cells. Mechanistically, we showed that the synergistic antitumor effect may be attributed to inhibition of the PI3K/AKT/mTOR signaling pathway, but not the RAF/MEK/ERK signaling pathway. With these results taken together, the current study demonstrates that inhibiting MMP-2 expression can enhance the antitumor effect of sorafenib in HCC cells with a high MMP-2 expression, which may provide a novel strategy to improve therapeutic efficiency in HCC.
Key words: Hepatocellular carcinoma (HCC), Sorafenib, Matrix metalloproteinase-2 (MMP-2), SB-3CT, PI3K/AKT/mTOR pathway
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third main cause of cancer-related deaths worldwide1. Although curative therapies such as liver transplantation, surgical resection, and local ablation have improved the outcome of early stage HCC, its overall prognosis remains unsatisfactory because most patients are diagnosed at an advanced stage. Therefore, more effective treatments for advanced HCC must be developed.
Targeted therapy has provided a novel therapeutic alternative for advanced HCC2,3. Sorafenib is a multikinase inhibitor whose targets include vascular endothelial growth factor receptor, RAF kinase, platelet-derived growth factor receptor, and c-Kit4. Several large randomized phase III studies have demonstrated the survival benefits of sorafenib in patients with unresectable HCC5,6. Currently, sorafenib has been globally approved as the standard treatment for patients with advanced HCC. Despite improvement in the overall survival of advanced HCC patients, the median survival benefit when using sorafenib is less than 3 months; furthermore, some patients exhibited tumor progression during sorafenib therapy7,8. Therefore, novel combined therapeutic strategies are required to enhance the antitumor efficiency of sorafenib.
Tumor metastasis is one of the main factors that limit the efficacy of sorafenib in HCC patients9. Intravasation and extravasation of HCC cells through basement membranes are essential steps in the metastatic cascade10. Matrix metalloproteinase-2 (MMP-2), also known as gelatinase and type IV collagenase, is an important regulator of cellular activities. It degrades extracellular matrix components and breaks down basement membrane tissue boundaries, thus facilitating cell migration11,12. High levels of MMP-2 have been correlated with enhanced metastasis and poor prognosis in HCC patients13,14. SB-3CT, a covalent mechanism-based MMP inhibitor that has high selectivity for MMP-2/9, holds the promise of effective intervention in the metastasis of various tumors, including breast cancer, prostate cancer, and lymphoma15–17. However, the effectiveness of MMP-2 inhibition by SB-3CT in suppressing HCC cell invasion and the underlying mechanisms remains to be clarified.
The current study was conducted to demonstrate the effects of inhibiting MMP-2 expression on HCC cell activity and to clarify whether inhibiting MMP-2 expression could enhance the antitumor efficacy of sorafenib. We found that inhibiting MMP-2 using SB-3CT combined with sorafenib might be a more effective therapy for advanced HCC. This synergistic antitumor effect may be attributed to inhibition of the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway, which is important for regulating HCC metastasis.
MATERIALS AND METHODS
Cell Lines and Cultures
Six HCC cell lines (Huh-7, Hep-3B, HepG2, PLC/PRF/5, SK-HEP-1, and SMMC-7721) and a normal hepatic cell line (LO2) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing, Zhejiang, P.R. China), 100 U/ml penicillin, and 100 U/ml streptomycin. Huh-7, Hep-3B, and PLC/PRF/5 cell lines were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, P.R. China). The other cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). All cells were maintained at 37°C in an atmosphere containing 5% CO2.
Reagents and Antibodies
Sorafenib (Nexavar®) and SB-3CT (MMP-2 inhibitor) were purchased from Selleck Chemicals (Houston, TX, USA). LY294002 (PI3K inhibitor), MK-2206 (AKT inhibitor), rapamycin (mTOR inhibitor), RG7204 (RAF inhibitor), U0126 (MEK inhibitor), and PD98059 (ERK inhibitor) were obtained from Selleck Chemicals. For in vitro experiments, these reagents were dissolved in dimethyl sulfoxide (DMSO) for long-term storage and then diluted to the working concentration with DMEM. The final DMSO concentration was less than 0.1%. For in vivo experiments, SB-3CT was dissolved in 10% DMSO, and sorafenib was dissolved in a mixture of Cremophor EL/ethanol/ddH2O (1:1:6). Primary antibodies for p/t-PI3K, p/t-AKT, p/t-mTOR, p/t-RAF, p/t-MEK, and p/t-ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-MMP-2 antibody was purchased from Abcam (Cambridge, UK). The antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and horseradish peroxidase (HRP)-labeled anti-rabbit secondary antibodies were obtained from CWBIO (Beijing, P.R. China).
Cell Viability
Cell viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega, Madison, WI, USA) in accordance with the manufacturer’s instructions. HepG2 and SK-HEP-1 cells were seeded into 96-well plates at a density of 2 × 103 cells/well and treated with serum-free medium containing various concentrations of sorafenib for 48 h. Next the wells were washed twice with phosphate-buffered saline (PBS), and 100 μl of DMEM containing 20 μl of MTS reagent was added into each well. The absorbance was measured at 490 nm, after 2 h of incubation at 37°C, using an automated enzyme-linked immunosorbent assay (ELISA) plate reader. The absorbance value obtained with cell lines without sorafenib treatment (control) was normalized as 100%.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Takara, Kusatsu, Japan). Reverse transcription was performed using a PrimeScript RT reagent kit (Takara) according to the manufacturer’s instructions. Quantitative real-time PCR to evaluate MMP-2 expression in different cell lines was performed using the following primers: MMP-2, 5′-GATACCCCTTTGACGGTAAGGA-3′ (forward) and 5′-CCTTCTCCCAAGGTCCATAGC-3′ (reverse); GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-TGGTGAAGACGCCAGTGGA-3′ (reverse). Relative gene expression levels were calculated using the comparative Ct (ΔΔCt) method, where the relative expression was calculated as 2−ΔΔCt, with Ct representing the threshold cycle.
Western Blot
Total protein was extracted from cells lysed with radioimmunoprecipitation lysis buffer (CWBIO) containing a protease inhibitor cocktail (Boehringer Mannheim, Lewes, UK) for 30 min at 4°C. After centrifugation at 14,000 × g for 20 min, protein concentration of the supernatant was measured using bicinchoninic acid assay (Beyotime, Shanghai, P.R. China) and equalized before loading. A total of 30 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking in Tris-buffered saline/Tween 20 (TBST) containing 5% nonfat dry milk for 1 h, membranes were washed with TBST three times. Immunoblot analysis was carried out using the appropriate antibodies. Finally, the bands were visualized using an enhanced chemiluminescence detection kit (Millipore).
Gelatin Zymography
SK-HEP-1 cells were treated with 0, 2, 4, and 8 μmol/L of SB-3CT for 48 h, and the supernatant was collected and centrifuged. Thirty micrograms of protein was then added to 8% SDS-PAGE for electrophoresis, and the gel was washed twice (30 min each) in 200 ml of 2.5% Triton X-100 and incubated in a 50-mM Tris-HCl containing 5 mM CaCl2, 0.2 M NaCl, and 0.02% Brij-35 for 18 h at 37°C. The gel was then stained with Commassie brilliant blue R for 1 h and destained in 30% methanol/10% acetic acid four times for 5, 15, 30, and 60 min. Quantification of bands was analyzed with ImageJ 1.48 software.
Invasion Assays
HepG2 or SK-HEP-1 cells were seeded into the upper compartment of a Transwell chamber (Corning Inc., Corning, NY, USA) at a density of 4 × 104 cells/well. The cells were cultured in 200 μl of serum-free DMEM containing sorafenib, SB-3CT, or a combination of the two drugs. The lower compartment was filled with 600 μl of DMEM containing 20% FBS. After 24 h of incubation at 37°C in 5% CO2, cells in the upper compartment were removed. Migrated cells in the lower compartment were fixed in 4% formalin, stained with crystal violet, and counted. For the wound healing assay, cells were seeded onto six-well plates and incubated in DMEM containing 10% FBS until they reached confluence. Scratches were introduced to the cell monolayer using a plastic pipette tip. After washing with PBS, serum-free medium (to inhibit cell proliferation) containing different drugs was added. The scratched area was photographed using a light microscope every 24 h for 2 days.
In Vivo Metastasic Analysis
Female nude mice 4–5 weeks old were purchased from the Sun Yat-Sen University Laboratory Animal Center (Guangzhou, P.R. China). Animal experiments were approved by the Animal Research Committee of Sun Yat-Sen University, and all procedures were performed according to the NIH Guide for Care and Use of Laboratory Animals. To evaluate the antimetastatic activities of SB-3CT plus sorafenib in vivo, SK-HEP-1 cells (1 × 106/0.2 ml) were injected into the tail vein of mice to simulate tumor metastasis. The mice were randomized into four groups (n = 8/group) and were treated with (a) vehicle, (b) sorafenib (30 mg/kg/day, PO), (c) SB-3CT (50 mg/kg/day, IP)15,17, or (d) SB-3CT plus sorafenib for 8 weeks. The mice were then sacrificed, and the lungs were excised and sampled for tissue sectioning. To quantify the metastases, 100 sequential sections of the lungs were prepared for each mouse, and every 10th section was stained with hematoxylin and eosin (H&E)3. The total number of foci in all 10 sections, as observed using a microscope, represented the degree of pulmonary metastasis.
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD). Comparisons of the mean values were performed with SPSS 20.0 software using a two-tailed Student’s t-test or one-way analysis of variance (ANOVA) with Bonferroni’s corrections for multiple comparisons. Statistical significance was concluded with values of p < 0.05 and p < 0.01.
RESULTS
Expression of MMP-2 Correlated With the Malignancy of HCC Cell Lines
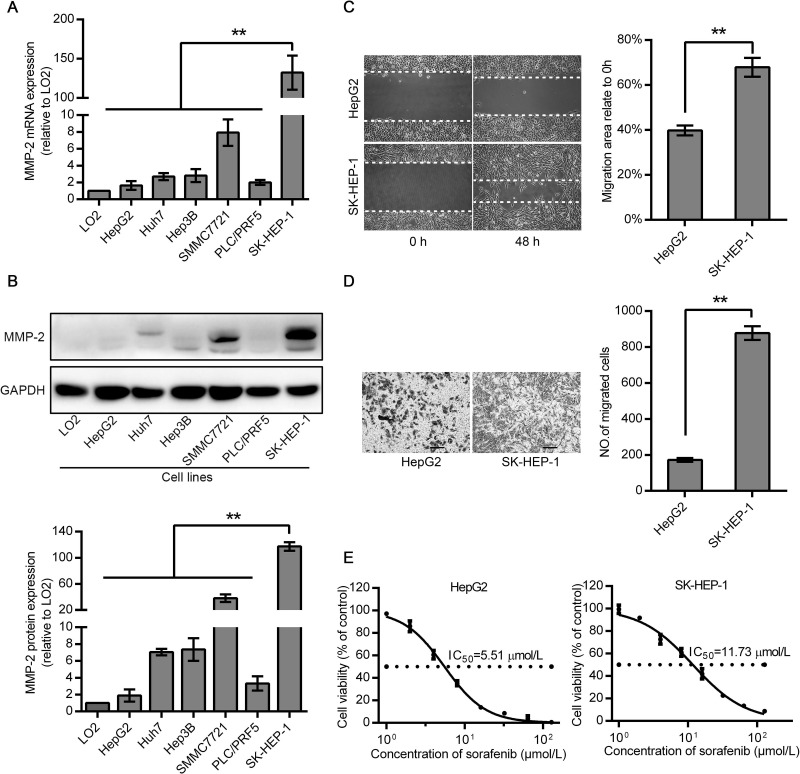
mRNA and protein expression levels of MMP-2 were determined in six HCC cell lines and a normal hepatic cell line using qRT-PCR and Western blot. The MMP-2 mRNA and protein levels were lowest in HepG2 cells and highest in SK-HEP-1 cells (Fig. 1A and B). Thus, we chose SK-HEP-1 and HepG2 cells for the subsequent experiments. The effect of sorafenib on the viability of HepG2 and SK-HEP-1 cells is shown in Figure 1E. SK-HEP-1 cells (IC50 = 11.73 μM), which expressed higher levels of MMP-2, were more resistant to sorafenib than HepG2 cells (IC50 = 5.51 μM). We then examined the migration ability with a wound healing assay in both cells. Consistent with the results of cell viability, SK-HEP-1 cells exhibited a higher motility than HepG2 cells. The migration area of SK-HEP-1 cells was about 1.7-fold greater than that of HepG2 cells after 48 h. Similar results were noted in the Transwell assay, where the number of migrated SK-HEP-1 cells was about 5.1-fold greater than that of HepG2 cells (Fig. 1C and D). Therefore, these results showed that the migration ability and sorafenib resistance of HCC cells were positively correlated with their MMP-2 expression level.
Figure 1.
Hepatocellular carcinoma (HCC) cells with a higher matrix metalloproteinase-2 (MMP-2) expression level exhibited greater viability and migration ability. (A, B) Expression levels of MMP-2 were determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR) or Western blot analysis in human HCC cell lines and a normal LO2 hepatic cell line. (C, D) Cell motility was measured by wound healing and Transwell migration assays. (E) Dose-dependent effects of sorafenib on the viability of HepG2 and SK-HEP-1 cells. Data are expressed as a percentage of control cells and represent the mean ± standard deviation (SD) of three separate experiments, each performed in triplicate. The IC50 value was calculated by nonlinear regression analysis. Scale bars: 100 μm. **p < 0.01.
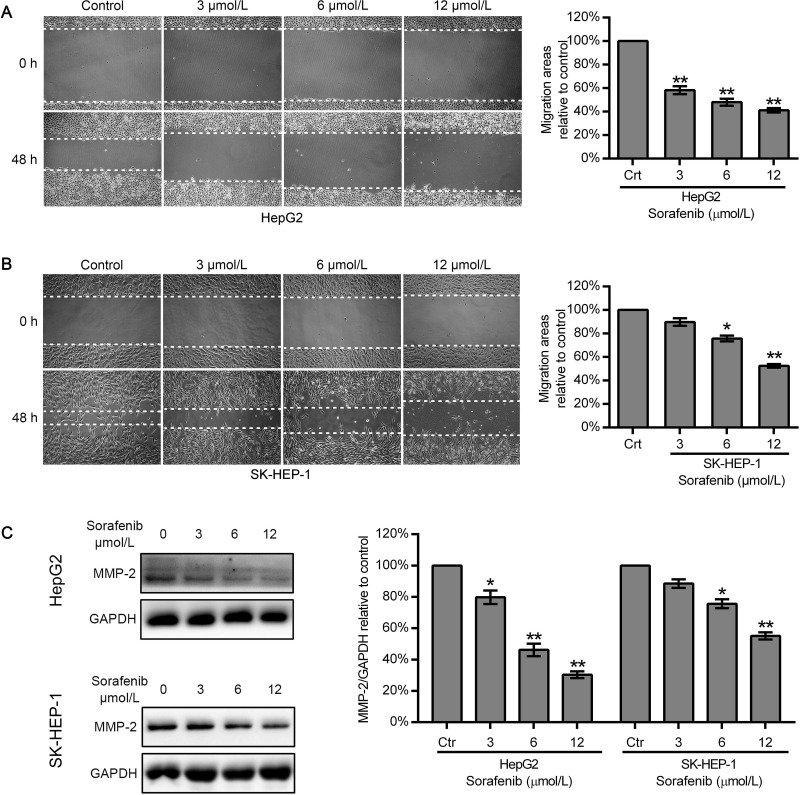
Sorafenib Downregulated the Expression of MMP-2 and Reduced the Motility of HepG2 Cells, But Had a Limited Effect on SK-HEP-1 Cells
To evaluate the effect of sorafenib on HCC cell migration, a wound healing assay was carried out with HepG2 and SK-HEP-1 cells. The cells were scratched and cultured in serum-free media for 48 h. Our results showed that sorafenib at 3, 6, and 12 μmol/L decreased the migration area of HepG2 cells to 58.26 ± 3.38%, 47.97 ± 3.03%, and 41.07 ± 1.82%, respectively, compared to that of the untreated HepG2 cells (Fig. 2A). However, for SK-HEP-1 cells, 3 and 6 μmol/L sorafenib only reduced the migration area to 89.73 ± 3.26% and 75.74 ± 2.51%, respectively, of that of control cells; even a high sorafenib concentration (12 μmol/L) could only decrease the migration area to 52.45 ± 1.60% (Fig. 2B). Western blot analysis demonstrated that sorafenib decreased MMP-2 expression in a dose-dependent manner in HepG2 cells but had a limited effect in SK-HEP-1 cells. Sorafenib at a low concentration (3 μmol/L) effectively suppressed MMP-2 expression in HepG2 cells. However, MMP-2 expression was not markedly affected even at a high dose (12 μmol/L) of sorafenib in SK-HEP-1 cells (Fig. 2C).
Figure 2.
Effect of sorafenib on the motility and MMP-2 expression of HepG2 and SK-HEP-1 cells. (A, B) Wound healing assay to analyze the migration of HepG2 and SK-HEP-1 cells after treatment with different concentrations of sorafenib (0, 3, 6, and 12 μmol/L). (C) Cells were exposed to 0, 3, 6, and 12 μmol/L sorafenib for 48 h, protein expression of MMP-2 was examined by Western blot. Data represent the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 compared with the control group.
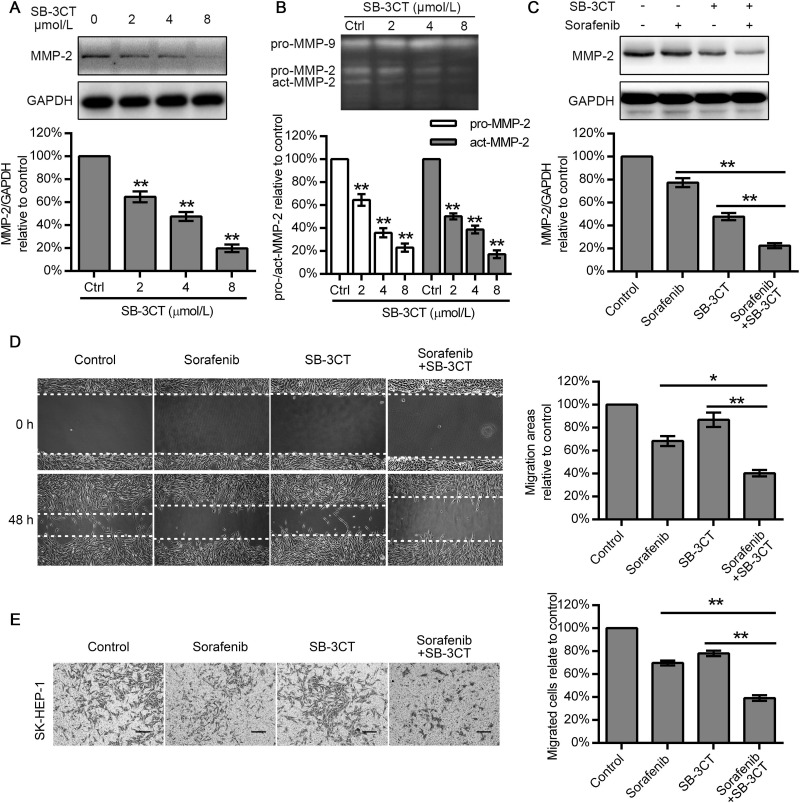
SB-3CT Synergized With Sorafenib to Inhibit MMP-2-Mediated Migration of HCC Cells
Considering that MMP-2 expression is positively correlated with the migration ability of cells and sorafenib treatment was ineffective against HCC cells with MMP-2 high expression (SK-HEP-1 cells), we explored whether combining an MMP-2 inhibitor with sorafenib might result in a cooperative effect in suppressing SK-HEP-1 cell migration. First, we treated SK-HEP-1 cells with 2, 4, and 8 μmol/L of SB-3CT for 48 h. A gelatin zymography assay demonstrated that SB-3CT treatment attenuated both the pro-MMP-2 expression and the level of active MMP-2 in a dose-dependent manner, but we did not observe any variation in the levels of the preform of MMP-9 (Fig. 3B). Western blot results showed that SB-3CT reduced the MMP-2 expression to 64.70 ± 4.72%, 47.60 ± 3.89%, and 19.82 ± 3.30%, respectively (Fig. 3A). We then treated SK-HEP-1 cells with 6 μmol/L sorafenib alone, 4 μmol/L SB-3CT alone18, and a combination of both agents. Western blot analysis revealed that sorafenib suppressed MMP-2 production to 64.41 ± 2.69%, compared with that in untreated SK-HEP-1 cells, while SB-3CT (4 μmol/L) attenuated MMP-2 expression to 47.75 ± 3.14%, which was superior to the effect of 6 μmol/L of sorafenib. In addition, combined SB-3CT and sorafenib therapy markedly decreased MMP-2 expression to 22.39 ± 2.19%, relative to the control values (Fig. 3C). A wound healing assay showed that sorafenib and SB-3CT alone moderately inhibited cell migration (68.32 ± 4.31% and 86.87 ± 6.35%). However, when the two drugs were combined, the resulting decrease in cell migration (40.33 ± 2.83%) was greater than that observed with either drug alone (Fig. 3D). Similarly, the Transwell assay showed that sorafenib reduced the number of migrated cells to 69.68 ± 2.15%, and SB-3CT decreased that number to 78.12 ± 2.38%, while cotreatment with both drugs exhibited a reduction to 39.10 ± 2.43% (Fig. 3E). Interestingly, we found that, for suppressing MMP-2, SB-3CT (4 μmol/L) was superior to sorafenib (6 μmol/L), but the ability exhibited by the wound healing and Transwell assays was just the opposite.
Figure 3.
SB-3CT and sorafenib synergistically inhibited HCC cell migration and MMP-2 expression. (A) SK-HEP-1 cells were exposed to 0, 2, 4, and 8 μmol/L SB-3CT for 48 h, and protein expression of MMP-2 was examined by Western blot. (B) Gelatin zymography assay was conducted to examine the effects of SB-3CT on the preform and active form of MMP-2/9 in SK-HEP-1 cells. (C) Sorafenib and SB-3CT synergistically inhibited MMP-2 expression of SK-HEP-1 cells. (D, E) Effect of sorafenib, SB-3CT, and combined sorafenib with SB-3CT therapy in wound healing and Transwell assays using SK-HEP-1 cells. Data represent the mean ± SD of three independent experiments. Scale bars: 100 μm. *p < 0.05, **p < 0.01.
Cotreatment of SB-3CT and Sorafenib Therapy Markedly Enhanced Suppression of the PI3K/AKT/mTOR Signaling Pathway, But Not the RAF/MEK/ERK Signaling Pathway
PI3K/AKT/mTOR is highly activated in HCC and plays an important role in cell migration and invasion. Since sorafenib is a RAF/MEK inhibitor, we treated SK-HEP-1 cells with 6 μmol/L sorafenib, 4 μmol/L SB-3CT, and their combination, and determined the expression level of the PI3K/AKT/mTOR and RAF/MEK/ERK pathways. Cells were also treated with their inhibitors as positive controls. Sorafenib obviously inhibited the expression of p-RAF, p-MEK, and p-ERK without obvious changes in the total RAF, MEK, and ERK expression, but it had a limited effect in suppressing the expression of p-PI3K, p-AKT, and p-mTOR. Our results further suggested that SB-3CT inhibited the expression of p-PI3K, p-AKT, and p-mTOR to a greater degree than the expression of p-RAF, p-MEK, and p-ERK. The combination of sorafenib and SB-3CT produced a stronger inhibitive effect in reducing the expression of p-PI3K, p-AKT, and p-mTOR than either drug alone. However, for the expression of p-RAF, p-MEK, and p-ERK, combined therapy did not show a stronger inhibition than sorafenib treatment alone. Thus, our findings showed that SB-3CT combined with sorafenib effectively suppressed the PI3K/AKT/mTOR signaling pathway, but not the RAF/MEK/ERK signaling pathway (Fig. 4).
Figure 4.
Sorafenib and SB-3CT combined therapy suppressed the PI3K/AKT/mTOR pathway but not the RAF/MEK/ERK pathway in SK-HEP-1 cells. (A–F) SK-HEP-1 cells were cultured in six-well plates and treated with 6 μmol/L sorafenib, 4 μmol/L SB-3CT, and a combination of sorafenib and SB-3CT for 48 h. Cells were incubated with PI3K inhibitor LY294002, AKT inhibitor MK-2206, mTOR inhibitor rapamycin, RAF inhibitor RG7204, MEK inhibitor U0126, and ERK inhibitor PD98059 as positive control. Protein expression was determined by Western blot. Gray value analysis was performed for every band, and data were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The values represent mean ± SD of three independent experiments. *p < 0.05, **p < 0.01.
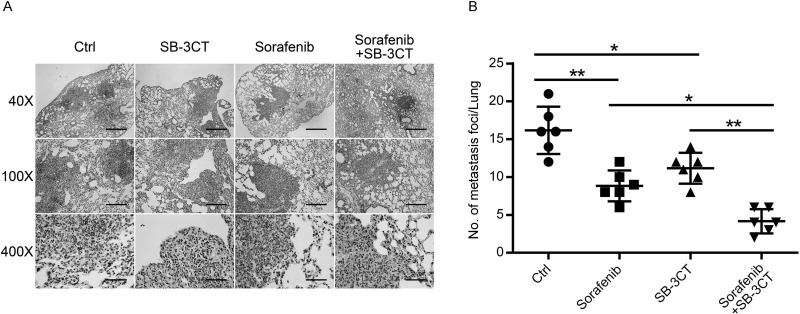
Effect of SB-3CT Combined With Sorafenib on In Vivo Antimetastatic Activity
To examine the therapeutic efficacy of SB-3CT plus sorafenib against metastasis in vivo, we established a lung metastasis model in nude mice. After 2 months of treatment, the average number of foci per mouse in the sorafenib and SB-3CT monotherapy groups was reduced to 54.64% and 69.07%, respectively, compared with that in the control group. On the other hand, cotreatment with sorafenib/SB-3CT significantly decreased lung metastasis nodules to 25.77% compared to that of the untreated group (Fig. 5).
Figure 5.
SB-3CT and sorafenib synergistically inhibited lung metastasis in vivo. (A) Representative hematoxylin and eosin (H&E) staining to assess pulmonary metastasis at 8 weeks. Scale bars: 1 mm (40×), 400 μm (100×), 100 μm (400×). (B) The average number of foci per mouse was calculated and presented as mean ± SD, n = 6. *p < 0.05, **p < 0.01.
DISCUSSION
Although sorafenib has been proven effective in the treatment of advanced HCC, the response rate of HCC patients to sorafenib is limited. This prompted the search for agents that can be combined with sorafenib to improve the treatment outcome. Previous studies have suggested that vitamin K, metformin, and diosmetin may enhance the efficacy of sorafenib against HCC19–21. However, as there exists significant tumor heterogenicity in HCC22, sensitivity to sorafenib varies in different HCC tissues and cells23. Because only a few studies have investigated the effect of combining sorafenib with other agents against HCC cell lines, the current study compared the synergistic antitumor effect of MMP-2 inhibition and sorafenib therapy in HCC cells with different MMP-2 expression levels. We demonstrated that inhibition of MMP-2 expression combined with sorafenib therapy might be a more effective therapeutic strategy for advanced HCC characterized by high MMP-2 expression.
MMP-2 plays a critical role in cell migration during cancer invasion by degrading extracellular matrix proteins. It is commonly upregulated in several types of cancer, including HCC24–27, and its inhibition was reported to suppress the invasion and metastasis of HCC both in vitro and in vivo21,28. We first examined the mRNA and protein expression of MMP-2 in six different HCC cell lines. Our results revealed that HCC cells with a higher expression of MMP-2 exhibited higher viability and migration ability. This is consistent with previous reports showing that HCC patients with a high level of MMP-2 are more susceptible to metastasis27. The effect of sorafenib on MMP-2 expression has also been reported previously. Ha et al.29 showed that sorafenib reduced hepatocyte growth factor-induced MMP-2 activity in HCC. Furthermore, Wei et al.30 reported that sorafenib (2–6 μmol/L) dose dependently decreased the expression of MMP-2 in HepG2 and Huh-7 cells. However, few studies have focused on the differential antitumor effect of sorafenib in HCC cells with different MMP-2 expression levels. Our results showed that 6 μmol/L sorafenib significantly reduced MMP-2 expression in HepG2 cells with a low expression of MMP-2, but was only slightly affected in SK-HEP-1 cells with a high MMP-2 expression. Even a high concentration of sorafenib (12 μmol/L) could only decrease the MMP-2 expression to 55.10% in SK-HE-1 cells. Similarly, 6 μmol/L sorafenib treatment more effectively reduced the migration of HepG2 cells compared with that of SK-HEP-1 cells. Furthermore, the cell migration rate was suppressed to only 52.45% in SK-HEP-1 cells treated with 12 μmol/L of sorafenib. These findings suggested that HCC cells with a high expression of MMP-2 are less sensitive to sorafenib treatment and prompted us to investigate the effect of inhibiting MMP-2 expression on the efficacy of sorafenib against HCC cells with high levels of MMP-2.
Gelatin zymography and Western blot assays revealed that treatment with SB-3CT, a competitive, mechanism-based inhibitor specific for MMP-2 and MMP-917, led to a significant decrease in both the MMP-2 expression and the level of active MMP-2 in SK-HEP-1 cells. This was consistent with previous findings that SB-3CT decreased the expression of MMP-2 and MMP-9 in vascular smooth muscle cells23. Other studies also indicated that SB-3CT significantly suppressed MMP-9 expression in ischemic brain tissues31,32. However, our results showed that SB-3CT had no obvious effect on the production of pro-MMP-9. This might be because active MMP-9 expresses at a low level in SK-HEP-1 cells (Fig. 3B). Moreover, although the inhibitory effect of SB-3CT on MMP-2 expression was superior to that of sorafenib, the inhibition of cell migration with sorafenib was stronger than that of SB-3CT in SK-HEP-1 cells with a high expression level of MMP-2. This observation suggests that sorafenib suppresses HCC cell migration mainly by inhibiting multikinases but not MMP-2. More importantly, our results showed that combined treatment with SB-3CT and sorafenib exerted a more significant antimigration action than either sorafenib or SB-3CT alone. The in vivo metastasis experiments further suggested that inhibition of MMP-2 expression by SB-3CT markedly enhanced the efficacy of sorafenib in reducing lung metastasis of SK-HEP-1 cells, compared with sorafenib treatment alone.
As a potent inhibitor of RAF kinase, sorafenib could markedly block the MAPK pathway33. However, cotreatment with sorafenib and SB-3CT showed no advantages in blocking the MAPK pathway. The results demonstrated that SB-3CT inhibited the expression and activity of MMP-2 and thus enhanced the antitumor effects of sorafenib via an MAPK-independent mechanism. The PI3K/AKT/mTOR signaling pathway plays an important role in the signal transduction activities associated with cell proliferation, apoptosis, and metastasis in multiple human malignancies34,35. In HCC tissues and cells, previous studies have demonstrated that inhibiting the PI3K/AKT/mTOR signaling pathway promoted antitumor effects34,36. The current study showed that sorafenib barely had an effect on the PI3K/AKT/mTOR pathway, while SB-3CT reduced the phosphorylation of PI3K, AKT, and mTOR in HCC cells. The combined treatment was even more effective in reducing the phosphorylation level of PI3K, AKT, and mTOR than their inhibitors. At the same time, previous studies demonstrated that downregulating MMP-2 expression by siRNA markedly suppressed the PI3K/AKT signaling pathway in A549 lung cancer cells and endothelial cells via αVβ3 integrin37,38. Thus, our results suggest the possible mechanism that SB-3CT inhibited MMP-2 expression and thus suppressed the PI3K/AKT/mTOR pathway and enhanced the antitumor effect of sorafenib. However, further studies are needed to clarify the exact molecular mechanism of such regulation in the inhibition of HCC metastasis.
Given all these results, the current study shows that, compared with HCC cells with a low expression of MMP-2, HCC cells with a high expression of MMP-2 are less sensitive to sorafenib. Inhibiting MMP-2 expression using SB-3CT enhanced the antitumor effect of sorafenib in HCC cells with both high and low expressions of MMP-2. This synergistic antitumor effect may be attributed to inhibition of the PI3K/AKT/mTOR signaling pathway. As the sensitivity to sorafenib varies in different HCC patients because of tumor heterogenicity, our study therefore provides a novel alternative strategy for individual therapy of HCC based on MMP-2 expression level.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81572398 and 81672419), the Science and Technology Planning Project of Guangdong Province (Nos. 2015A050502023 and 2016A020216010), the Natural Science Foundation of Guangdong Province (Nos. 2014A030313061 and 2013B021800101), and the Young Teacher Foundation of Sun Yat-Sen University (No. 14ykpy21). Yajin Chen and Changzhen Shang designed the experiments; Wenliang Tan and Sicong Zhu performed the experiments and wrote the manuscript; Jun Cao and Lei Zhang revised the manuscript; Wenda Li and Kairui Liu analyzed and interpreted the data; and Jinyi Zhong made the pictures and graphs. All the authors have read and approved the manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Peng S, Wang Y, Peng H, Chen D, Shen S, Peng B, Chen M, Lencioni R, Kuang M. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology 2014;60(4):1264–77. [DOI] [PubMed] [Google Scholar]
- 3. Xiang Q, Chen W, Ren M, Wang J, Zhang H, Deng DY, Zhang L, Shang C, Chen Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20(11):2959–70. [DOI] [PubMed] [Google Scholar]
- 4. Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66(24):11851–8. [DOI] [PubMed] [Google Scholar]
- 5. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 6. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 7. Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: Emergence of resistance to sorafenib. Clin Cancer Res. 2012;18(7):1824–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanoff HK, Chang Y, Lund JL, O’Neil BH, Dusetzina SB. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist 2016;21(9):1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baffy G. Decoding multifocal hepatocellular carcinoma: An opportune pursuit. Hepatobiliary Surg Nutr. 2015;4(3):206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tauro M, McGuire J, Lynch CC. New approaches to selectively target cancer-associated matrix metalloproteinase activity. Cancer Metastasis Rev. 2014;33(4):1043–57. [DOI] [PubMed] [Google Scholar]
- 11. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002;295(5564):2387–92. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Chen X, Lu H. Knockdown of SLC34A2 inhibits hepatocellular carcinoma cell proliferation and invasion. Oncol Res. 2016;24(6):511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniele A, Divella R, Quaranta M, Mattioli V, Casamassima P, Paradiso A, Garrisi VM, Gadaleta CD, Gadaleta-Caldarola G, Savino E, Maci R, Bellizzi A, Fazio V. Clinical and prognostic role of circulating MMP-2 and its inhibitor TIMP-2 in HCC patients prior to and after trans-hepatic arterial chemo-embolization. Clin Biochem. 2014;47(3):184–90. [DOI] [PubMed] [Google Scholar]
- 14. Ebata M, Fukuda Y, Nakano I, Katano Y, Fujimoto N, Hayakawa T. Serum levels of tissue inhibitor of metalloproteinases-2 and of precursor form of matrix metalloproteinase-2 in patients with liver disease. Liver 1997;17(6):293–9. [DOI] [PubMed] [Google Scholar]
- 15. Bonfil RD, Sabbota A, Nabha S, Bernardo MM, Dong Z, Meng H, Yamamoto H, Chinni SR, Lim IT, Chang M, Filetti LC, Mobashery S, Cher ML, Fridman R. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer 2006;118(11):2721–6. [DOI] [PubMed] [Google Scholar]
- 16. Ke Z, Lin H, Fan Z, Cai TQ, Kaplan RA, Ma C, Bower KA, Shi X, Luo J. MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int J Cancer 2006;119(1):8–16. [DOI] [PubMed] [Google Scholar]
- 17. Kruger A, Arlt MJ, Gerg M, Kopitz C, Bernardo MM, Chang M, Mobashery S, Fridman R. Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res. 2005;65(9):3523–6. [DOI] [PubMed] [Google Scholar]
- 18. Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J. Ethanol-induced in vitro invasion of breast cancer cells: The contribution of MMP-2 by fibroblasts. Int J Cancer 2004;112(5):738–46. [DOI] [PubMed] [Google Scholar]
- 19. Ha TY, Hwang S, Hong HN, Choi YI, Yoon SY, Won YJ, Song GW, Kim N, Tak E, Ryoo BY. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res. 2015;35(4):1985–95. [PubMed] [Google Scholar]
- 20. Hsieh SC, Tsai JP, Yang SF, Tang MJ, Hsieh YH. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the ERK/JNK-mediated NF-kappaB-dependent pathway that reduces uPA and MMP-9 expression. Amino Acids 2014;46(12):2809–22. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Wen X, Liu B, Zhang Q, Zhang J, Miao H, Zhu R. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13(3):2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu L, Kim Y, Spolverato G, Gani F, Pawlik TM. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr. 2016;5(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao YG, Meng FX, Li BW, Sheng YM, Liu MM, Wang B, Li HW, Xiu RJ. Gelatinases promote calcification of vascular smooth muscle cells by up-regulating bone morphogenetic protein-2. Biochem Biophys Res Commun. 2016;470(2):287–93. [DOI] [PubMed] [Google Scholar]
- 24. Peng H, Liu L, Zhao X. Prognostic significance of matrix metalloproteinase-2 in gynecological cancer: A systemic review of the literature and meta-analysis. J BUON 2013;18(1):202–10. [PubMed] [Google Scholar]
- 25. Qian Q, Wang Q, Zhan P, Peng L, Wei SZ, Shi Y, Song Y. The role of matrix metalloproteinase 2 on the survival of patients with non-small cell lung cancer: A systematic review with meta-analysis. Cancer Invest. 2010;28(6):661–9. [DOI] [PubMed] [Google Scholar]
- 26. Wang HL, Zhou PY, Zhang Y, Liu P. Relationships between abnormal MMP2 expression and prognosis in gastric cancer: A meta-analysis of cohort studies. Cancer Biother Radiopharm. 2014;29(4):166–72. [DOI] [PubMed] [Google Scholar]
- 27. Wang B, Ding YM, Fan P, Wang B, Xu JH, Wang WX. Expression and significance of MMP2 and HIF-1alpha in hepatocellular carcinoma. Oncol Lett. 2014;8(2):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, Duan HB, Yang YS. Knockdown of Rap2B inhibits the proliferation and invasion in hepatocellular carcinoma cells. Oncol Res. 2017;25(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ha TY, Hwang S, Moon KM, Won YJ, Song GW, Kim N, Tak E, Ryoo BY, Hong HN. Sorafenib inhibits migration and invasion of hepatocellular carcinoma cells through suppression of matrix metalloproteinase expression. Anticancer Res. 2015;35(4):1967–76. [PubMed] [Google Scholar]
- 30. Wei JC, Meng FD, Qu K, Wang ZX, Wu QF, Zhang LQ, Pang Q, Liu C. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin. 2015;36(2):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, Hadass O, Lehmidi T, Blair GJ, Lee M, Chang M, Mobashery S, Sun GY, Gu Z. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener. 2012;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, Lipton SA. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong J, Xiu P, Dong X, Wang F, Wei H, Wang X, Xu Z, Liu F, Li T, Wang Y, Li J. Meloxicam combined with sorafenib synergistically inhibits tumor growth of human hepatocellular carcinoma cells via ER stress-related apoptosis. Oncol Rep. 2015;34(4):2142–50. [DOI] [PubMed] [Google Scholar]
- 34. Samarin J, Laketa V, Malz M, Roessler S, Stein I, Horwitz E, Singer S, Dimou E, Cigliano A, Bissinger M, Falk CS, Chen X, Dooley S, Pikarsky E, Calvisi DF, Schultz C, Schirmacher P, Breuhahn K. PI3K/AKT/mTOR-dependent stabilization of oncogenic far-upstream element binding proteins in hepatocellular carcinoma cells. Hepatology 2016;63(3):813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003;4(4):257–62. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Nie H, Zhao X, Qin Y, Gong X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer 2016;16(1):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maddirela DR, Kesanakurti D, Gujrati M, Rao JS. MMP-2 suppression abrogates irradiation-induced microtubule formation in endothelial cells by inhibiting alphavbeta3-mediated SDF-1/CXCR4 signaling. Int J Oncol. 2013;42(4):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer 2010;127(5):1081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]