Abstract
Zinc and ring finger 3 (ZNRF3), which belongs to the E3 ubiquitin ligase family, is involved in the progression and development of cancer. However, the expression and function of ZNRF3 in human nasopharyngeal carcinoma (NPC) remain unclear. Thus, the aim of this study was to investigate the role of ZNRF3 in human NPC. Our results showed that ZNRF3 was downregulated in NPC cell lines. Restoration of ZNRF3 significantly inhibited the proliferation of NPC cells and tumor xenograft growth in vivo. In addition, overexpression of ZNRF3 suppressed migration and invasion, as well as attenuated the epithelial–mesenchymal transition (EMT) process in NPC cells. Furthermore, restoration of ZNRF3 obviously downregulated the expression levels of β-catenin, cyclin D1, and c-Myc in NPC cells. In conclusion, these data suggest that ZNRF3 inhibited the metastasis and tumorigenesis via suppressing the Wnt/β-catenin signaling pathway in NPC cells. Thus, ZNRF3 may act as a novel molecular target for the treatment of NPC.
Key words: Zinc and ring finger 3 (ZNRF3), Nasopharyngeal carcinoma (NPC), Proliferation, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer that has a high prevalence, 20–50 cases per 100,000 individuals, in the southern China region1. Currently, radiotherapy is the most effective treatment for NPC, but the prognosis is often not satisfactory due to the rates of recurrence and metastasis2. The majority of NPC patients are diagnosed in the late stages, and 70% of patients present with cervical lymph node metastasis at first consultation3. Thus, the investigation of molecular mechanisms of NPC progression and metastasis is imperative in order to identify potential targets for the treatment of NPC.
E3 ubiquitin ligases, a large family of proteins, regulate the turnover and activity of many target proteins4. A large body of evidence has shown that E3 ubiquitin ligases regulate a variety of biological processes including cell cycle regulation, proliferation, and apoptosis5–7. Zinc and ring finger 3 (ZNRF3) belongs to the E3 ubiquitin ligase family, which negatively regulates Wnt signaling. It is associated with the Wnt receptor complex and inhibits Wnt signaling by promoting the turnover of frizzled and LRP6 receptors8. Accumulating data have strongly suggested that ZNRF3 is involved in cancer development and is lowly expressed in many human cancers9–11. Shi et al. confirmed that the ZNRF3 level is reduced in lung carcinoma, and its expression level is positively correlated with the survival of lung cancer patients. Restoration of ZNRF3 inhibited the proliferation and cell cycle progression in vitro, as well as attenuated the growth of lung cancer xenografts12. However, the expression and function of ZNRF3 in human NPC remain unclear. Thus, the aim of this study was to investigate the role of ZNRF3 in human NPC. Our data showed that ZNRF3 inhibited the metastasis and tumorigenesis via suppressing the Wnt/β-catenin signaling pathway in NPC cells.
MATERIALS AND METHODS
Cell Culture
Human NPC cell lines (SUNE-6-10B, SUNE-5-8F, and CNE-1) and the control cell line NP69 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All of the cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD), 100 IU/ml streptomycin, and 100 IU/ml penicillin (Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere (37°C, 5% CO2).
Total RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted from NPC cell lines using TRIzol reagent (Takara, Dalian, P.R. China) following the manufacturer’s instructions, and 5 μg of RNA of each sample was reverse transcribed using SuperScript RT kit (Invitrogen). All the qPCRs were performed on a StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The following primers were used: ZNRF3, 5′-GCGGGTCATCCCCTGTAC-3′ (sense) and 5′-GCTTGGGTTTCCCTTTTGTT-3′ (antisense); β-actin, 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ (sense) and 5′-CTGTCACCTTCACCGTTCCAGTTT-3′ (antisense). The band intensities of amplification products were measured by a densitometer, and the results were normalized with β-actin.
Western Blotting Analysis
Proteins were extracted from NPC cell lines using RIPA lysis buffer containing phosphatase and protease inhibitors (Sigma-Aldrich). A total of 20 μg of proteins was separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. After blocking with 5% nonfat milk in Tris-buffered saline with Tween (TBST; 10 mM Tris-HCl, pH of 7.5, 150 mM NaCl, and 0.05% Tween 20) for 1 h at room temperature, the membranes were incubated with various primary antibodies against ZNRF3, E-cadherin, N-cadherin, β-catenin, cyclin D1, c-Myc, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The following day, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The target protein was visualized using an enhanced chemiluminescence (ECL) detection system (Amersham, Little Chalfont, UK).
Construction of Plasmids and Transfection
The full-length ZNRF3 cDNA was cloned into the pcDNA3.1 vector (Genechem, Shanghai, P.R. China). CNE-1 cells were transfected with ZNRF3 or vector using Lipofectamine™ 2000 (Invitrogen), according to the manufacturer’s protocols.
Cell Proliferation Assay
Cell proliferation was detected using a cell counting kit-8 (CCK-8) assay. Infected cells at a density of 3 × 104 cells/well were seeded into 96-well culture plates. Next, we added 10 μl of reagent from CCK-8 (Dojindo, Kumamoto, Japan) to each well for detection at days 1, 2, 3, and 4. After 1 h of incubation at 37°C, the absorbance was measured at 490 nm with a microplate reader (Bio-Rad, San Diego, CA, USA).
In Vitro Migration and Invasion Assays
For the Transwell migration assay, infected CNE-1 cells (5 × 104 cells/well) suspended in 0.1% FBS medium were placed in the top chamber, while the lower chamber of the Transwell plates was filled with 600 μl of RPMI medium containing 10% FBS. After 24 h, cells on the upper membrane of the inserts were removed using cotton swabs. Cells that migrated to the lower surface of the filters were fixed, stained with crystal violet, and counted under a microscope (Olympus Corp., Tokyo, Japan). The invasion assay was done using the same procedure, except that the membrane was coated with Matrigel (BD Biosciences, Bedford, MA, USA) to form a matrix barrier.
Tumorigenesis in Nude Mice In Vivo
All of the animal experiments were approved by the Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Xi’an Jiaotong University. Female Balb/c nude mice (4–5 weeks of age, 18–20 g) were purchased from the Laboratory Animal of The Second Affiliated Hospital of Xi’an Jiaotong University (P.R. China). CNE-1 cells transfected with ZNRF3 and the corresponding control cells (5 × 106) were suspended in 200 μl of PBS and then injected subcutaneously into the left axilla of the mice (five mice/group). Tumor volumes were measured with calipers every 5 days after injection. The tumor volume was calculated according to the following formula: V = L × W 2/2, where V is the volume (mm3), L is the biggest diameter (mm), and W is the smallest diameter (mm). After 25 days, all of the mice were sacrificed, and the tumor tissues were excised and weighed.
Statistics Analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA). A value of p < 0.05 was considered to be statistically significant.
RESULTS
The Expression of ZNRF3 in NPC Cell Lines
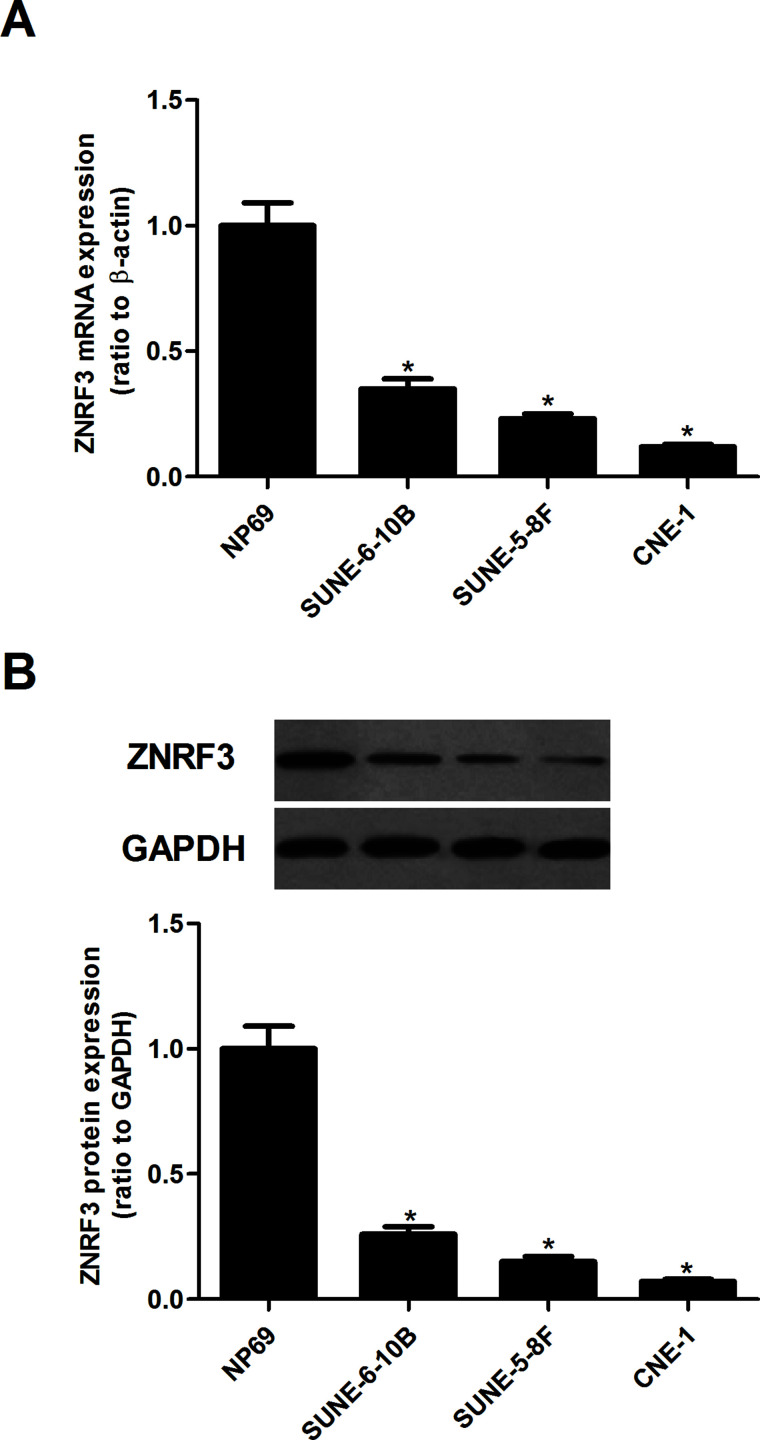
We first examined the expression of ZNRF3 in human NPC cell lines using qRT-PCR and Western blotting. The results of the qRT-PCR analysis indicated that the mRNA expression of ZNRF3 was significantly decreased in human NPC cell lines, compared with the normal laryngeal epithelia cell line (Fig. 1A). Similarly, the Western blotting analysis showed that ZNRF3 protein expression was also downregulated in human NPC cell lines (Fig. 1B).
Figure 1.
The expression of ZNRF3 in NPC cell lines. (A) qRT-PCR was performed to analyze the mRNA expression of ZNRF3 in human NPC cell lines (SUNE-6-10B, SUNE-5-8F, and CNE-1). (B) Western blotting analysis was used for the detection of the protein expression of ZNRF3 in human NPC cell lines. Data were expressed as mean ± SD (n = 3). *p < 0.05 versus NP69.
Overexpression of ZNRF3 Inhibits the Proliferation of NPC Cells
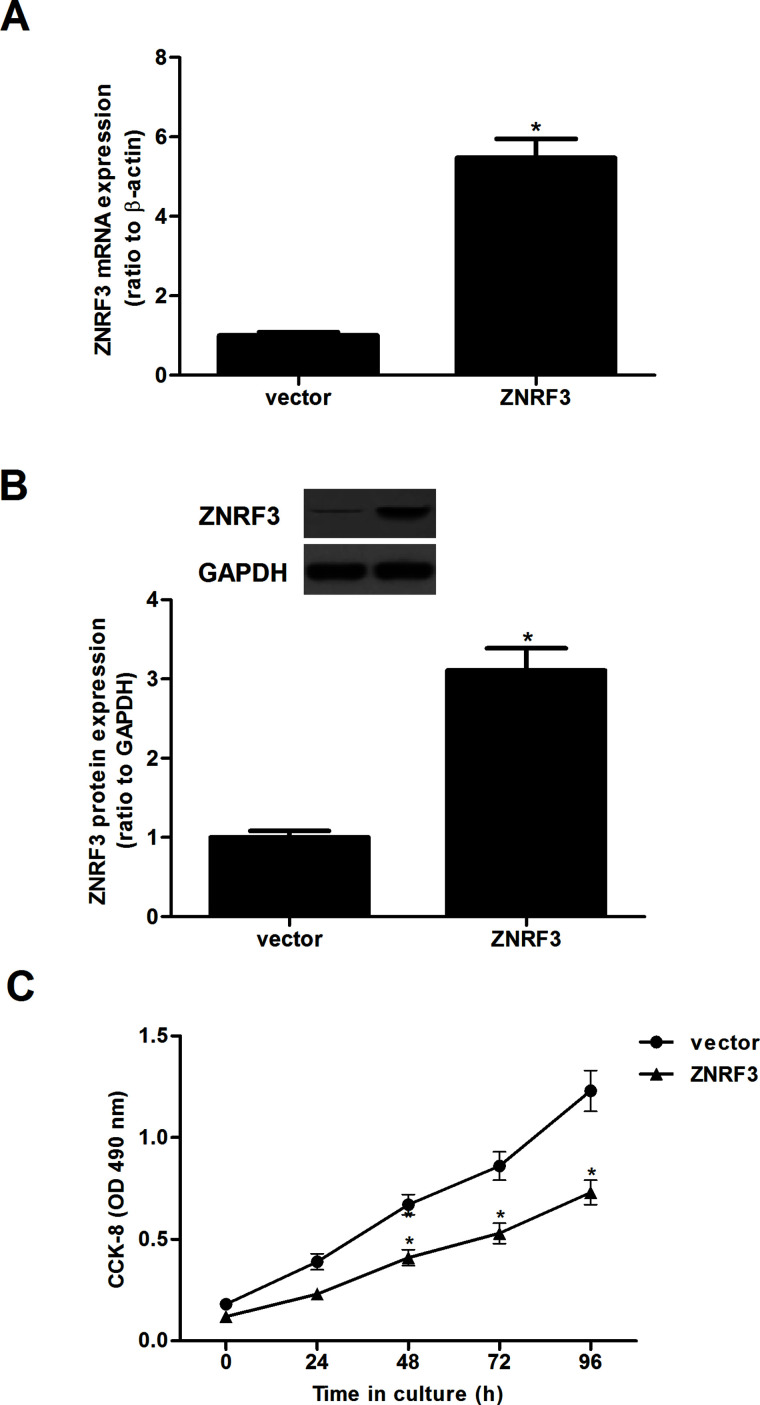
To investigate the role of ZNRF3 in NPC, CNE-1 cells were transfected with ZNRF3 or vector for 24 h, respectively. The transfection efficiency was confirmed by qRT-PCR and Western blot. CNE-1 cells transfected with ZNRF3 showed a significant upregulation of ZNRF3 expression at both the mRNA and protein levels (Fig. 2). In addition, the effect of ZNRF3 on NPC cell proliferation was evaluated by the CCK-8 assay. Overexpression of ZNRF3 greatly suppressed the proliferation of CNE-1 cells, compared with the negative control group (Fig. 2C).
Figure 2.
Overexpression of ZNRF3 inhibits the proliferation of NPC cells. CNE-1 cells were transfected with ZNRF3 or vector for 24 h. (A) qRT-PCR was performed to analyze the mRNA expression of ZNRF3 in CNE-1 cells. (B) Western blotting analysis was used for the detection of the protein expression of ZNRF3. (C) Detection of cell proliferation by CCK-8 assay. Data were expressed as mean ± SD (n = 3). *p < 0.05 versus vector.
Overexpression of ZNRF3 Inhibits the Migration and Invasion of NPC Cells
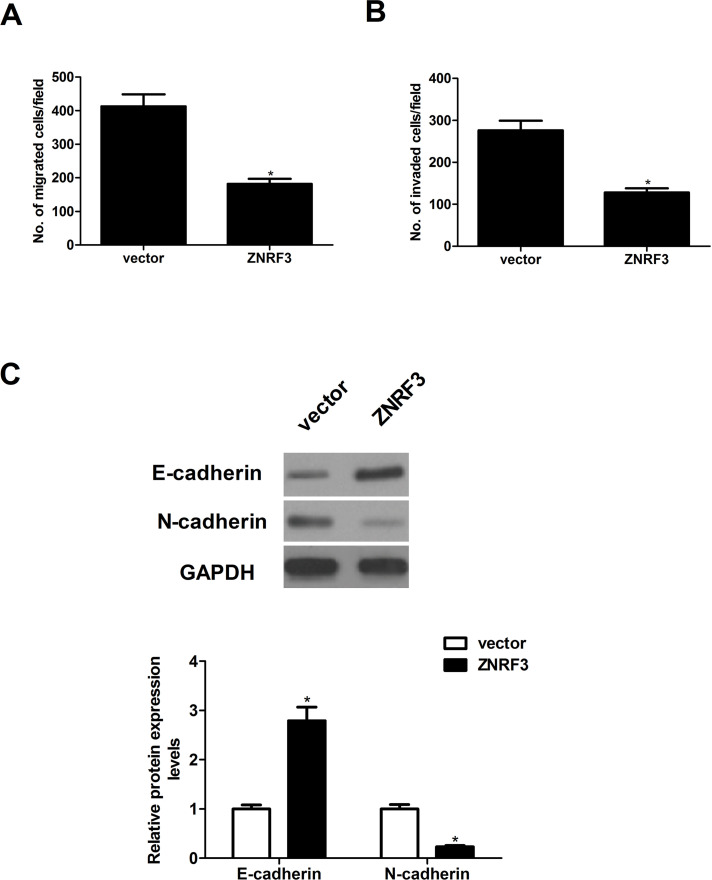
We next determined the potential impact of ZNRF3 on NPC cell migration and invasion. The results of the Transwell migration assay showed that the number of migrated CNE-1 cells was greatly reduced in ZNRF-3-transfected CNE-1 cells when compared with the vector group (Fig. 3A). In the Matrigel invasion assay, the average invading cell count in the ZNRF-3-transfected CNE-1 cells was lower than that in the vector group (Fig. 3B). Then we detected the effect of ZNRF3 on EMT-related molecule expression in CNE-1 cells. The results of the Western blot analysis demonstrated that overexpression of ZNRF3 significantly increased the protein expression of E-cadherin and decreased the protein expression of N-cadherin protein, compared with the vector group (Fig. 3C).
Figure 3.
Overexpression of ZNRF3 inhibits the migration and invasion of NPC cells. CNE-1 cells were transfected with ZNRF3 or vector for 24 h. (A) Cell migration was measured by Transwell migration assay. (B) Cell invasion was assessed by the Matrigel invasion chamber. (C) The protein expression levels of E-cadherin, N-cadherin, and vimentin were evaluated by Western blotting. Data were expressed as mean ± SD (n = 3). *p < 0.05 versus vector.
Overexpression of ZNRF3 Inhibits Xenografted Tumor Growth In Vivo
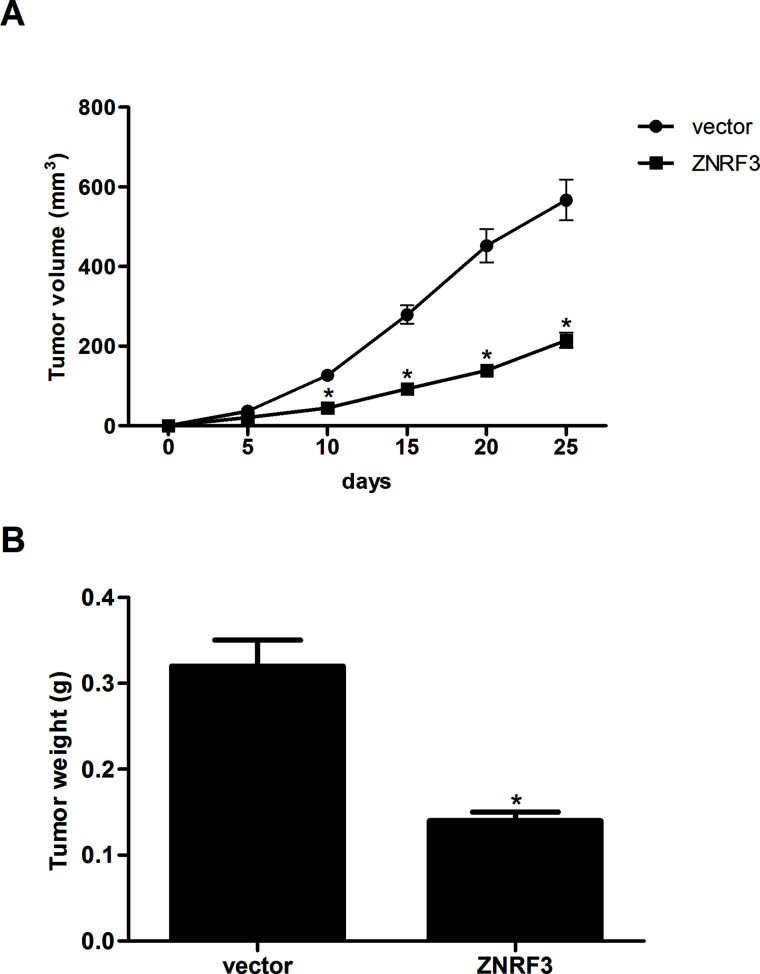
To further examine the role of ZNRF3 on NPC growth in vivo, the xenografted tumor in nude mouse was employed. We observed that overexpression of ZNRF3 remarkably reduced the volume (Fig. 4A) and weight (Fig. 4B) of the xenografted tumor, compared with the vector group.
Figure 4.
Overexpression of ZNRF3 inhibits xenografted tumor growth in vivo. (A) CNE-1 cells transfected with ZNRF3 and the corresponding control cells (5 × 106) were suspended in 200 μl of PBS and then injected subcutaneously into the left axilla of the mice. The tumor volume was measured every 5 days. (B) Twenty-five days after inoculation, mice were euthanized, and the tumors were weighed. Data were expressed as mean ± SD (n = 6). *p < 0.05 versus vector.
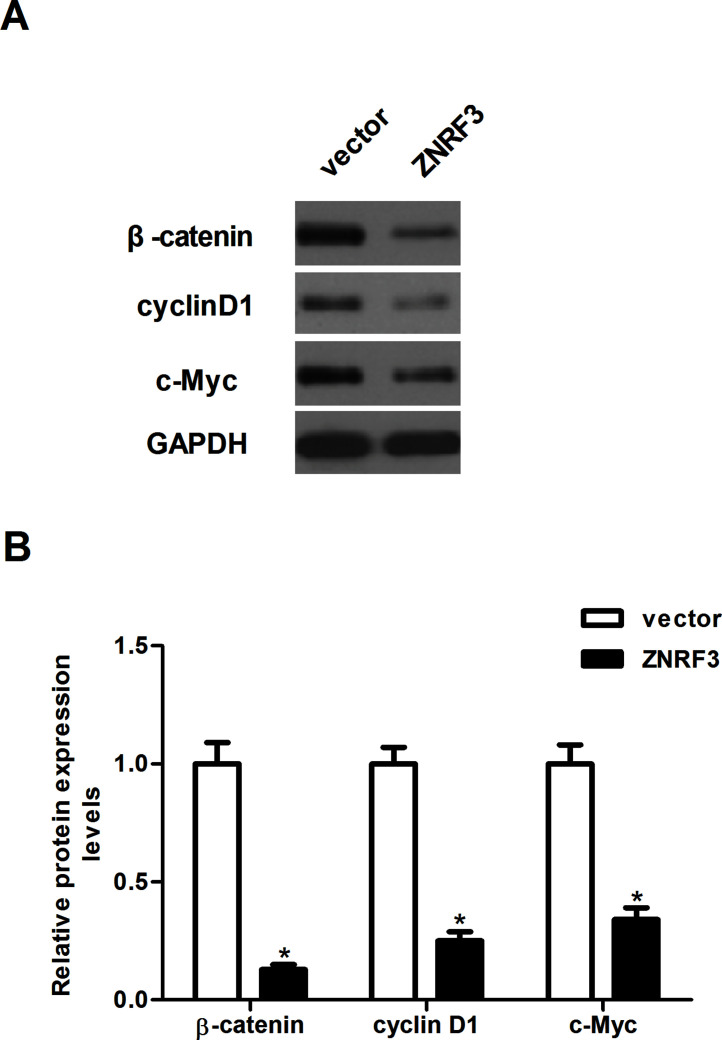
Overexpression of ZNRF3 Inhibits the Activation of Wnt/β-Catenin Pathway in NPC Cells
The Wnt/β-catenin signaling pathway plays an important role in the development and progression of tumors13. Thus, we examined the effect of ZNRF3 on the activation of the Wnt/β-catenin signaling pathway in CNE-1 cells. The results of the Western blotting analysis indicated that overexpression of ZNRF3 significantly downregulated the protein expression levels of β-catenin, cyclin D1, and c-Myc in CNE-1 cells, compared with the vector group (Fig. 5).
Figure 5.
Overexpression of ZNRF3 inhibits the activation of the Wnt/β-catenin pathway in NPC cells. CNE-1 cells were transfected with ZNRF3 or vector for 24 h. (A) The protein expression levels of β-catenin, cyclin D1, and c-Myc were evaluated by Western blotting. (B) Relative protein levels of β-catenin, cyclin D1, and c-Myc were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. GAPDH was used as the internal control. Data were expressed as mean ± SD (n = 3). *p < 0.05 versus vector.
DISCUSSION
In this study, we showed that the expression levels of ZNRF3 were significantly reduced in human NPC cell lines. Overexpression of ZNRF3 suppressed the proliferation of NPC cells and tumor xenograft growth in vivo. In addition, we found that overexpression of ZNRF3 inhibited the migration and invasion of NPC cells, as well as prevented the EMT process. Furthermore, overexpression of ZNRF3 greatly downregulated the expression levels of β-catenin, cyclin D1, and c-Myc in NPC cells.
ZNRF3 has been shown to play a role in cancer development and progression. Qiu et al. reported that ZNRF3 is downregulated in papillary thyroid carcinoma (PTC) compared to normal thyroid tissues, and overexpression of ZNRF3 significantly suppressed cell proliferation, migration, and invasion in vitro, as well as tumor growth in vivo14. Zhou et al. confirmed that the expression of ZNRF3 was significantly decreased in gastric adenocarcinoma tissues compared with adjacent normal gastric tissues, and overexpression of ZNRF3 greatly inhibited gastric adenocarcinoma cell proliferation9. In agreement with the previous studies, we observed that the expression levels of ZNRF3 were significantly reduced in human NPC cell lines. Overexpression of ZNRF3 suppressed the proliferation of NPC cells and tumor xenograft growth in vivo. These data suggest that ZNRF3 may act as a tumor suppressor in the development and progression of NPC.
NPC has a high rate of local invasion and early metastasis15. EMT is required for tumor invasion and metastasis. During the EMT procedure, tumor cells lose epithelial E-cadherin expression and cellular adhesion and acquire increased potential for local invasion and ability to evade to distant organs16. Reduction or a loss of E-cadherin expression is one of the well-established hallmarks of EMT. One study showed that the levels of membrane E-cadherin protein expression were obviously reduced, whereas the mesenchymal marker vimentin was upregulated in NPC samples compared with those in nasopharyngitis samples17. In the present study, we observed that overexpression of ZNRF3 significantly inhibited the migration and invasion of NPC cells, increased the protein expression of E-cadherin, and decreased the protein expression of N-cadherin protein in NPC cells. These data imply that ZNRF3 inhibited the migration and invasion of NPC cells via preventing the EMT process.
Previous studies indicate that the Wnt/β-catenin signaling pathway is overactivated in several human cancers, including NPC18–20. β-Catenin is a central factor in canonical Wnt signaling and induces the transcription of several target genes, which are involved in cell proliferation, metastasis, and EMT21. Xu et al. reported that there was a significantly higher expression of β-catenin in NPC with lymph node metastasis than in NPC without lymph node metastasis22. Therefore, inhibition of the Wnt/β-catenin signaling pathway may represent a promising approach to the treatment of NPC23–25. Most importantly, it has been reported that ZNRF3 partly attenuated protein levels of β-catenin and TCF-4 in gastric cancer cells9. In this study, we found that overexpression of ZNRF3 greatly downregulated the protein expression levels of β-catenin, cyclin D1, and c-Myc in NPC cells. These data suggest that ZNRF3 inhibits the metastasis and tumorigenesis in NPC cells through the inactivation of the Wnt/β-catenin signaling pathway.
In conclusion, the present study revealed that ZNRF3 could inhibit the metastasis and tumorigenesis of NPC cells both in vivo and in vitro. Therefore, ZNRF3 may be a potential molecular target for the treatment of NPC.
REFERENCES
- 1. Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell 2004;5:423–8. [DOI] [PubMed] [Google Scholar]
- 2. Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx 2012;39:137–44. [DOI] [PubMed] [Google Scholar]
- 3. Hui EP, Leung SF, Au JSK, Zee B, Tung S, Chua D, Sze WM, Law CK, Leung TW, Chan ATC. Lung metastasis alone in nasopharyngeal carcinoma: A relatively favorable prognostic group. Cancer 2004;101:300–6. [DOI] [PubMed] [Google Scholar]
- 4. Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature 2009;458:438–44. [DOI] [PubMed] [Google Scholar]
- 5. Buetow L, Huang DT. Structural insights into the catalysis and regulation of e3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vittal V, Stewart MD, Brzovic PS, Klevit RE. Regulating the regulators: Recent revelations in the control of E3 ubiquitin ligases. J Biol Chem. 2015;290:21244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inoue Y, Itoh Y, Sato K, Kawasaki F, Sumita C, Tanaka T, Morishita D, Hayashi H. Regulation of epithelial-mesenchymal transition by E3 ubiquitin ligases and deubiquitinase in cancer. Curr Cancer Drug Targets 2016;16:110–8. [DOI] [PubMed] [Google Scholar]
- 8. Zebisch M, Xu Y, Krastev C, Macdonald BT, Chen M, Gilbert RJC, He X, Jones EY. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4:2787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y, Lan J, Wang W, Shi Q, Yang L, Cheng Z, Guan H. ZNRF3 acts as a tumour suppressor by the Wnt signalling pathway in human gastric adenocarcinoma. J Mol Histol. 2013;44:555–63. [DOI] [PubMed] [Google Scholar]
- 10. Qin H, Cai A, Xi H, Jing Y, Lin C. ZNRF3 induces apoptosis of gastric cancer cells by antagonizing Wnt and hedgehog signaling. Panminerva Med. 2015;73:1–7. [PubMed] [Google Scholar]
- 11. Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, Fan Y. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell Physiol Biochem. 2015;35:71–82. [DOI] [PubMed] [Google Scholar]
- 12. Shi J, Jiang X, Yu Z, He G, Ning H, Wu Z, Cai Y, Yu H, Chen A. ZNRF3 contributes to the growth of lung carcinoma via inhibiting Wnt/β-catenin pathway and is regulated by miR-93. Tumor Biol. 2015;37:1–7. [DOI] [PubMed] [Google Scholar]
- 13. Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Tar. 2011;15:873–7. [DOI] [PubMed] [Google Scholar]
- 14. Qiu W, Yang Z, Fan Y, Qi Z. ZNRF3 is downregulated in papillary thyroid carcinoma and suppresses the proliferation and invasion of papillary thyroid cancer cells. Tumor Biol. 2016;37(9):12665–72. [DOI] [PubMed] [Google Scholar]
- 15. Ke L, Xiang Y, Xia W, Yang J, Yu Y, Ye Y, Liang H, Guo X, Lv X. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment interleukin 6 and clinical stage. Clin Immunol. 2016;164:45–51. [DOI] [PubMed] [Google Scholar]
- 16. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang H, Gao M, Shen Z, Luo B, Li R, Jiang X, Ding R, Ha Y, Wang Z, Jie W. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep. 2014;32:559–66. [DOI] [PubMed] [Google Scholar]
- 18. Shi W, Bastianutto C, Li A, Perez-Ordonez B, Ng R, Chow KY, Zhang W, Jurisica I, Lo KW, Bayley A, Kim J, O’Sullivan B, Siu L, Chen E, Liu FF. Multiple dysregulated pathways in nasopharyngeal carcinoma revealed by gene expression profiling. Int J Cancer 2006;119:2467–75. [DOI] [PubMed] [Google Scholar]
- 19. MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W. Wnt/β-catenin signaling pathway as novel cancer drug targets. Curr Cancer Drug Targets 2005;4:653–71. [DOI] [PubMed] [Google Scholar]
- 21. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192–205. [DOI] [PubMed] [Google Scholar]
- 22. Xu L, Jiang Y, Zheng J, Xie G, Li J, Shi L, Fan S. Aberrant expression of β-catenin and e-cadherin is correlated with poor prognosis of nasopharyngeal cancer. Human Pathol 2013;44:1357–64. [DOI] [PubMed] [Google Scholar]
- 23. Guan Z, Zhang J, Wang J, Wang H, Zheng F, Peng J, Xu Y, Yan M, Liu B, Cui B. SOX1 down-regulates β-catenin and reverses malignant phenotype in nasopharyngeal carcinoma. Mol Cancer 2014;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ren XY, Zhou GQ, Jiang W, Sun Y, Xu YF, Li YQ, Tang XR, Wen X, He QM, Yang XJ. Low SFRP1 expression correlates with poor prognosis and promotes cell invasion by activating the Wnt/β-catenin signaling pathway in NPC. Cancer Prev Res. 2015;8:968–77. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Wen X, Ren XY, Li YQ, Tang XR, Wang YQ, He QM, Yang XJ, Sun Y, Liu N. YPEL3 suppresses epithelial–mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 2016;35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]