Abstract
Cervical cancer is among the most common cancers inflicting women worldwide. Understanding the pathological mechanisms of cervical cancer development is critical for identifying novel targets for cervical cancer treatment. MicroRNAs (miRs) have various roles in regulating cancer development. In this study, we investigated the potential role of miR-181a and its target in regulating cervical cancer development and chemotherapy resistance. The expression of miR-181a was evaluated and modulated in several human cervical cancer cell lines. The role of miR-181a in regulating cervical cancer growth and chemotherapy sensitivity was investigated in cell culture models and mouse tumor xenograft models. The target of miR-181a and its function were identified in cervical cancer models. We found a distinct expression profile for miR-181a in cervical cancer cell lines. Low expression of miR-181a was closely related to cervical cancer growth and oxaliplatin resistance. HSPA5/GRP78 was identified as a target of miR-181a in cervical cancer cells. Upregulation of GRP78 led to a high cell proliferation rate and oxaliplatin resistance in cervical cancer models. In a retrospective cervical cancer cohort, high GRP78 expression was correlated with poor survival. miR-181a suppressed cervical cancer development via downregulating GRP78. High expression of GRP78 is a tumor-promoting factor in cervical cancer and is thus a potential target for novel treatment.
Key words: Cervical cancer, MicroRNA-181a, Oxaliplatin, Glucose-regulated protein 78 (GRP78), Tumor development
INTRODUCTION
MicroRNAs (miRs) are small (approximately 20–22 nucleotides in length), noncoding, RNA molecules that regulate the expression of their target genes1. Combined with the 3′-untranslated regions (3′-UTRs) of their target genes, miRs negatively regulate gene expression via inhibiting mRNA translation1. In cancer biology, depending on their target genes, miRs can be positive or negative in regulating tumor development1. In view of the clinical application, miRs have been identified as diagnostic and prognostic markers in various cancers1–3. Therefore, understanding the function of miRs would be critical in deciphering the tumor development regulatory network.
Glucose-regulated protein 78 (GRP78), also known as the immunoglobulin heavy-chain-binding protein (BiP), is a member of the heat shock protein (HSP) 70 family4,5. As one of the best-characterized endoplasmic reticulum (ER) chaperone proteins, GRP78 is involved in many cellular processes, including translocating newly synthesized polypeptides across the ER membrane, facilitating misfolded proteins for proteasome degradation, maintaining calcium homeostasis, and serving as a sensor for ER stress4,5. In cancer biology, studies have shown that stress induction of GRP78 was an important prosurvival mechanism because of its antiapoptotic property in the unfolded protein response4,5. Upregulation of GRP78 in cancer cell lines was correlated with elevated tumor growth, invasion, and metastasis. In cancer treatment, a high expression of GRP78 was associated with chemotherapy resistance in gastric cancer, prostate cancer, breast cancer, and lung cancer6–9.
Cervical cancer is among the most common cancers in women10. Human papillomavirus (HPV) infection is the major risk factor for cervical cancer and is associated with more than 90% of cervical cancer incidence11. Although HPV 9-valent vaccine has shown promising efficacy in preventing HPV infection, women who were previously infected by HPV were still at a high risk for cervical cancer12. A previous study has identified miR-181a as a key regulator of GRP78 protein in cerebral ischemia tissue13. However, the roles of miR-181a and GRP78 in determining cervical cancer growth and drug sensitivity were still not clear. Based on the studies mentioned above, we hypothesized that miR-181a could inhibit cervical cancer progression via downregulating GRP78.
MATERIALS AND METHODS
Cell Culture and Cell Viability Assay
Human cervical cancer cell lines HeLa, SiHa, C-33A, and HT-3 (Beijing Institute for Tumor Prevention and Treatment, P.R. China) were used in this study. The HeLa, SiHa, and C-33A cell lines were cultured in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum (FBS). The HT-3 cell line was cultured in McCoy’s 5a medium with 10% FBS. A final concentration of 100 U/ml penicillin and 100 μg/ml streptomycin was used to control cell culture contamination. All the cell lines were maintained in a humidified incubator with 5% CO2 at 37°C. After oxaliplatin (OXA) treatment, the cell lines were subjected to test cell viability using a CCK-8 kit (Sigma-Aldrich, St. Louis, MO, USA). The manufacturer suggested the procedures that were followed for cell viability testing.
qRT-PCR
Total RNA was extracted by mirVana miRNA Isolation Kit and was reverse transcribed using miScript Reverse Transcription Kit (Qiagen, Hilden, Germany). The expression measurement was performed with SYBR Green (Qiagen) and the LightCycler® 480 System (Roche Life Science, Indianapolis, IN, USA). The comparative threshold cycle (Ct) method was used to determine relative gene expression. The 18S rRNA was chosen as an internal control for comparison. The primer for miR-181a was purchased from Applied Biosystems (Cat. No. 000480; Foster City, CA, USA).
Invasion Assay
Tumor cell invasion was measured by counting the number of tumor cells that invaded through Matrigel precoated Transwell chambers with 8-mm pores (BD Biosciences, San Jose, CA, USA). On the top of inserts, 12-h FBS-starved HeLa tumor cells with different miR-181a levels were placed. On the bottom chamber, 5% FBS cell culture medium was added. After incubation for 24 h for invasion, cells that invaded were fixed with 70% ethanol, stained with crystal violet, and counted in five random fields under a light microscope.
Animal Model
A subcutaneous cervical cancer mouse model was established using the HeLa cell line and 6-week-old female nude mice (18–20 g; Chinese Academy of Science, P.R. China). All animals were housed in the SPF environment and got unlimited water and food. To test the effects of miR-181a on tumor growth, 5 × 106 HeLa cells with different miR-181a expression levels (wild type, transfection control, high expression, and low expression) were injected subcutaneously into the flank of each mouse. Eight mice were included in each group for this experiment. To investigate the role of GRP78 on tumor development and drug sensitivity, we subcutaneously inoculated 2 × 106 HeLa cells with different GRP78 expression status (wild-type expression, transfection control, overexpression, knockdown) into the flank of each mouse. Eight mice were used in each group. The tumor volume (calculated as width2 × length × π/6) of each mouse was recorded every 10 days. A single dose of OXA (20 mg/kg) was given to the tumors with different GRP78 expressions on day 20 to test drug response. The animal study was approved by the local committee of The Experimental Animal Use and Care of Jiangsu Province Hospital.
Dual-Luciferase Assay
The luciferase reporter assay was performed as described by the manufacturer’s instructions. HeLa cells were plated at a density of 2 × 104 cells/well into 96-well plates 1 day before transfection. On day 2, cells were cotransfected with 0.5 ng of firefly luciferase control reporter plasmid, 0.01 ng of Renilla luciferase target reporter, and 40 ng of miRNA expression vector using Lipofectamine® 2000 Transfection Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Dual-luciferase assay was performed 48 h after transfection. Results are presented as relative luciferase activity by normalizing to the Renilla/firefly value of the empty control vector. The sequence of miR-181a wild type was 5′-AACAUUCAACGCUGUCGGUGAGU-3′. The sequence of miR-181a seed mutation (SM) was 5′-AUGUUAGUACGCUGUCGGUGAGU-3′.
Western Blotting
The expression of GRP78 in the Hela cell line was measured by Western blotting assay. The cells were collected and lysed by RIPA buffer with proteinase inhibitor and phosphatase inhibitor. The total protein amount was measured and normalized by BCA assay and then separated by sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to a PVDF membrane by a half-dry transferring system. The GRP78 protein was detected by the anti-GRP78 antibody (1:1,000 dilution; Abcam, Cambridge, MA, USA). The anti-GAPDH antibody (1:1,000 dilution; Abcam) was used for internal control. Horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; Abcam) and ECL Western blotting detection reagents (Thermo Fisher Scientific) were used for imagining.
Cell Infection
Human GRP78 overexpression lentivirus vector, GRP78 shRNA lentivirus vector, and control vector were purchased from Origene Technologies (Rockville, MD, USA). Cells (293) were transfected with either GRP78 overexpression, GRP78 shRNA, or control vector for producing lentivirus to infect HeLa cells. After 48 h of transfection, lentivirus was harvested and used for infecting HeLa cells. Expression of GRP78 was measured by Western blotting in each group to validate infection success.
ELISA
The amount of activated caspase 3 and BrdU in the tumor cells and tissues were measured by enzyme-linked immunosorbent assay (ELISA; kits were purchased from Thermo Fisher Scientific). The procedures suggested by the manufacturer were followed. The total protein amount in each sample was measured and normalized by BCA assay before loading into the ELISA plates.
Immunohistochemistry
A total of 105 cervical cancer patients diagnosed between October 2006 and September 2010 in Jiangsu Province Hospital were included for a retrospective survival analysis. Sixty-five patients were in stages III–IV when diagnosed. Informed consent was signed by each patient. This study was approved by the ethics committee of Jiangsu Province Hospital. Tumor tissues were obtained before chemoradiation. None of the patients had undergone chemotherapy or radiotherapy before the surgery.
GRP78 expression was detected in the collected cervical cancer tissues by immunohistochemistry (IHC). Briefly, the formalin-fixed paraffin-embedded sections were deparaffined by xylene and rehydrated with ethanol. The antigen was retrieved by heating the citric acid buffer (pH 6.0) and submerging slides in a water bath for 20 min. Incubation with 5% BSA–PBS for 30 min at room temperature was performed for blocking nonspecific antibodies. Primary antibodies (1:500 dilution; Abcam) were added to the slides and incubated overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies (1:1,000 dilution; Abcam) were then added and incubated for 1 h at room temperature. Slides were then washed and mounted.
Statistical Analysis
All statistical analyses were performed using the GraphPad Prism version 6.0 software (GraphPad Software, La Jolla, CA, USA). A two-sample t-test or one-way ANOVA was used to analyze the difference among means of experimental groups. Tukey’s multiple comparisons test were performed for two-group comparison after one-way ANOVA. The log rank test was used for retrospective survival analysis. A value of p < 0.05 for each hypothesis test was considered as statistical significance.
RESULTS
Expression of miR-181a Was Diverse in Cervical Cancer Cell Lines
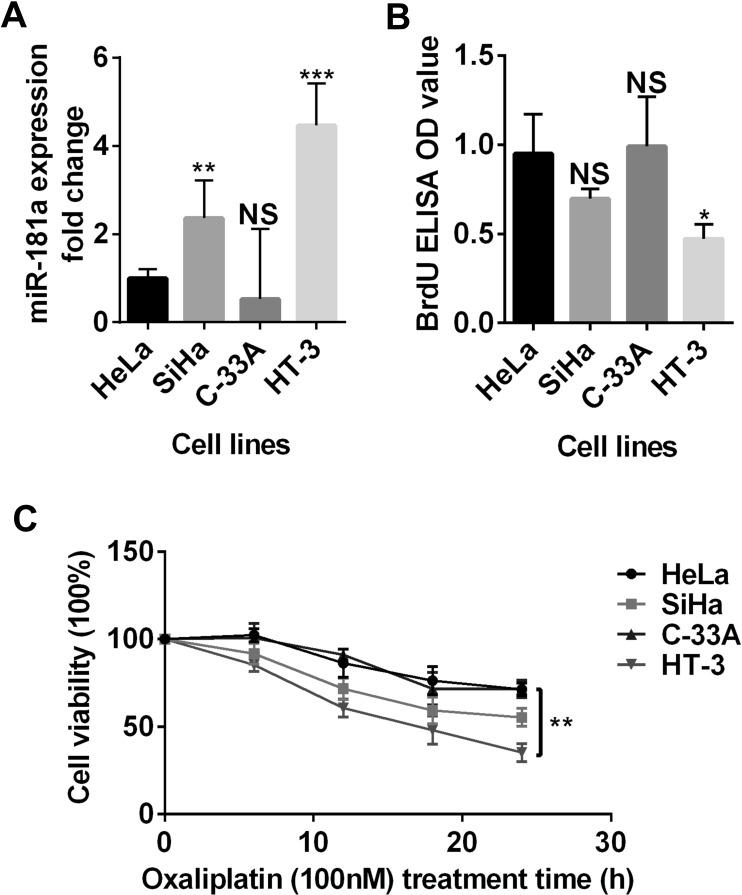
To have a comprehensive view of miR-181a expression in cervical cancer, we measured the miR-181a level in four human cervical cancer cell lines. We found that HeLa cells had a moderate miR-181a expression compared with the SiHa, HT-3, and C-33A cell lines (Fig. 1A). We further evaluated the proliferation rate and chemotherapy (OXA) sensitivity of these four cell lines. Interestingly, the cell lines (SiHa and HT-3) that had a higher miR-181a expression tended to have a slower proliferation rate and were more sensitive to OXA treatment (Fig. 1B and C). These results showed that cervical cancer cell lines had diverse miR-181a expression levels and that miR-181a expression was likely related to cell proliferation and drug sensitivity.
Figure 1.
Expression of miR-181a in cervical cancer cell lines. (A) The miR-181a expression level of four cervical cancer cell lines (HeLa, SiHa, C-33A, and HT-3) was evaluated by qRT-PCR. The miR-181a expressions of the four cervical cancer cell lines were significantly different. (B) The BrdU incorporation was measured by enzyme-linked immunosorbent assay (ELISA). The difference of the BrdU incorporations between the four cervical cancer cell lines was also significant. (C) Response to the oxaliplatin (OXA) treatment was evaluated by cell viability. A significant difference was observed among the mean viabilities of each cell lines. NS, nonsignificant. *p < 0.05; **p < 0.01; ***p < 0.001.
Upregulation of miR-181a Suppressed Cervical Cancer Development
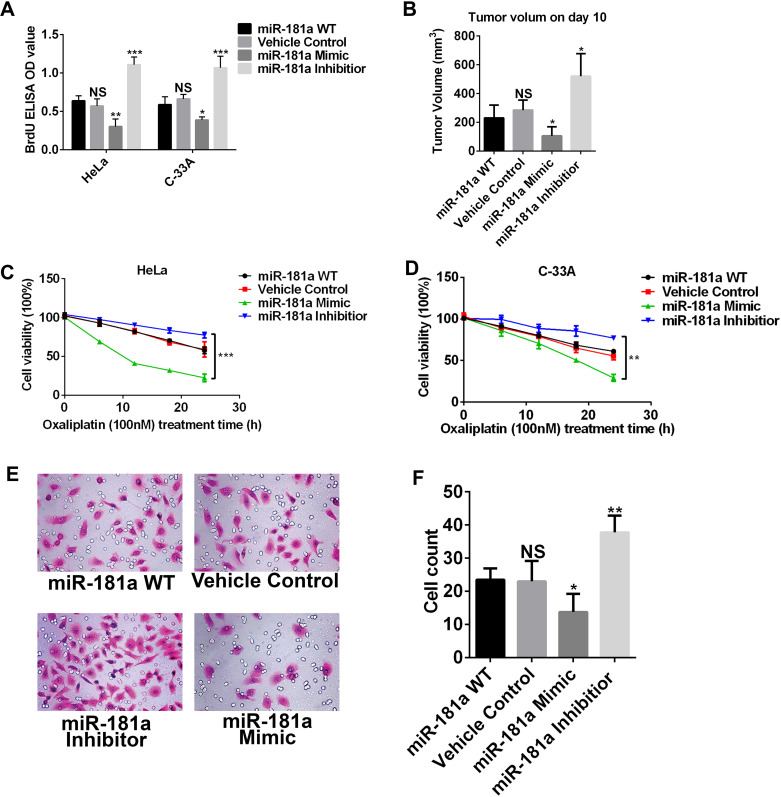
To have a better understanding of miR-181a in regulating cervical cancer development and drug sensitivity, we manipulated miR-181a expression in two cervical cancer cell lines: HeLa and C-33A. We treated cancer cells with either miR-181a mimic or miR-181a inhibitor to modulate the miR-181a level. To test cell proliferation, we performed a BrdU incorporation assay on day 7 after miR-181a mimic or inhibitor treatment (Fig. 2A). After upregulating the miR-181a level, HeLa and C-33A cells showed a decreased cell proliferation rate, while downregulating the miR-181a level increased the cell proliferation rate (Fig. 2A). In the subcutaneous tumor model, we found that the miR-181a mimic-treated HeLa cells had the lowest tumor volume on day 10 after tumor inoculation, whereas the miR-181a inhibitor-treated HeLa cells developed the largest tumors (Fig. 2B).
Figure 2.
High expression of miR-181a suppressed the development of cervical cancer in vitro and in vivo. (A) BrdU incorporation of HeLa cells and C-33A cells with different miR-181a expression levels. The high-miR-181a cells had low BrdU incorporation, while the low-miR-181a cells had high BrdU incorporation. (B) HeLa cell subcutaneous mouse model was established. Tumor volume was measured on the 10th day after inoculation. Tumors with high miR-181a had the smallest mean volume. (C, D) Cell viability of HeLa cells and C-33A cells treated with OXA at different time points. The miR-181a high expression cells were sensitive to OXA treatment, while the miR-181a low expression cells were resistant. (E, F) Invasion assay of HeLa cells with different miR-181a levels. *p < 0.05; **p < 0.01; ***p < 0.001.
We investigated more potential functions of miR-181a on cancer cells, including OXA sensitivity and invasion. Interestingly, HeLa and C-33A cells treated with the miR-181a mimic became much more sensitive to OXA when compared with the wild-type and vehicle control groups (Fig. 2C and D). However, cells treated with miR-181a became more resistant to OXA (Fig. 2C and D). The invasion assay indicated that HeLa cells had the lowest invasive ability when treated with the miR-181a mimic (Fig. 2E and F). To summarize, our data clearly indicated that miR-181a could suppress cervical cancer development via decreasing tumor growth and invasion and increasing drug sensitivity.
miR-181a Targeted HSPA5 in Cervical Cancer Cells
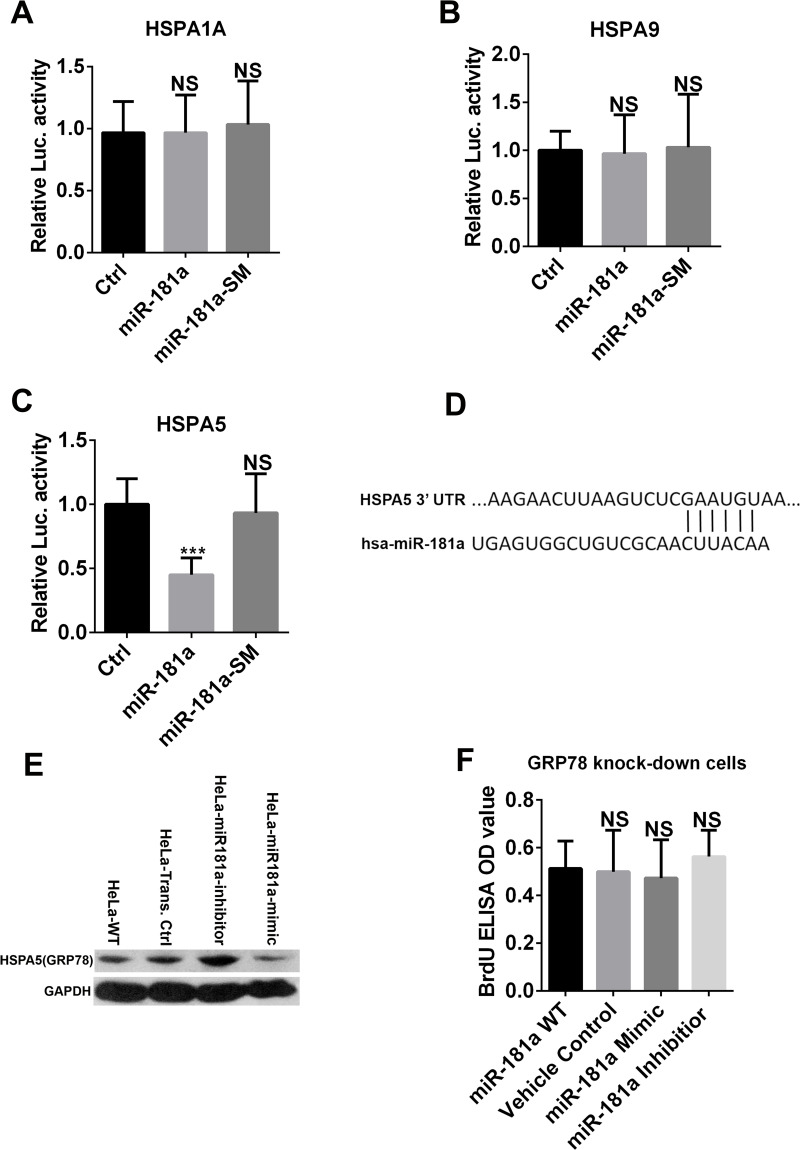
Based on the computational miR target prediction, we decided to test three predicted target genes of miR-181a: HSPA1A, HSPA9, and HSPA5 in HeLa cells. According to the results of the dual-luciferase assay, neither HSPA1A nor HSPA9 3′-UTR was targeted in HeLa cells (Fig. 3A and B). However, the 3′-UTR of the HSAP5 gene was targeted by miR-181a, and the luciferase signal decreased significantly (Fig. 3C). The Western blotting assay further validated that the expression of HSPA5 (GRP78) in HeLa cells was decreased by the miR-181a mimic treatment but increased by the miR-181a inhibitor treatment, suggesting that HSPA5 (GRP78) was a target of miR-181a in cervical cancer cells (Fig. 3D). Since miR-181a could suppress cell proliferation in HeLa cells, we modulated the miR-181a level in GRP78 knockdown HeLa cells and tested their cell proliferation rate. In GRP78 knockdown HeLa cells, changing the miR-181a level did not change cell proliferation (Fig. 3E), further validating that GRP78 was the major effective target of miR-181a in cervical cancer cells.
Figure 3.
HSPA5 is the target of miR-181a in HeLa cells. (A–D) HSPA1A, HSPA9, and HSPA5 were predicted targets of miR-181a. Dual-luciferase activity assays were performed in HeLa cells cotransfected with the plasmid containing Renilla luciferase and the HSPA1A or HSPA9 or HSPA5 3′-untranslated region (3′-UTR; WT) and the plasmids encoding either pri-miR-181a or its SM. The results demonstrated that miR-181a did not recognize HSPA1A and HSPA9 3′-UTRs, as there is no reduction of luciferase activity compared to the SM control. However, HSPA5 was recognized by miR-181a in dual-luciferase activity. (E) Western blot assays showed that the expression of HSPA5 (GRP78) in HeLa cells was negatively correlated with miR-181a expression level. (F) Modulating miR-181a level in GRP78 knockdown HeLa cells did not change cell proliferation rate. ***p < 0.001.
GRP78 Promoted Cervical Cancer Development and Was Correlated With Poor Survival
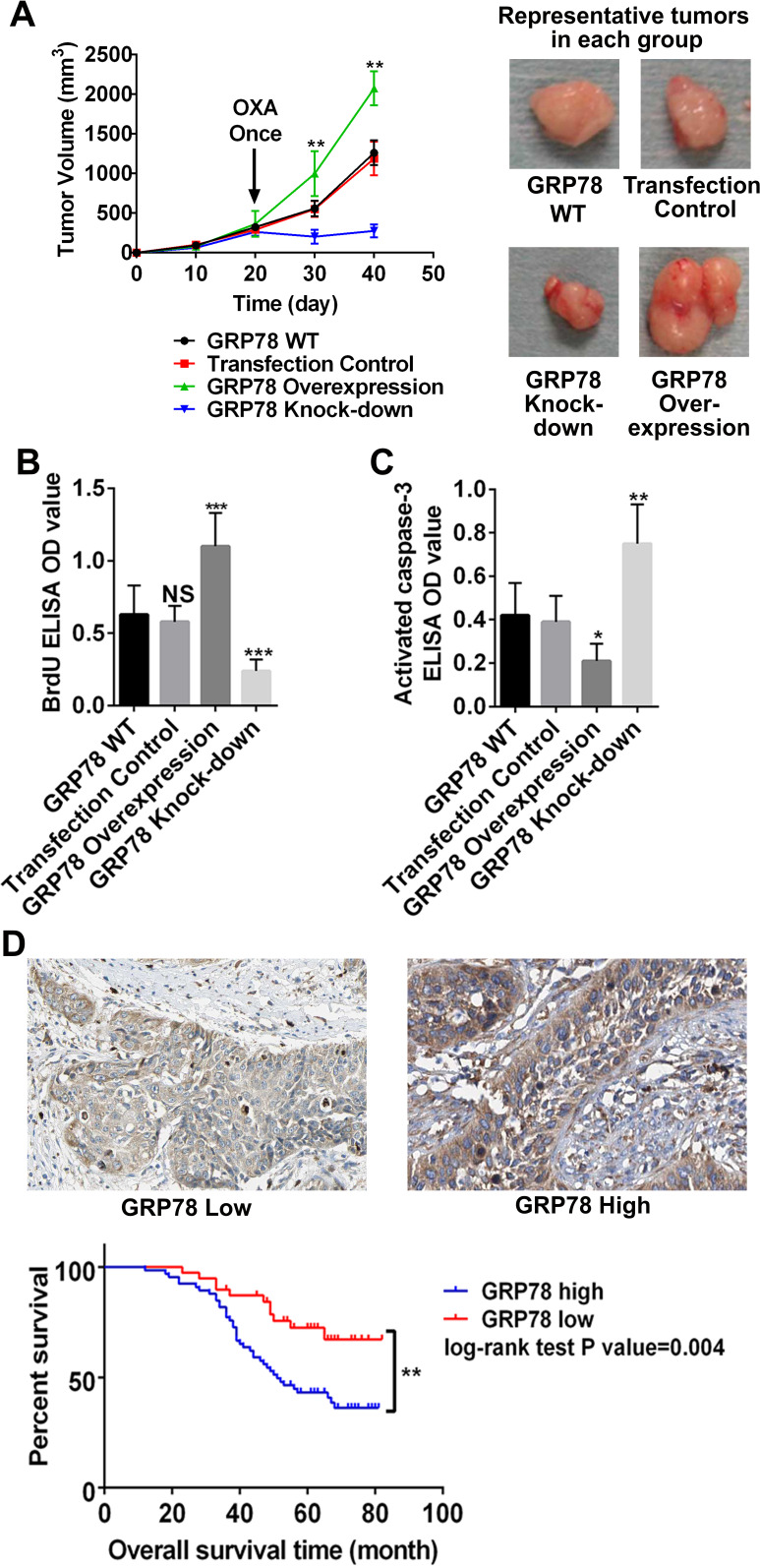
Since miR-181a targeted GRP78 in cervical cancer cells and could suppress tumor development, we further investigated the role of GRP78 in cervical cancer. By regulating GRP78 expression in HeLa cells, we found that upregulation of GRP78 could increase cervical cancer growth and drug resistance in the subcutaneous model (Fig. 4A). When we downregulated GRP78 expression, HeLa cell subcutaneous tumors could be well controlled by OXA. BrdU and activated caspase 3 ELISA of tumor tissues showed that the GRP78 upregulated subcutaneous tumors had the highest proliferation rate and the lowest apoptotic rate, and vice versa (Fig. 4B and C). To validate that miR-181a’s target, GRP78, is a tumor-promoting factor, we also included a retrospective survival analysis of cervical cancer patients with diverse GRP78 expression levels. Tumor tissues of 105 cervical cancer patients were used to evaluate their GRP78 expression. In line with our data in experimental models, in clinical samples we found that a high GRP78 expression was correlated with poor survival (Fig. 4D). In conclusion, our data suggest that GRP78, a major target of miR-181a, could accelerate cervical cancer development. Therefore, one of the mechanisms of miR-181a-induced cervical cancer suppression depends on GRP78.
Figure 4.
Survival curves of cervical cancer patients with different GRP78 expression levels. (A) Subcutaneous cervical cancer mouse model was established with HeLa cells having different GRP78 levels. OXA treatment was given on day 20 after tumor inoculation. The GRP78 knockdown group had the best response to OXA treatment. Representative tumors from each group were shown. (B, C) BrdU and activated caspase 3 levels in tumor tissues were quantified by ELISA assays. GRP78 knockdown group had the highest activated caspase 3 and lowest BrdU incorporation. (D) Representative figures of high and low GRP78 expression in patient samples. The patients with high GRP78 expression tended to have shorter overall survival time than the patients with low GRP78 expression. *p < 0.05; **p < 0.01 (p = 0.004); ***p < 0.001.
DISCUSSION
Cervical cancer is a common malignant disease in women that has around 12,820 new incidences in the US10. Although comprehensive treatments have been developed, treatment of cervical cancer is still a tough clinical problem. Investigating the pathological mechanisms and potential novel targets of cervical cancer is therefore significant. miRs are single-stranded RNAs approximately 20–22 nucleotides in length and constitute a new class of gene regulatory mechanism1. Diverse functions of miRs in cancers have been identified, including as a tumor promoter or a suppressive factor, as prognostic markers, and as diagnostic markers1–3. In the present study, we aimed to clarify the functions of miR-181a and its targets in cervical cancer growth and chemotherapy sensitivity.
miR-181a has long been correlated with a large range of human diseases. In mouse cerebral ischemic injury models, miR-181 was found to be a major contributor13. In cancers, dependent on the tumor types and experimental conditions, miR-181a showed different functions via targeting diverse genes. In cervical cancer, a previous study showed that miR-181a confers resistance of cervical cancer to radiation therapy14. However, when we tested the role of miR-181a in tumor cell proliferation and chemotherapy sensitivity, we identified its tumor-suppressive function in cervical cancer. We found that miR-181a was very distinctively expressed in the human cervical cancer cell lines HeLa, C-33A, SiHa, and HT-4. A high expression of miR-181a was correlated with lower tumor growth as shown by the BrdU incorporation assay and subcutaneous tumor model. More interestingly, upregulation of miR-181a sensitized cervical cancer cells to OXA treatment. Taken together, in terms of tumor growth and chemotherapy, miR-181a inhibits cervical cancer development. Our data were in line with previous studies, which reported a similar effect in other cancers15–18.
GRP78, an important ER chaperone and signaling pathway regulator, is commonly overexpressed in cancers19,20. In terms of chemotherapy, GRP78 could be induced by a variety of anticancer drugs, thereby conferring chemoresistance to cancers and contributing to tumorigenesis6–9. GRP78 is predicted to be a target of miR-181a. Therefore, we hypothesized that the tumor growth- and chemoresistance-reducing functions of miR-181a in cervical cancer might be mediated by downregulation of GRP78. By screening the targeting effects of miR-181a on HSPA5/GRP78 and similar genes, we validated that GRP78 is a major target of miR-181a in cervical cancer cells. Further experiments focused on illustrating the potential tumor-promoting function of GRP78 on cervical cancer. As expected, upregulation of GRP78 enhances tumor growth and chemotherapy resistance in a subcutaneous model. In a retrospective patient cohort, we found that GRP78 was highly expressed in certain patients and correlated with poor survival. These data suggested that the downregulation of GRP78 mediated the tumor-suppressive function of miR-181a in cervical cancer and is thus a promising target for future investigation.
In conclusion, our study indicates that miR-181a is a tumor suppressive factor in cervical cancer by downregulating GRP78 expression. GRP78 contributed to cervical cancer growth and drug resistance and is a prognosticator of poor survival in cervical cancer. Further studies investigating the targeting value of GRP78 in cervical cancer treatment are expected.
ACKNOWLEDGMENT
The authors would like to thank the Internal Scientific Grant from Jiangsu Province Hospital, P.R. China.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. MicroRNA cancer regulation. New York (NY): Springer; 2013. p. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao C, Lu F, Chen H, Zhao F, Zhu Z, Zhao X, Chen H. Clinical significance of circulating miRNA detection in lung cancer. Med Oncol. 2016;33:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs—The micro steering wheel of tumour metastases. Nat Rev Cancer 2009;9:293–302. [DOI] [PubMed] [Google Scholar]
- 4. Lee AS. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. [DOI] [PubMed] [Google Scholar]
- 5. Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): Functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1993;4:1–18. [DOI] [PubMed] [Google Scholar]
- 6. Pootrakul L, Datar RH, Shi S-R, Cai J, Hawes D, Groshen SG, Lee AS, Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–93. [DOI] [PubMed] [Google Scholar]
- 7. Lee E, Nichols P, Spicer D, Groshen S, Mimi CY, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. [DOI] [PubMed] [Google Scholar]
- 8. Koomägi R, Mattern J, Volm M. Glucose-related protein (GRP78) and its relationship to the drug-resistance proteins P170, GST-pi, LRP56 and angiogenesis in non-small cell lung carcinomas. Anticancer Res. 1998;19:4333–6. [PubMed] [Google Scholar]
- 9. Kang J, Zhao G, Lin T, Tang S, Xu G, Hu S, Bi Q, Guo C, Sun L, Han S. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 2013;339:247–59. [DOI] [PubMed] [Google Scholar]
- 10. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 11. Patadji S, Li Z, Pradhan D, Zhao C. Significance of high-risk HPV detection in women with atypical glandular cells on Pap testing: Analysis of 1857 cases from an academic institution. Cancer 2017;125:205–11. [DOI] [PubMed] [Google Scholar]
- 12. Lopalco PL. Spotlight on the 9-valent HPv vaccine. Drug Des Devel Ther. 2017;11:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouyang Y-B, Lu Y, Yue S, Xu L-J, Xiong X-X, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis. 2012;45:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ke G, Liang L, Yang J, Huang X, Han D, Huang S, Zhao Y, Zha R, He X, Wu X. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene 2013;32:3019–27. [DOI] [PubMed] [Google Scholar]
- 15. Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, Servant N, Paccard C, Hupé P, Robert T. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70:1793–803. [DOI] [PubMed] [Google Scholar]
- 16. Jiao X, Zhao L, Ma M, Bai X, He M, Yan Y, Wang Y, Chen Q, Zhao X, Zhou M. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2). Breast Cancer Res Treat. 2013;139:717–30. [DOI] [PubMed] [Google Scholar]
- 17. Liu M, Wang J, Huang H, Hou J, Zhang B, Wang A. miR-181a–Twist1 pathway in the chemoresistance of tongue squamous cell carcinoma. Biochem Biophys Res Commun. 2013;441:364–70. [DOI] [PubMed] [Google Scholar]
- 18. Bai H, Cao Z, Deng C, Zhou L, Wang C. miR-181a sensitizes resistant leukaemia HL-60/Ara-C cells to Ara-C by inducing apoptosis. J Cancer Res Clin Oncol. 2012;138:595–602. [DOI] [PubMed] [Google Scholar]
- 19. Niu Z, Wang M, Zhou L, Yao L, Liao Q, Zhao Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci Rep. 2015;5:16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaira K, Toyoda M, Shimizu A, Mori K, Shino M, Sakakura K, Takayasu Y, Takahashi K, Oyama T, Asao T. Expression of ER stress markers (GRP78/BiP and PERK) in patients with tongue cancer. Neoplasma 2016;63:588–94. [DOI] [PubMed] [Google Scholar]