Abstract
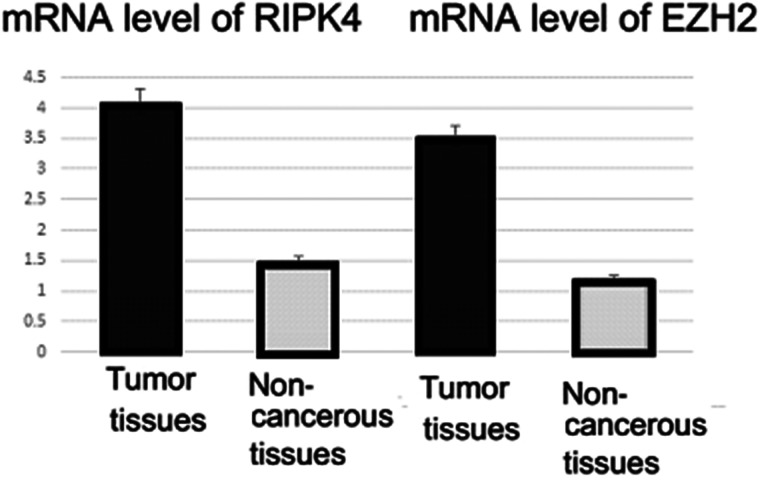
The investigation of specific genes will establish more useful biomarkers for accurate detection and management of gynecological cancers, especially patients with cervical cancer (CCP). The aim of this study was to evaluate the expression level of RIPK4 and EZH2 messenger RNA (RIPK4 and EZH2 mRNA) in CCP. Expression of RIPK4 and EZH2 in the tissues was determined by immunohistochemistry and qRT-PCR methods. Correlations of RIPK4 and EZH2 mRNA with clinical and pathological parameters were analyzed using the Fisher’s exact test. The mRNA level of RIPK4 was significantly upregulated in tumor tissues compared with matched adjacent normal tissues (4.10 ± 0.89 vs. 1.5 ± 0.82; p = 0.021). EZH2 mRNA was increased in cancer tissues compared to normal tissues (3.54 ± 0.71 vs. 1.2 ± 0.65; p = 0.003). High expression of RIPK4 was observed in 25 patients (64.1%), whereas weak expression was seen in 14 cases (35.9%). Furthermore, the expression of RIPK4 was overexpressed in matched adjacent normal tissues (p = 0.004). FIGO stage and lymph node metastasis were significantly linked to a higher expression of RIPK4 (p < 0.05). Overexpression of EZH2 was found in 30 patients (76.9%) and was associated with FIGO stage, histological type, and lymph node metastasis (p < 0.05). In conclusion, our data suggest that RIPK4/EZH2 markers might be used as potential predictors of prognosis in cervical cancer.
Key words: Cervical cancer, Marker, Patient, Pathology, Immunohistochemistry (IHC)
INTRODUCTION
Cervical cancer is the third most prevalent cancer in women worldwide. The complex molecular mechanism in the pathogenesis of cervical cancer is poorly understood1,2. Despite advances in therapeutic strategies, the clinical outcome in patients with advanced-stage disease remains poor. Clinical outcomes are different between patients and can be difficult to predict.
Thus, identification of molecular mechanisms, genetics, and other valuable factors in the pathogenesis of cervical cancer is important for improvement of therapeutic strategies3. It is urgent to find markers that supply data for the prognosis of patients. Receptor-interacting serine/threonine-protein kinase 4 (RIPK4) belongs to the RIP kinase family and is encoded by the RIPK4 gene4–6. Ripk4 mutant is associated with autosomal recessive Bartsocas–Papas syndrome7,8. Moreover, RIPK4 was found to regulate the Wnt signaling pathway by phosphorylating Dishevelled proteins, where it promotes the stabilization of β-catenin via phosphorylating DVL2. As a matter of fact, current evidence recently indicated that RIPK4 can promote Wnt signaling, and increased expression of RIPK4 might have a remarkable impact on the growth of Wnt-dependent tumors.
Previous studies indicated that RIPK4 was overexpressed in many kinds of cancers, such as skin, ovarian, cervical, squamous cell, and colorectal cancers9,10. Huang9 found that RIPK4 knockdown is associated with the suppression of growth in Wnt-dependent N-Tera2 xenograft tumor model, indicating that RIPK4 overexpression may be involved in the development of certain tumor types. These findings indicate the oncogenic role of RIPK4. Low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs) are a series of precancerous lesions for cervical squamous cell carcinoma. It has been demonstrated that RIPK4 might be a valuable biomarker for distinguishing HSIL from chronic cervicitis/LSIL, with a high sensitivity and specificity. Studies explored that high expression of RIPK4 can act as a potential prognostic marker for cervical squamous cell cancer10. p16INK4A, a surrogate marker of HPV infection, and MIB-1, a marker of cell proliferation, have been recently suggested to be the most useful in daily practice. These two biomarkers have been shown to identify whether an SIL is present, differentiating it from normal, atrophic, reactive, or immature metaplastic epithelium10. On the other hand, specific molecular biomarkers are required for predicting patient prognosis in cervical cancer. However, evaluation is needed to find the association of RIPK4 expression with clinical outcome in patients with cervical cancer. Enhancer of zeste homolog 2 (EZH2) is a Polycomb group of genes that play an important role in cell cycle regulation. EZH2 has been implicated in different cell processes, including cell cycle, senescence, tumor proliferation, metastasis, and angiogenesis11–13. EZH2 expression has been reported to be increased in several types of tumors including prostate, breast, and bladder cancers, as well as brain tumors14–17. Moreover, EZH2 is correlated with epidermal–mesenchymal transformation and angiogenesis, leading to an advanced metastatic form of tumor cells. It has been reported that EZH2 can be linked to tumorigenesis via silencing of tumor suppressor genes. However, its entire function in the development of cancer needs further details18,19. The present study was aimed to evaluate the mRNA and protein expression levels of RIPK4/EZH2, and their clinical significance was investigated in patients with cervical cancer.
MATERIALS AND METHODS
Patient and Tissue Specimens
The samples of cervical cancer tissue and matched adjacent normal tissues were collected from 39 patients who underwent surgical resection between 2007 and 2013 in Tehran, Tabriz, and Mashhad Hospitals, Iran. None of the patients received chemotherapy or radiotherapy or had other treatment history. The samples were put immediately into liquid nitrogen and stored at −80°C until use. For histological examination, all tumorous and adjacent normal tissue portions were collected. Pathological tumor staging refers to the FIGO (Federation of Gynecology and Obstetrics) stage. The clinical pathological data of the patients are summarized in Table 1.
Table 1.
Correlation of RIPK4 Expression With Clinicopathological Parameters
| Variables | No. of Cases | No. Expression of RIPK4 | p Value of RIPK4 | |
|---|---|---|---|---|
| High = 25 | Low = 14 | |||
| Age | 0.57 | |||
| <50 | 23 | 15 | 8 | |
| ≥50 | 16 | 10 | 6 | |
| Tumor size (cm) | 0.63 | |||
| <4 | 20 | 13 | 7 | |
| ≥4 | 19 | 12 | 7 | |
| Histological grades | 0.43 | |||
| Well/moderate differentiated | 18 | 13 | 5 | |
| Poorly differentiated | 21 | 12 | 9 | |
| FIGO stage | <0.05 | |||
| Ib–Iia | 22 | 9 | 13 | |
| IIb–IIIa | 17 | 16 | 1 | |
| Lymph node metastasis | <0.05 | |||
| No | 25 | 12 | 13 | |
| Yes | 14 | 13 | 1 | |
RNA Extraction and qRT-PCR
Total RNA was extracted from tissues using TRIzol reagent (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The markers cDNA was synthesized from total RNA using the Reverse Transcription Kit (Applied Biosystems). Real-time quantitative polymerase chain reactions (qRT-PCRs) were performed using the SYBRGreen PCR Master Mix on Applied Biosystems 7500 Real-Time PCR System. GAPDH was used as a loading control, and the comparative cycle threshold (CT) method was established for the evaluation of relative mRNA expression levels.
Immunohistochemistry
Immunohistochemical staining for RIPK4/EZH2 expressions was performed on 4-μm formalin-fixed paraffin-embedded tissue sections. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min at room temperature. After antigen retrieval in citrate buffer (0.01 M, pH 6.0), primary antibodies were applied to the sections overnight at 4°C [mouse monoclonal anti-RIPK (Abcam, Cambridge, MA, USA); rabbit polyclonal anti-EZH2]. The sections were incubated with a biotinylated secondary antibody for 20 min at 37°C. Sections were stained using DAB. The percentage of positive tumors was scored as follows: stained cell ≤5%, scored 0; between 6% and 25%, scored 1; between 26% and 50%, scored 2; between 51% and 75%, scored 3; >75%, scored 4. Staining intensity was graded as follows: negative (0) and weak staining (1+) were grouped together as negative, and moderate (2+) and strong staining (3+) were grouped together as positive.
Statistical Analysis
SPSS 18.0 software was applied for statistical analysis (SPSS Inc., Chicago, IL, USA). The differences between expression levels were analyzed using the Fisher’s exact test. Correlations between expression and the clinicopathological characteristics were also evaluated using the chi-square test. Log-rank test and Kaplan–Meier method were used for survival analysis. Multivariate analyses of prognostic values were evaluated by Cox proportional hazards model. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
mRNA and Protein Expression
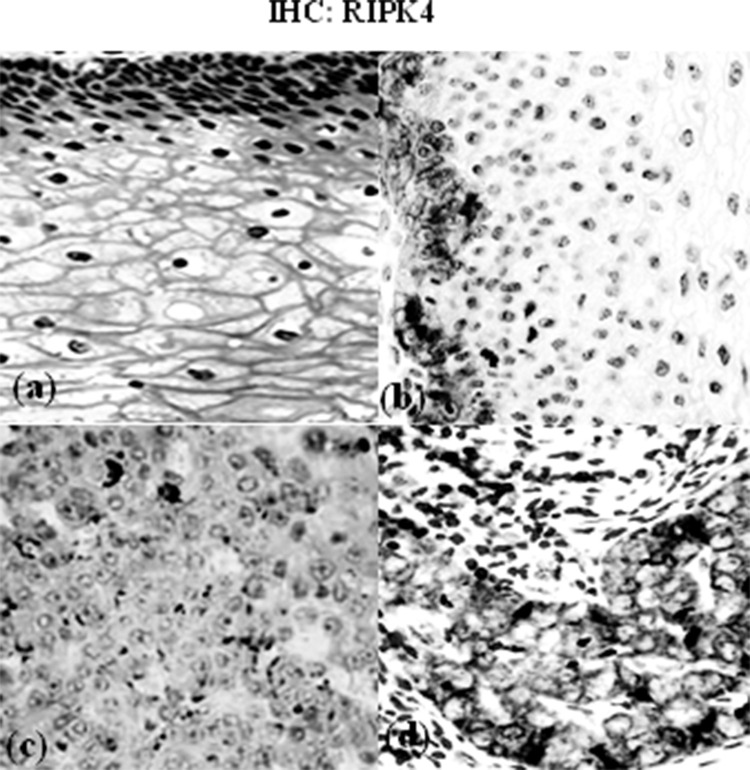
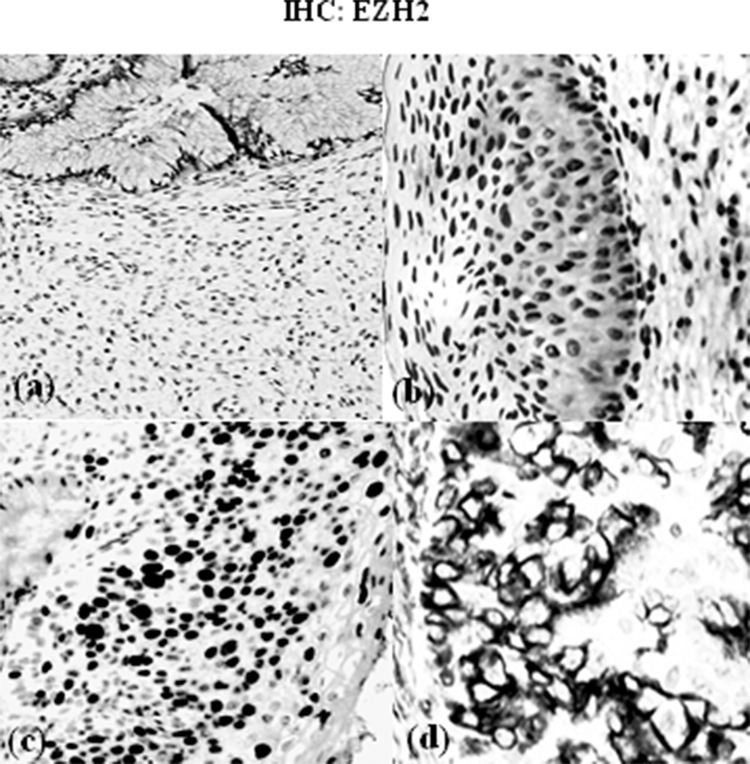
The mRNA levels of two markers were evaluated in cancer tissues and matched adjacent normal tissues using qRT-PCR assay. The mRNA level of RIPK4 was significantly upregulated in tumor tissues compared with matched adjacent normal tissues (4.10 ± 0.89 vs. 1.5 ± 0.82; p = 0.021) (Figs. 1 and 2). EZH2 mRNA was increased in cancer tissues compared to normal tissues (3. 54 ± 0.71 vs. 1.2 ± 0.65; p = 0.003) (Fig. 1). By immunohistochemistry, we observed that protein expression of RIPK4 was increased in 25 patients (64.1%), while weak expression rate of the protein was 35.9% (14 cases) (Figs. 1 and 3). Moreover, our results indicated that the expression of RIPK4 was decreased in matched adjacent normal tissues (p = 0.004). The clinicopathological factors of patients can be seen in Tables 1 and 2. Statistical analyses indicated that FIGO stage and lymph node metastasis were significantly associated with higher expression of RIPK4 (p < 0.05). However, There was no significant association with age, gender, histological grade, or tumor size (all p > 0.05) (Table 1). Increased expression of EZH2 was found in 30 patients (76.9%). Meanwhile, these results suggested that weak expression rate of the protein was found in 23% of the patients (nine cases). On the other hand, expression of EZH2 was remarkably decreased in matched adjacent normal tissues (p = 0.002). Statistical analyses indicated that high expression of EZH2 was positively associated with FIGO stage, histological type, and lymph node metastasis (p < 0.05) (Table 2).
Figure 1.
Immunohistochemistry: RIPK4. Microphotographs showing the immunohistochemical expression of RIPK4 in cervical tissues. (a) Negative expression in nontumor tissue (400×), (b) weak expression in tumor tissue (200×), (c) moderate expression in tumor tissue (200×), and (d) strong expression in tumor tissue (400×).
Figure 2.
Immunohistochemistry: EZH2. Microphotographs showing the immunohistochemical expression of EZH2 in cervical tissues. (a) Negative expression in nontumor tissue (100×), (b) weak expression in tumor tissue (400×), (c) moderate expression in tumor tissue (200×), and (d) strong expression in tumor tissue (400×).
Figure 3.
Relative expression of the mRNA level of RIPK4 and EZH2 in tumor tissues compared with matched adjacent normal tissues.
Table 2.
Correlation of EZH2 Expression With Clinicopathological Parameters
| Variables | No. of Cases | No. Expression of EZH2 | p Value of EZH2 | |
|---|---|---|---|---|
| High = 30 | Low = 9 | |||
| Age | 0.44 | |||
| <50 | 23 | 16 | 7 | |
| ≥50 | 16 | 14 | 2 | |
| Tumor size (cm) | 0.46 | |||
| <4 | 20 | 13 | 7 | |
| ≥4 | 19 | 17 | 2 | |
| Histological grades | <0.05 | |||
| Well/moderate differentiated | 18 | 9 | 9 | |
| Poorly differentiated | 21 | 21 | 0 | |
| FIGO stage | <0.05 | |||
| Ib–IIa | 22 | 13 | 9 | |
| IIb–IIIa | 17 | 17 | 0 | |
| Lymph node metastasis | <0.05 | |||
| No | 25 | 17 | 8 | |
| Yes | 14 | 13 | 1 | |
Association of Protein Expression With Overall Survival
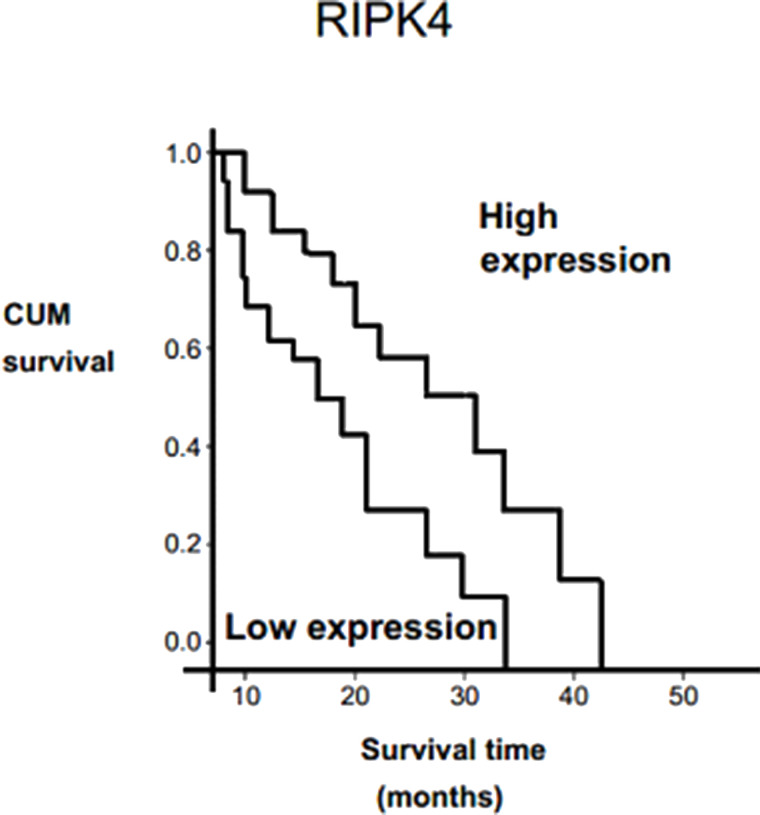
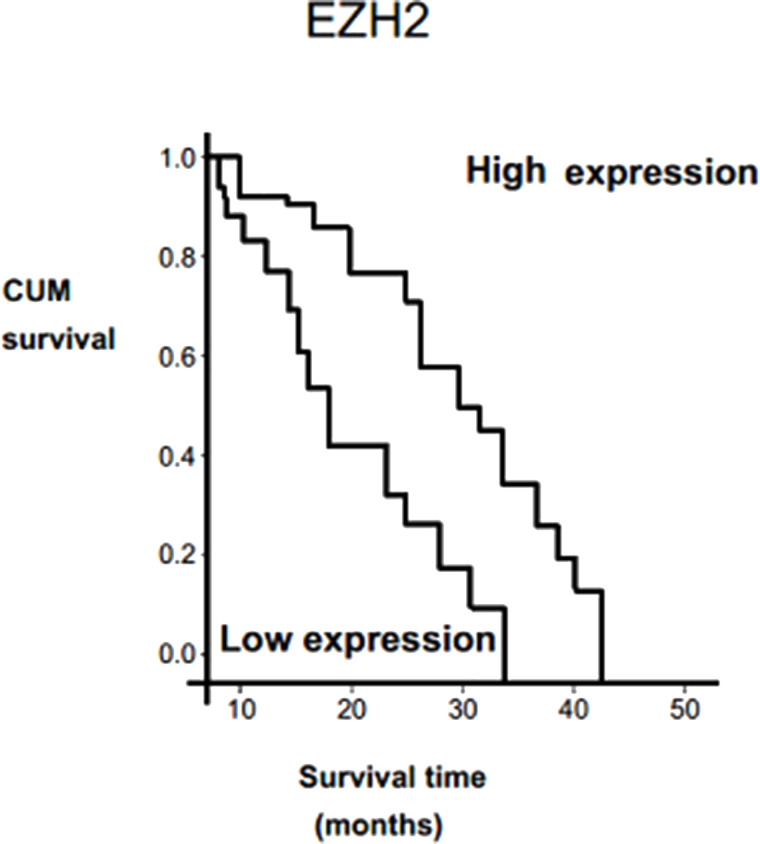
In order to identify the prognostic value of markers for cervical cancer, we evaluated the correlation between the high expressions of RIPK4/EZH2 and the patient’s survival time using Kaplan–Meier analysis with the log-rank test. Our findings indicated that high expression of RIPK4 was associated with shorter overall survival (p < 0.021) (Fig. 4). Furthermore, patients with increased expression of EZH2 had shorter overall survival (p = 0.102) (Fig. 5). Univariate analysis indicated that high expressions of RIPK4/EZH2, FIGO stage, and lymph node metastasis were associated with overall survival (p = 0.001, 0.005, 0.002, and 0.035, respectively). Finally, multivariate Cox proportional hazards model revealed that high RIPK4/EZH2 and FIGO stage expressions were independent prognostic factors for cervical cancer by overall survival (p = 0.023, p = 0.37) (Table 3).
Figure 4.
Survival analyses for RIPK4. Overall survival between patients with expression levels of RIPK4 was estimated using the Kaplan–Meier method and compared by the log-rank test.
Figure 5.
Survival analyses for EZH2. Overall survival between patients with expression levels of EZH2 was estimated using the Kaplan–Meier method and compared by the log-rank test.
Table 3.
Multivariate Analysis for Overall Survival by Cox Proportional Hazards Model
| Clinicopathological Characteristics | HR | 95% CI | p Value |
|---|---|---|---|
| Gender | 0.80 | 0.7232–1.25 | 0.73 |
| Age | 0.67 | 0.553–1.415 | 0.67 |
| Tumor size (cm) | 0.57 | 0.71–1.13 | 0.75 |
| Histologic type | 1.03 | 0.94–1.62 | 0.37 |
| FIGO stage | 3.01 | 1.46–9.34 | 0.003 |
| Lymph node metastasis | 1.27 | 1.1–1.76 | 0.511 |
| RIPK4 expression | 3.4 | 1.81–11.4 | 0.002 |
| EZH2 expression | 2.91 | 1.51–9.32 | 0.005 |
DISCUSSION
We investigated the expression level of RIPK4 in cervical cancer by semiquantitative PCR, in addition to the clinicopathologic and prognostic significance of RIPK4. RIPK4 could be a useful biomarker to improve diagnostic specificity for HSIL in cervical squamous cell carcinoma patients. Furthermore, the diagnostic efficiency of RIPK4 for HSIL with p16INK4a and Ki-67 has been recently indicated. Moreover, combined staining of RIPK4 and p16INK4a was revealed to be associated with the highest diagnostic value for distinguishing HSIL from LSIL and chronic cervicitis10.
With regard to RIPK4 usefulness as a diagnostic marker for HSILs, we focused our efforts on investigating RIPK4 expression in cervical cancer and further explored the clinical significance of RIPK4 in the development and progression of cervical cancer. Our results showed that the mRNA level of RIPK4 was significantly upregulated in tumor tissues compared to that in matched adjacent normal tissues. High expression of RIPK4 was observed in 64.1% of cervical cancer tissues, whereas expression of RIPK4 was decreased in matched adjacent normal tissues. High expression of RIPK4 can act as a potential prognostic marker for cervical squamous cell cancer10.
Previous research has shown RIPK4 overexpression in many types of cancers, such as skin, ovarian, cervical squamous cell carcinoma, and colorectal cancers9,10. High RIPK4 mRNA level has been defined in ovarian, skin, and colorectal cancers9,10.
It has been indicated that phosphorylation of Dishevelled proteins by RIPK4 regulates the Wnt signaling pathway, and increased RIPK4 expression contributes to the progression of ovarian cancer in a xenograft tumor model20, indicating an oncogenic role for RIPK4.
On the other hand, correlation of low RIPK4 expression with the loss of differentiation has been reported in tongue squamous cell cancer, indicating a tumor suppressor role for RIPK421,22. RIPK4 expression was found to be correlated with tongue squamous cell carcinoma due to degree of differentiation, age, and gender22. This explanation can account for this apparently conflicting finding. RIPK4 may be involved in various carcinogenic mechanisms in various types of tumors. In the present study, FIGO stage and lymph node metastasis were significantly linked to high expression of RIPK4. Furthermore, high RIPK4 expression was reported to be correlated with clinical stage in cervical squamous cell carcinoma patients10. This is in accordance with the present study.
Our findings indicated that a high expression of RIPK4 was associated with shorter overall survival. RIPK4 overexpression was also observed in several types of human cancers. Our data are in agreement with previous studies, reporting that RIPK4 expression was significantly related to overall survival and disease-free survival10. However, further studies are needed to clarify the role RIPK4 in different kinds of cancers, and little is known about RIPK4 in cervical cancer patients.
Our results showed that EZH2 mRNA was increased in cancer tissues compared to normal tissues. Overexpression of EZH2 was found in 76.9% of patients, whereas expression of EZH2 was decreased in matched adjacent normal tissues. Moreover, overexpression of EZH2 was positively associated with FIGO stage, histological type, and lymph node metastasis.
EZH2 expression has been reported to be linked to the invasive and aggressive nature of tumors23. EZH2 expression has been reported in clinical specimens of several kinds of tumors including prostate, breast, and bladder cancers, as well as brain tumors14–17. EZH2 is correlated with epidermal–mesenchymal transformation and angiogenesis, leading to an advanced metastatic form of tumor cells. Moreover, it has been suggested that EZH2 can be linked to tumorigenesis via silencing of tumor suppressor genes. However, its entire function in the development of cancer needs further details18,19,24. High expression of EZH2 was related to aggressive tumor subgroups, clinical progress, and shorter survival in cutaneous melanoma and cancers of the endometrium, prostate, and breast25. A previous study in cervical cancer indicates that EZH2 was strongly related to clinical stage, infiltration depth, lymph node metastasis, and histologic differentiation in cervical cancer26. This result was more or less in accordance with the findings of our study. Furthermore, high EZH2 expression was significantly related to differentiation, depth, and lymphatic invasion in cervical cancer23.
Although previous studies have reported a significant correlation between EZH2 and tumor differentiation in cervical squamous cell carcinoma patients, we did not find any correlation between the EZH2 expression and tumor differentiation in cervical cancer. The discordance in this study may be associated with clinical short cohort of cervical cancer specimens.
We found that patients with increased expression of EZH2 had shorter overall survival, which is entirely consistent with the findings of Liu et al. in cervical cancer. They indicated that EZH2+ expression was correlated with shorter overall survival compared with those with EZH2− expression26. EZH2 function in the development of cancers needs further details. Univariate analysis indicated that a high expression of RIPK4/EZH2, FIGO stage, and lymph node metastasis were associated with overall survival. The multivariate Cox proportional hazards model revealed that high RIPK4/EZH2 and FIGO stage expressions were independent prognostic factors for cervical cancer by overall survival.
In conclusion, our results suggest that these markers might be used as potential predictors of prognosis in cervical cancer.
REFERENCES
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MHF, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 2011;378:1461–84. [DOI] [PubMed] [Google Scholar]
- 3. Shen SN, Wang LF, Jia YF, Hao YQ, Zhang L, Wang H. Upregulation of microRNA-224 is associated with aggressive progression and poor prognosis in human cervical cancer. Diagn Pathol. 2013;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang LQ, Zhang Y, Yan H, Liu KJ, Zhang S. MicroRNA-373 functions as an oncogene and targets YOD1 gene in cervical cancer. Biochem Biophys Res Commun. 2015;459:515–20. [DOI] [PubMed] [Google Scholar]
- 5. Holland P. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation. Curr Biol. 2002;12:1424–8. [DOI] [PubMed] [Google Scholar]
- 6. Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS. The DNA sequence of human chromosome 21. Nature 2000;405:311–9. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell K. Exome sequence identifies RIPK4 as the Bartsocas-Papas syndrome locus. Am J Hum Genet. 2012;90:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalay E. Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am J Hum Genet. 2012;90:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang X. Phosphorylation of Dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science 2013;339:1441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu DQ, Li FF, Zhang JB, Zhou TJ, Xue WQ, Zheng XH. Increased RIPK4 expression is associated with progression and poor prognosis in cervical squamous cell carcinoma patients. Scientific Reports 2015;5:11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi JH, Song YS, Yoon JS. Enhancer of zeste homolog 2 expressions is associated with tumor cell proliferation and metastasis in gastric cancer. APMIS 2010;118:196–202. [DOI] [PubMed] [Google Scholar]
- 12. Lu C, Han HD, Mangala LS. Regulation of tumor angiogenesis by EZH2. Cancer Cell 2010;18:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan T, Jiang S, Chung N. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer Res. 2011;9:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varambally S, Dhanasekaran SM, Zhou M. The Polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624–29. [DOI] [PubMed] [Google Scholar]
- 15. Weikert S, Christoph F, Köllermann J. Expression levels of the EZH2 Polycomb transcriptional repressor correlates with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med. 2005;16:349–53. [PubMed] [Google Scholar]
- 16. Kleer CG, Cao Q, Varambally S. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003;100:11606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol Cancer 2010;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 2006;6:846–56. [DOI] [PubMed] [Google Scholar]
- 19. Crea F, Fornaro L, Bocci G, Sun L, Farrar WL, Falcone A, Danesi R. EZH2 inhibition: Targeting the crossroad of tumor invasion and angiogenesis. Cancer Metastasis Rev. 2012;31:753–61. [DOI] [PubMed] [Google Scholar]
- 20. Huang X, McGann JC, Liu BY, Hannoush RN, Lill JR, Pham V. Phosphorylation of Dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science 2013;339:1441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heim D, Cornils K, Schulze K, Fehse B, Lohse AW, Brümmendorf TH. Retroviral insertional mutagenesis in telomerase-immortalized hepatocytes identifies RIPK4 as novel tumor suppressor in human hepatocarcinogenesis. Oncogene 2015;34:364–72. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Zhu W, Zhou Y, Xu W, Wang H. RIPK4 is downregulated in poorly differentiated tongue cancer and is associated with migration/invasion and cisplatin-induced apoptosis. Int J Biol Markers 2014;29:e150–9. [DOI] [PubMed] [Google Scholar]
- 23. Fang J, Zhang M, Li Q. Enhancer of zeste homolog 2 expression is associated with tumor cell proliferation and invasion in cervical cancer. Am J Med Sci. 2011;342:198–204. [DOI] [PubMed] [Google Scholar]
- 24. Varambally S, Dhanasekaran SM, Zhou M. The Polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624–9. [DOI] [PubMed] [Google Scholar]
- 25. Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Liu T, Bao X, He M, Li L, Yang X. Increased EZH2 expression is associated with proliferation and progression of cervical cancer and indicates a poor prognosis. Int J Gynecol Pathol. 2014;33:218–24 [DOI] [PubMed] [Google Scholar]