Abstract
Protein disulfide isomerases A6 (PDIA6) belongs to the PDI family. Recently, PDIA6 was found to have a close association with various cancers. However, there has been little investigation into the biological functions of PDIA6 in bladder cancer (BC). In this study, we explored the expression pattern and functional significance of PDIA6 in BC. We found that PDIA6 was overexpressed in BC tissues and cell lines. The in vitro study showed that PDIA6 downregulation significantly inhibited BC proliferation and invasion. In addition, the in vivo experiment demonstrated that PDIA6 downregulation decreased the volume, weight, and metastasis of tumors. Furthermore, PDIA6 downregulation reduced the protein expression of β-catenin, cyclin D1, and c-Myc and thus suppressed the Wnt/β-catenin signaling pathway. In conclusion, we suggest that PDIA6 could be targeted for the treatment of BC.
Key words: Protein disulfide isomerases A6 (PDIA6), Bladder cancer (BC), Proliferation, Invasion
INTRODUCTION
Bladder cancer (BC), a common malignancy in the urinary system, has a high incidence in both developed and developing countries1. The disease is classified into two types, which are non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC)2. NMIBC will develop, with a probability of 30%, into MIBC due to a high rate of recurrence3,4. Many factors may contribute to the progression of BC, such as chronic irritation, molecular abnormalities, and exposure to chemicals5. In spite of increasingly advanced therapies, the 5-year survival rate is still far from satisfactory for patients with metastatic BC, which varies from 6% to 36%6–9. Therefore, great effort needs to be made in exploring an effective intervention so as to improve the poor outcome for BC patients.
Protein disulfide isomerases (PDIs), first isolated from rat livers, are abundantly found in many tissues10,11. PDIs belong to a family of ER-resident chaperones that assist in forming and isomerizing disulfide bonds of the ER lumen12. The PDI family contains at least 20 members, and all the members conduct disulfide formation and cyclic oxidation to participate in ER protein folding and maturation13–15. Recently, PDIA6, a member of the PDI family, was reported to be implicated in the progression of several kinds of cancers. For example, Ramos et al. suggested the expression of PDIA6 as an aggressiveness marker in breast cancer16. In addition, PDIA6 was found to have a promoting effect on the proliferation of cervical cancer cells17. However, there has been little investigation into the biological functions of PDIA6 in BC.
This study aimed to explore the expression pattern and functional significance of PDIA6 in BC. We found that PDIA6 was overexpressed in BC tissues and cell lines. We conducted in vitro and in vivo experiments and observed an inhibitory effect of PDIA6 downregulation on the proliferation and invasion of BC cells. In addition, PDIA6 downregulation reduced the protein expression of β-catenin, cyclin D1, and c-Myc, thus suppressing the Wnt/β-catenin signaling pathway.
MATERIALS AND METHODS
Tissue Samples
BC tissues and adjacent noncancerous tissues were obtained from 24 BC patients who underwent surgery in Huaihe Hospital, Henan University (P.R. China). Tissue samples were immediately frozen in liquid nitrogen at −80°C until use. The study was performed with written consent from each patient and with the approval of the ethics committee of Huaihe Hospital.
Cell Lines and Cell Culture
Human BC cell lines (T24 and RT4) and human bladder epithelial cell line SV-HUC-1 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cell lines were cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Hyclone), streptomycin (100 μg/ml), and penicillin (100 U/ml), followed by incubation at 37°C in a humidified atmosphere with 5% CO2.
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated from tissue samples or cell lines using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and then reversely transcribed into cDNA with a reverse transcription kit (Takara Biotechnology, Dalian, P.R. China). The following primers were used for RT-PCR: PDIA6, 5′-TGGATCCAACAAAAACAGACC-3′ (forward) and 5′-CTCAGCGCAGCATCTACAAT-3′ (reverse); β-actin, 5′-CTCCATCCTGGCCTCGCTGT-3′ (forward) and 5′-GCTGTCACCTTCACCGTTCC-3′ (reverse). The reaction was conducted under the following conditions: 95°C for 15 min, 40 cycles of 95°C for 10 s, and 65°C for 30 s. The relative expression level of genes was normalized to β-actin and analyzed using the comparative CT method (2−ΔΔCT)18.
Protein Extraction and Western Blot Analysis
Total protein was extracted from tissue samples or cell lines using RIPA lysis buffer. Protein concentration was calculated with a BCA protein assay kit (Beyotime, Haimen, P.R. China). After separation by 10% SDS-PAGE, protein was transferred to nitrocellulose membranes and blocked for 1 h in 5% nonfat milk. Subsequently, membranes were incubated overnight at 4°C with primary antibodies against PDIA6, β-catenin, cyclin D1, c-Myc, and β-actin (Invitrogen, Carlsbad, CA, USA), followed by incubation with the corresponding secondary antibody. All antibodies used in the study were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protein bands were visualized by an ECL detection system and analyzed by BandScan5.0.
Cell Transfection
PDIA6 siRNA (siPDIA6) and negative control siRNA (siNC) were obtained from RiBo Biotech (Guangzhou, P.R. China). T24 and RT4 cells were seeded into six-well plates, cultured for 24 h, and then transfected with siPDIA6 or siNC using transfection reagents (Invitrogen) according to the manufacturer’s protocol. The sequences were as follows: siPDIA6, 5′-UCGAUUUGUUCUCUGAUAA-3′; siNC, 5′-TCTTAATCGCGTATAAGGC-3′.
Cell Proliferation Assay
The proliferative capacity of BC cells was evaluated by the MTT assay. After transfection for 48 h, cells were plated into 96-well plates at a density of 2 × 103 cells/well and then incubated for different times. Subsequent to adding MTT (Sigma-Aldrich, St. Louis, MO, USA) to each well, cell incubation was continued for another 4 h before removal of culturing medium and adding of DMSO (Sigma-Aldrich). The absorbance was measured at 570 nm using a microplate reader.
Cell Invasion Assay
Transwell chambers with Matrigel-coated membranes were used to detect cell invasion. Transfected cells (4 × 104) were plated in the upper chamber with serum-free medium. The lower chamber contained DMEM supplemented with 10% FBS. After incubation for 48 h at 37°C, cells invading to the lower surface of the membrane were fixed and stained with cold methanol and crystal violet, respectively. Five fields were randomly selected to count the number of invading cells under a microscope.
In Vivo Xenograft Experiments
Female BALB/c nude mice (4 to 5 weeks old) were used for in vivo experiments. All animals were handled with the approval of the Institutional Animal Care and Use Committee of Henan University. Mice were divided into two groups, and each group consisted of eight mice. For the tumor growth assay, 5 × 105 transfected RT4 cells, suspended in 200 μl of RPMI-1640, were subcutaneously injected into the right flank of the mice. Tumor size was measured every week and calculated by the following formula: tumor volume = (length × width2)/2. After 4 weeks, mice were sacrificed, and tumors were weighed.
For the tumor metastasis assay, 100 μl of suspension of transfected RT4 cells (5 × 104) was intravenously injected into the tail of nude mice (n = 8). Four weeks later, mice were sacrificed, and tumor metastasis to the lungs was checked.
Statistical Analysis
Student’s t-tests were used for data analysis and the SPSS16.0 software (Chicago, IL, USA) for statistical analysis. All values were expressed as means ± standard deviation (SD). A comparison was made between different groups via the one-way analysis of variance. A value of p < 0.05 indicated statistically significant difference.
RESULTS
Expression of PDIA6 in BC Tissues and Cell Lines
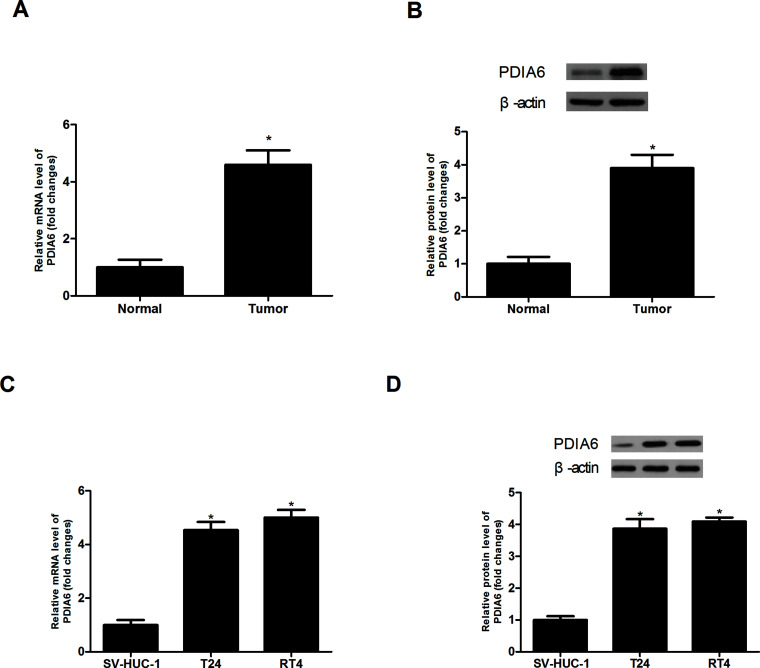
We examined the expression of PDIA6 in BC tissues and adjacent normal tissues by RT-PCR and Western blot assays. The results showed that PDIA6 had a higher expression level in BC tissues than in normal tissues (Fig. 1A and B). Next, we detected the expression of PDIA6 in the BC cell lines T24 and RT4 and in the normal bladder epithelial cell line SV-HUC-1. Consistently, PDIA6 was more highly expressed in T24 and RT4 cells than in SV-HUC-1 cells (Fig. 1C and D).
Figure 1.
Expression of PDIA6 in BC tissues and cell lines. (A, B) The RT-PCR and Western blot assays showed much higher expression levels of PDIA6 in BC tissues than in corresponding normal tissues. (C, D) The RT-PCR and Western blot assays showed much higher expression levels of PDIA6 in BC cell lines T24 and RT4 than in the normal bladder epithelial cell line SV-HUC-1. *p < 0.05.
The Effect of PDIA6 Downregulation on BC Cell Proliferation In Vitro and Tumor Growth In Vivo
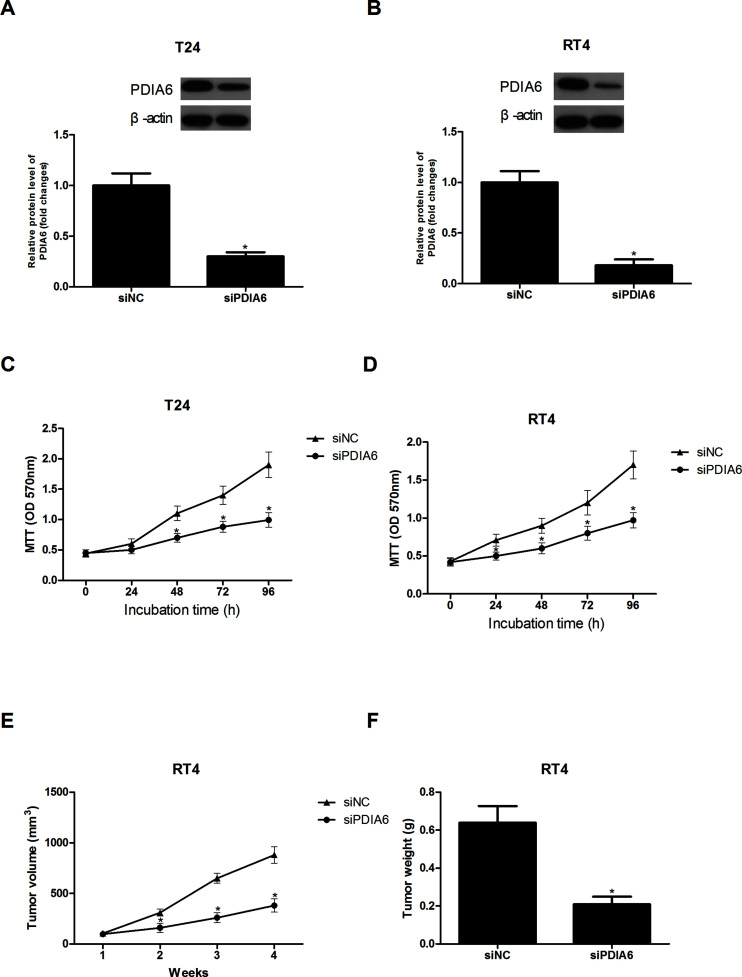
In order to explore the functional role of PDIA6 in the development of BC, we transfected T24 and RT4 cells with siPDIA6 to downregulate PDIA6. Forty-eight hours after transfection, Western blot analysis was performed to confirm the decreased expression of PDIA6 in T24 and RT4 cells (Fig. 2A and B).
Figure 2.
The effect of PDIA6 downregulation on BC cell proliferation in vitro and tumor growth in vivo. (A, B) The Western blot assay showed downregulated PDIA6 in T24 and RT4 cells after transfection of siPDIA6. (C, D) The MTT assay showed that PDIA6 downregulation greatly reduced the proliferative capacity of T24 and RT4 cells. (E, F) The volume and weight of tumors were remarkably decreased after injection of siPDIA6-transfected RT4 cells into nude mice. *p < 0.05.
The MTT assay was conducted to examine the effect of PDIA6 downregulation on BC cell proliferation. The proliferative capacity of T24 cells was greatly reduced by PDIA6 downregulation in comparison with the control cells (Fig. 2C). In addition, we obtained a similar result for RT4 cells (Fig. 2D). To confirm whether PDIA6 downregulation affected BC cell growth in vivo, we subcutaneously injected siPDIA6-transfected RT4 cells into the right flank of nude mice. Consistent with the in vitro results, the in vivo experiments showed that the volume and weight of tumors formed by siPDIA6-transfected RT4 cells were remarkably decreased in comparison with the control group (Fig. 2E and F).
The Effect of PDIA6 Downregulation on BC Cell Invasion In Vitro and Tumor Metastasis In Vivo
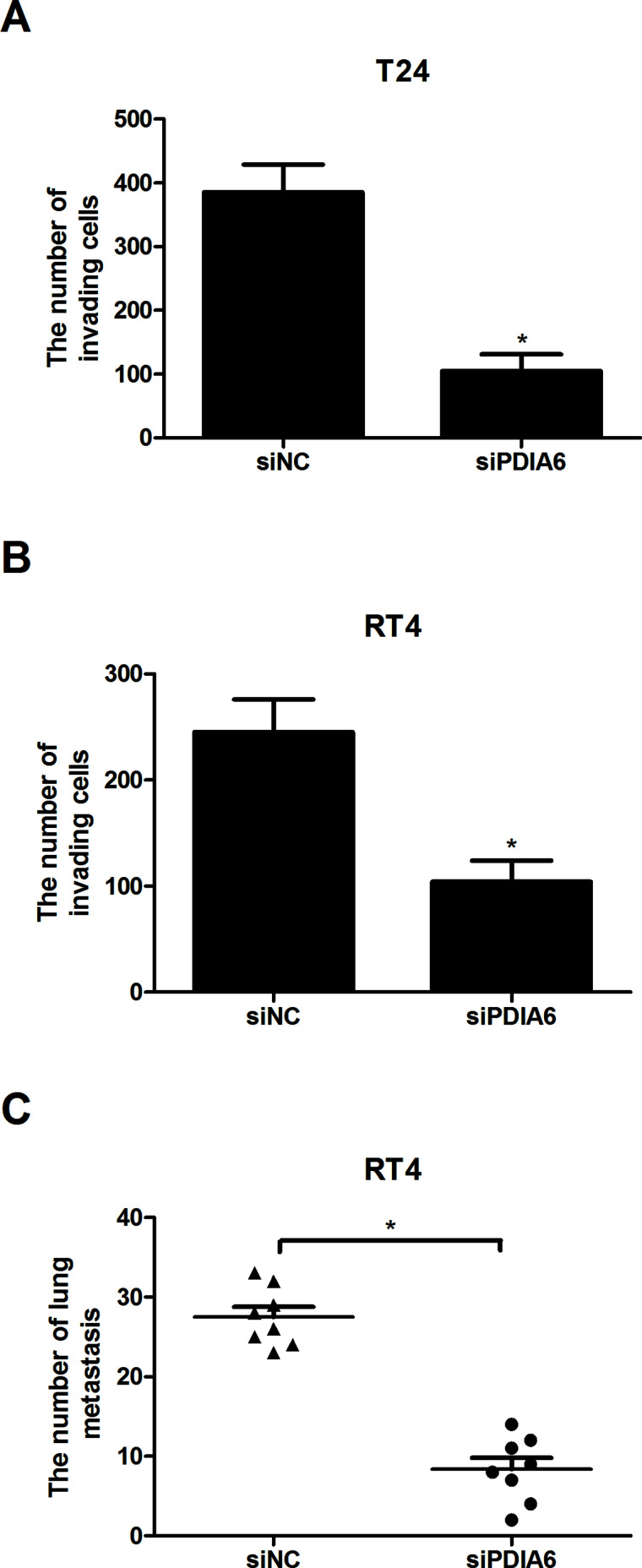
The Transwell assay was performed to measure the effect of PDIA6 downregulation on BC cell invasion. The assay results indicated that PDIA6 downregulation significantly reduced the number of T24 and RT4 cells that invaded through the Matrigel-coated membranes (Fig. 3A and B). Further in vivo experiments were conducted to test the effect of PDIA6 downregulation on tumor metastasis. Tumor metastasis to the lungs of nude mice in the PDIA6 downregulated group was markedly lower in comparison with the control group (Fig. 3C). These results suggested that the invasive and metastatic capabilities of BC cells were inhibited by PDIA6 downregulation in vitro and in vivo.
Figure 3.
The effect of PDIA6 downregulation on BC cell invasion in vitro and tumor metastasis in vivo. (A, B) The Transwell assay showed that the invasion of T24 and RT4 cells was significantly inhibited by PDIA6 downregulation in comparison with the control group. (C) The metastatic capability of RT4 cells was greatly weakened by PDIA6 downregulation in comparison with the control group. *p < 0.05.
The Effect of PDIA6 Downregulation on the Activity of Wnt/β-Catenin Signaling Pathway
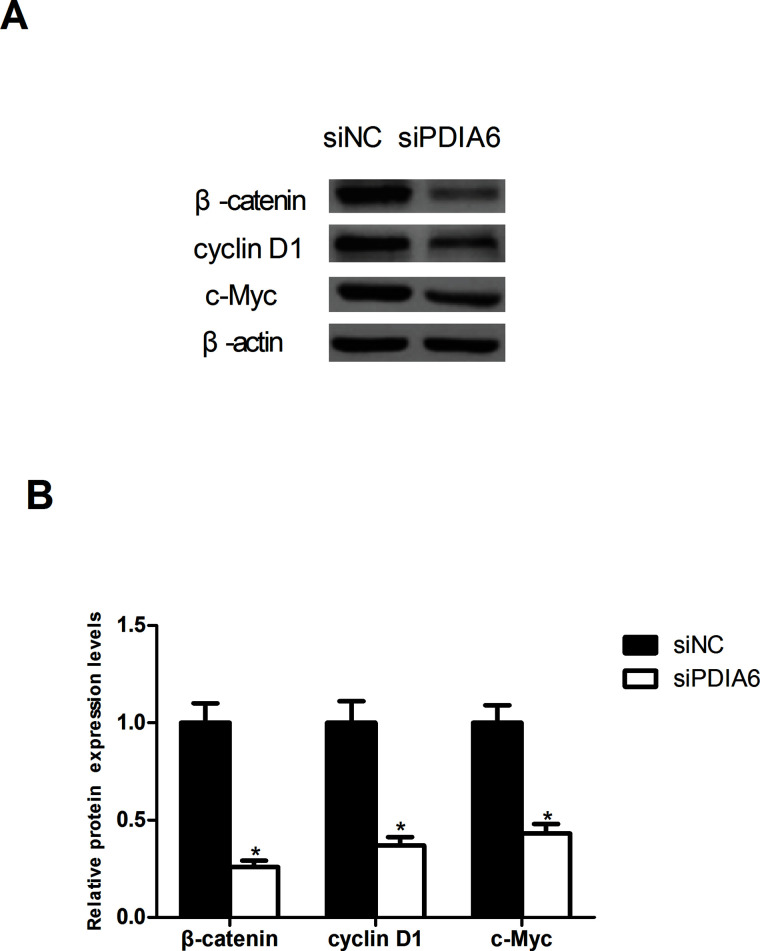
To explore whether PDIA6 downregulation exerted any effect on the Wnt/β-catenin signaling pathway, we measured the protein expression of β-catenin, cyclin D1, and c-Myc in RT4 cells. PDIA6 downregulation significantly decreased the protein expression of β-catenin, cyclin D1, and c-Myc in RT4 cells in comparison with the control cells, indicating a suppressive effect of PDIA6 downregulation on the activity of the Wnt/β-catenin signaling pathway (Fig. 4).
Figure 4.
The effect of PDIA6 downregulation on the activity of Wnt/β-catenin signaling pathway. (A) The Western blot assay showed that the protein expression of β-catenin, cyclin D1, and c-Myc was remarkably decreased in RT4 cells by PDIA6 downregulation in comparison with the control group. (B) Quantification of the protein expression was conducted with the BandScan5.0 software. *p < 0.05.
DISCUSSION
A great number of studies have been carried out at both the genetic and molecular levels for BC. However, the progress made in improving the survival rate of BC patients has been unsatisfactory. Thus, exploration of novel therapeutic targets for BC is desperately needed.
PDIA6, also known as ERp5, P5, or TXNDC7, belongs to the PDI family19. Similar to other members of the PDI family, PDIA6 is often found to have a ubiquitous expression in various human tissues20. In addition, PDIA6 is induced by ischemic and hypoxic stress in different kinds of tissues and cells such as head and neck carcinoma15,21. Recently, PDIA6 was found to have a close association with the development of various cancers. For example, one study suggested that PDIA6 could be a helper in the resistance to cell death in lung adenocarcinoma22. Another study reported the role of PDIA6 in marking the aggressiveness of breast cancer16. Both studies mentioned above found overexpressed PDIA6 in corresponding cancer tissues. In our study, we observed a similar overexpression pattern of PDIA6 in BC tissues. We further verified this result by detecting the expression of PDIA6 in BC cell lines. As expected, we found that PDIA6 was highly expressed in BC cell lines in comparison with the normal bladder epithelial cell line. With regard to biological functions of PDIA6 in cancer development, Gao et al. demonstrated a promoting effect of PDIA6 on the proliferation of cervical cancer cells17. We also found that PDIA6 played a tumor-promoting role in the proliferation and invasion of BC cells. These findings, confirmed by our in vivo results, were that PDIA6 downregulation inhibited BC cell growth and metastasis in nude mice. In accordance with our study results, we suggest that PDIA6 functions as a tumor promoter during the progression of BC. However, the mechanisms underlying the promoting function remain unknown.
The Wnt/β-catenin signaling pathway is known for its important role in cellular processes such as proliferation, migration, differentiation, and apoptosis23,24. Increasing evidence has shown that deregulated Wnt/β-catenin signaling pathway is a great contributor to the development of various cancers, including BC25–28. More importantly, PDIA6 was found to promote cervical cancer cell proliferation via activating the Wnt/β-catenin signaling pathway17. Therefore, we reasonably inferred in our study that PDIA6 exerted its promoting effect on BC cell proliferation and invasion through the Wnt/β-catenin signaling pathway. To prove our inference, we examined whether PDIA6 downregulation affected the expression of β-catenin and its downstream targets cyclin D1 and c-Myc in BC cells. The assay results showed that PDIA6 downregulation significantly inhibited the Wnt/β-catenin signaling pathway via reducing the protein expression of β-catenin, cyclin D1, and c-Myc in BC cells. These results were consistent with our hypothesis.
In summary, our study found overexpressed PDIA6 in BC tissues and cell lines. Downregulation of PDIA6 inhibited BC cell proliferation and invasion in vitro as well as tumor growth and metastasis in vivo. In addition, PDIA6 downregulation suppressed the Wnt/β-catenin signaling pathway. Taken together, we suggest that PDIA6 could be targeted as a treatment for BC.
REFERENCES
- 1. Ploeg M, Aben KKH, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. N Engl J Med. 1990;322:1129–38. [DOI] [PubMed] [Google Scholar]
- 3. Rhijn BWGV, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, Witjes JA, Zlotta AR. Recurrence and progression of disease in non-muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur Urol. 2009;56:430–42. [DOI] [PubMed] [Google Scholar]
- 4. Stenzl A, Cowan NC, Santis MD, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur Urol. 2012;62:e45–6. [DOI] [PubMed] [Google Scholar]
- 5. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009;374:239–49. [DOI] [PubMed] [Google Scholar]
- 6. Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71:3802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Huang Z, Zhu Z, Zheng X, Liu J, Han Z, Ma X, Zhang Y. Upregulated UHRF1 promotes bladder cancer cell invasion by epigenetic silencing of KiSS1. PLoS One 2014;9:e104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarver T. Cancer facts & figures 2012. American Cancer Society (ACS). J Consum Health Internet 2012;16:366–7. [Google Scholar]
- 9. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. [DOI] [PubMed] [Google Scholar]
- 10. Goldberger RF, Epstein CJ, Anfinsen CB. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963;238:628–35. [PubMed] [Google Scholar]
- 11. Freedman RB, Hirst TR, Tuite MF. Protein disulphide isomerase: Building bridges in protein folding. Trends Biochem Sci. 1994;19:331–6. [DOI] [PubMed] [Google Scholar]
- 12. Ferrari DM, Söling HD. The protein disulphide-isomerase family: Unravelling a string of folds. Biochem J. 1999;339(Pt1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 13. Schwaller M, Wilkinson B, Gilbert HF. Reduction-reoxidation cycles contribute to catalysis of disulfide isomerization by protein-disulfide isomerase. J Biol Chem. 2003;278:7154–9. [DOI] [PubMed] [Google Scholar]
- 14. Appenzeller-Herzog C, Ellgaard L. The human PDI family: Versatility packed into a single fold. Biochim Biophys Acta 2008;1783:535–48. [DOI] [PubMed] [Google Scholar]
- 15. Guennadi K, Pekka M, Thomas DY, Kalle G. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–36. [DOI] [PubMed] [Google Scholar]
- 16. Ramos FS, Serino LT, Carvalho CM, Lima RS, Urban CA, Cavalli IJ, Ribeiro EM. PDIA3 and PDIA6 gene expression as an aggressiveness marker in primary ductal breast cancer. Genet Mol Res. 2015;14:6960–7. [DOI] [PubMed] [Google Scholar]
- 17. Gao H, Sun B, Fu H, Chi X, Wang F, Qi X, Hu J, Shao S. PDIA6 promotes the proliferation of HeLa cells through activating the Wnt/β-catenin signaling pathway. Oncotarget 2016;7(33):53289–98. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19. Sha ZX, Hong L, Wang QL, Yang L, Yang L, Min L, Chen SL. Channel catfish (Ictalurus punctatus) protein disulphide isomerase, PDIA6: Molecular characterization and expression regulated by bacteria and virus inoculation. Fish Shellfish Immunol. 2012;33:220–8. [DOI] [PubMed] [Google Scholar]
- 20. Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 2001;99:4465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vekich JA, Belmont PJ, Thuerauf DJ, Glembotski CC. Protein disulfide isomerase-associated 6 is an ATF6-inducible ER stress response protein that protects cardiac myocytes from ischemia/reperfusion-mediated cell death. J Mol Cell Cardiol. 2012;53:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tufo G, Jones AWE, Wang Z, Hamelin J, Tajeddine N, Esposti DD, Martel C, Boursier C, Gallerne C, Migdal C. The protein disulfide isomerases PDIA4 and PDIA6 mediate resistance to cisplatin-induced cell death in lung adenocarcinoma. Cell Death Differ. 2014;21:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–305. [DOI] [PubMed] [Google Scholar]
- 24. Robinson JA, Chatterjeekishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8. [DOI] [PubMed] [Google Scholar]
- 25. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13:11–26. [DOI] [PubMed] [Google Scholar]
- 26. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192–205. [DOI] [PubMed] [Google Scholar]
- 27. Macdonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 2011;138:4341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]