Abstract
Forkhead box R2 (FOXR2), a member of the FOX gene family, has not been very well investigated for its role in cancer. A recent study has shown that FOXR2 is highly expressed in breast cancer samples and is associated with poor prognosis. In addition, FOXR2 was identified as an oncogene in medulloblastoma. Nevertheless, whether FOXR2 plays a role in colorectal cancer (CRC) remains unclear. In the present study, we conducted several in vitro and in vivo studies to investigate the expression and effect of FOXR2 in CRC. The study results demonstrated that FOXR2 was upregulated in CRC tissues and cells. Downregulation of FOXR2 inhibited CRC cell proliferation, invasion, and the epithelial–mesenchymal transition (EMT) phenotype in vitro and also suppressed CRC cell growth and metastasis in vivo. Furthermore, downregulation of FOXR2 remarkably reduced the protein expression of Shh, Gli1, and Ptch1 in SW480 cells. Taken together, our data suggested that FOXR2 significantly promoted proliferation, invasion, and EMT of CRC cells. All these findings provided evidence for the role of FOXR2 as an oncogene in CRC development.
Key words: Forkhead box R2 (FOXR2), Proliferation, Invasion, Epithelial–mesenchymal transition (EMT), Colorectal cancer (CRC)
INTRODUCTION
Colorectal cancer (CRC), a common gastrointestinal tract malignancy, is characterized by an increasing incidence and a high mortality1–3. The occurrence of CRC has been found to be associated with several factors such as genetics, habit, ethnicity, and diet4–8. According to statistics, about 950,000 cases are newly diagnosed every year, making CRC a global public health problem9. So far, the diagnostic methods available for CRC include colonoscopy and fecal occult blood test10. With improvements in clinical practice, CRC could be treated via modified surgical techniques and chemotherapy in combination with radiation therapy11. However, about 50% of CRC patients succumb to the disease because of distant metastasis or multidrug resistance12. Therefore, it is urgent to identify a novel effective target for CRC treatment.
The forkhead box (FOX) gene family consists of 43 members from FOXA1 to FOXQ113. All the members are involved in a series of biological processes such as proliferation, apoptosis, differentiation, transformation, and metabolic homeostasis14–16. A growing number of studies have indicated the role of the FOX gene family in carcinogenesis, either as a suppresser or a promoter of cancer progression. For instance, FOXD3 has been found to be downregulated in melanoma and is important in suppressing cancer development17. In contrast, FOXM1 has been reported to be upregulated in a wide range of neoplasms and functions as an oncogene18,19.
FOXR2, another member of the FOX gene family, has not been investigated much for its role in cancer. A recent study has shown that FOXR2 is highly expressed in breast cancer and is associated with poor prognosis20. In addition, FOXR2 was identified as an oncogene in medulloblastoma21. Nevertheless, whether FOXR2 plays a role in CRC remains unclear.
In the present study, we conducted several functional studies to investigate the expression and effect of FOXR2 in CRC. The study results demonstrated that FOXR2 was upregulated in CRC tissues and cells. In addition, downregulation of FOXR2 inhibited CRC cell proliferation, invasion, and epithelial–mesenchymal transition (EMT) and also reduced the protein expression of Shh, Gli1, and Ptch1 in CRC cells, suggesting an oncogenic role of FOXR2 in CRC.
MATERIALS AND METHODS
Tissue Specimens
Human CRC tissues and matching adjacent noncancerous tissues were obtained from 36 patients at the Hubei Cancer Hospital (P.R. China). All patients provided written consent prior to participation in the study and received no therapy before tissue samples were collected. All specimens were handled in accordance with ethical and legal standards. The study was carried out with the approval of the ethics committee of the Hubei Cancer Hospital.
Cell Lines and Cell Culture
Human CRC cell lines (SW480, HT29, and LOVO) and the normal colonic epithelial cell line NCM460 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were maintained in RPMI-1640 (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and cultured at 37°C in a humidified incubator with 5% CO2.
Quantitative Real-Time PCR
Total RNA was extracted from tissue samples or cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription of RNA into cDNA was performed using the PrimeScript RT Reagent Kit (Takara Biotechnology, Dalian, P.R. China) according to the manufacturer’s recommendations. The cyclical conditions of PCR were 35 cycles of denaturation at 94°C for 2 min, annealing at 56°C for 30 s, and extension at 72°C for 10 min. The primers for cDNA amplification were as follows: FOXR2, 5′-TCAGTGTGCAGGAGATCTAC-3′ (forward) and 5′-TACCAAGATCAAAGAGAGAGGTCAAC-3′ (reverse); β-actin, 5′-CCTGGCACCCAGCACAATG-3′ (forward) and 5′-GGGCCGGACTCGTCATACT-3′ (reverse). The relative mRNA expression levels were normalized to β-actin and measured using the comparative CT method (2−ΔΔCT)22.
Western Blot
Tissues or cells were lysed in RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA). Lysates were electrophoresed in 10% SDS-PAGE (Sigma-Aldrich) and then transferred to PVDF membranes (Sigma-Aldrich). After blocking for 1 h in 5% nonfat milk, the membranes were incubated overnight at 4°C with the primary antibodies against FOXR2, E-cadherin, vimentin, N-cadherin, Shh, Ptch1, Gli1, or β-actin. Then the membranes were washed three times with TBST and incubated at room temperature for 1 h with secondary HRP-conjugated antibodies. All antibodies for experiments were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protein bands were visualized by a chemiluminescence system (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s instructions. The band intensities were quantified using analysis software (Life Technologies, Gaithersburg, MD, USA).
Small Interfering RNA (siRNA) Transfection
Human FOXR2 siRNA (siFOXR2) and negative control siRNA (siNC) were purchased from Ambion (Grand Island, NY, USA). The sense sequences of siFOXR2 and siNC were 5′-GCUCCCUAGAUGAGAUACAdTdT-3′ and 5′-UUCUCCGAACGUGUCACGUdTdT-3′, respectively. SW480 and HT29 cells were transfected with siFOXR2 or siNC using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s protocol. After 24 h of transfection, the cells were harvested for additional experiments. Western blot analysis was performed to confirm the transfection efficiency.
Cell Proliferation Assay In Vitro
After transfection, cells were seeded into 96-well plates at a density of 3 × 103 cells/well and incubated for 24, 48, 72, and 96 h, respectively. Then 20 μl of MTT (5 mg/ml; Sigma-Aldrich) was added to each well, and the cells were further incubated for 4 h at 37°C. Next, the culture medium was removed, and 150 μl of DMSO (Sigma) was added. The absorbance was measured at a wavelength of 570 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Cell Invasion Assay In Vitro
Transwell chambers (Millipore, Boston, MA, USA) with Matrigel were used to perform the invasion assay. Transfected cells (3 × 104 cells/well) were seeded in the upper chamber coated with Matrigel. Serum-free medium (200 μl) was added to the upper chamber, and 800 μl of culture medium containing 20% FBS was added to the lower chamber. After 24 h of incubation, cells remaining on the upper surface of the filter were scraped off with a cotton swab and those invading the lower surface of the filter were fixed and stained with 0.1% crystal violet. The invading cells were counted in four random visual fields under a microscope (200×).
Xenograft Tumor Model In Vivo
Male BALB/c nude mice (4 to 6 weeks old) were obtained from the Animal Center of the Chinese Academy of Science (Shanghai, P.R. China). All animal studies were performed with the approval of the Institutional Animal Care and Use Committee of the Hubei Cancer Hospital. For the tumor growth assay, 200 μl of suspension of transfected cells at a concentration of 1 × 107 cells/ml was subcutaneously inoculated into nude mice (six mice per group). Tumor size was measured every week. Four weeks later, all mice were sacrificed, and tumors were weighed. Tumor volume (cm3) was determined by the following formula: length × width2/2.
For the tumor metastasis assay, 200 μl of suspension of transfected cells at a concentration of 1 × 107 cells/ml was intravenously injected into the tails of nude mice (six mice per group). Four weeks later, all mice were sacrificed, and all organs were checked for macroscopic metastasis. Tumor metastasis was mainly found in the lungs.
Statistical Analysis
Data were obtained from at least three independent experiments and expressed as the means ± standard deviation (SD). The SPSS 16.0 software was applied for statistical analysis, and the GraphPad Prism 5 software was used for graphical representation. Differences between different groups were analyzed by Student’s t-tests. A value of p < 0.05 was considered statistically significant.
RESULTS
Expression of FOXR2 Is Upregulated in CRC Tissues and Cell Lines
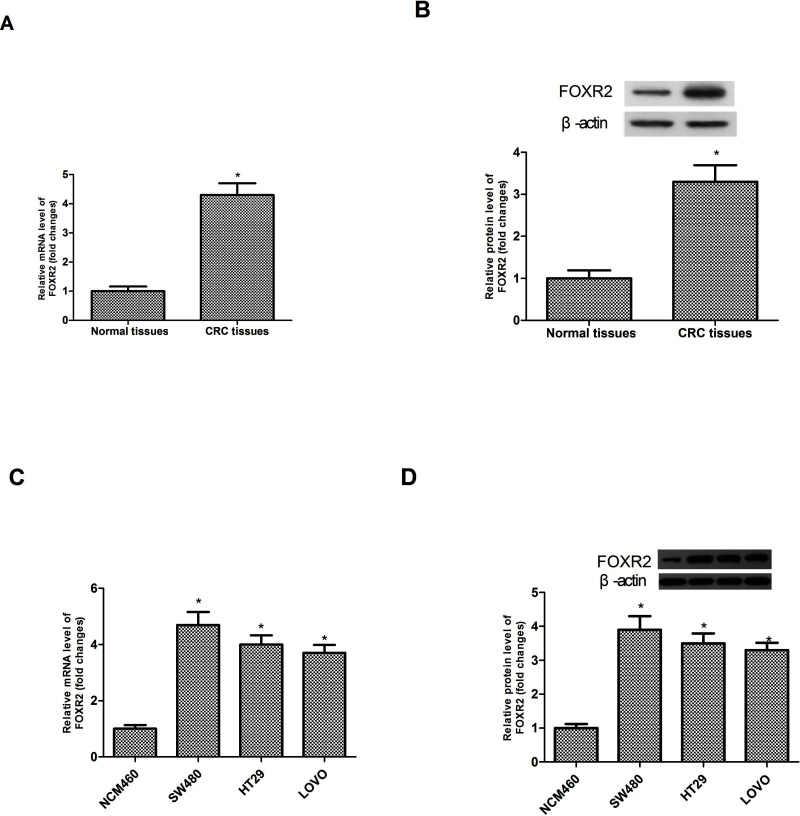
We conducted RT-PCR and Western blot analysis to measure the expression levels of FOXR2 in CRC tissues and matching adjacent noncancerous tissues. We observed significantly increased mRNA and protein expression levels of FOXR2 in CRC tissues compared to corresponding noncancerous tissues (Fig. 1A and B). We also detected the expression levels of FOXR2 in three CRC cell lines (SW480, HT29, and LOVO) and the normal colonic epithelial cell line NCM460. FOXR2 was highly expressed in three CRC cell lines but lowly expressed in NCM460 cells (Fig. 1C and D).
Figure 1.
Expression of FOXR2 is upregulated in CRC tissues and cell lines. The mRNA (A) and protein (B) expression levels of FOXR2 were significantly higher in CRC tissues than in the corresponding noncancerous tissues. The mRNA (C) and protein (D) expression levels of FOXR2 were remarkably upregulated in three CRC cell lines (SW480, HT29, and LOVO) when compared to the normal colonic epithelial cell line NCM460. *p < 0.05.
Downregulation of FOXR2 Inhibits CRC Cell Proliferation and Invasion In Vitro
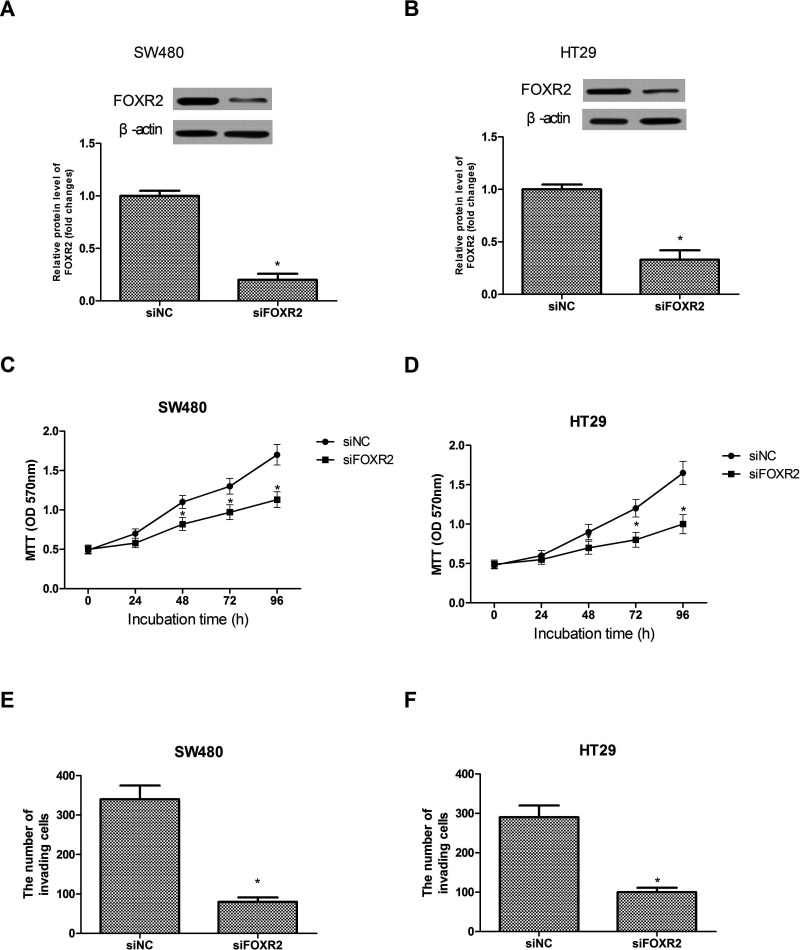
We downregulated the expression of FOXR2 in SW480 and HT29 cells by transfecting siFOXR2. Western blot analysis was performed to confirm the transfection efficiency. siFOXR2 transfection dramatically decreased the expression of FOXR2 in the SW480 (Fig. 2A) and HT29 (Fig. 2B) cell lines at the protein levels.
Figure 2.
Downregulation of FOXR2 inhibits CRC cell proliferation and invasion in vitro. Western blot assay showed decreased FOXR2 protein expression after siRNA-mediated downregulation in SW480 (A) and HT29 (B) cells. Downregulation of FOXR2 remarkably inhibited the proliferation rate of SW480 (C) and HT29 (D) cells as measured by the MTT assay. Downregulation of FOXR2 significantly inhibited the invasive capability of SW480 (E) and HT29 (F) cells as measured by the Transwell assay. *p < 0.05.
We performed MTT assay to examine the effect of FOXR2 downregulation on cell proliferation. The assay indicated that downregulation of FOXR2 remarkably inhibited the proliferation rate of the SW480 (Fig. 2C) and HT29 (Fig. 2D) cells compared to the control group. In addition, we determined the invasion rate. The assay results showed that downregulation of FOXR2 significantly inhibited the invasive capability of the SW480 (Fig. 2E) and HT29 (Fig. 2F) cells compared to the control group.
Downregulation of FOXR2 Inhibits CRC Cell Growth and Metastasis In Vivo
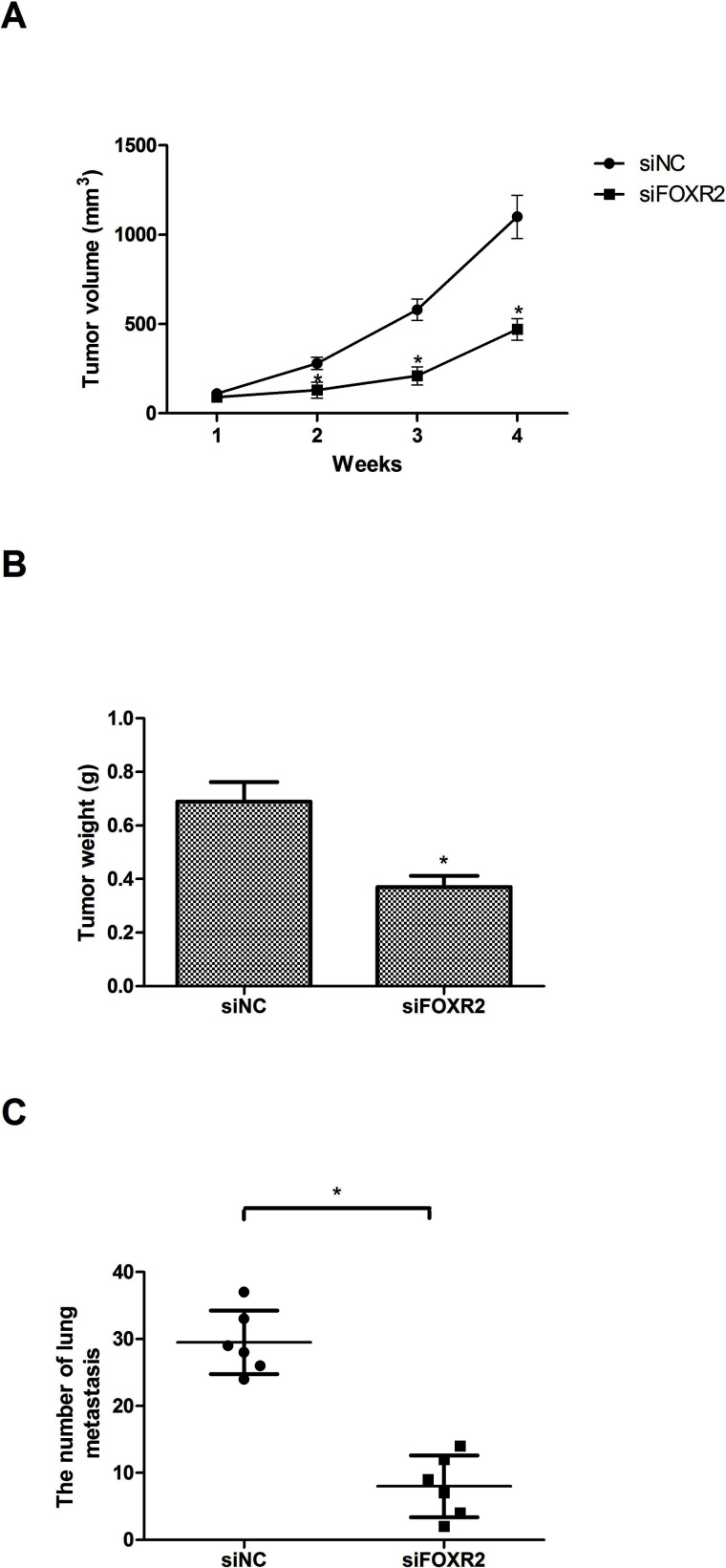
We further investigated the effect of FOXR2 downregulation on the growth and metastasis of CRC cells in vivo. SW480 cells transfected with siFOXR2 or siNC were subcutaneously inoculated into nude mice, and tumor size was measured every week. Four weeks later, all mice were sacrificed, and tumors were weighed. Tumor volume (Fig. 3A) and weight (Fig. 3B) were distinctly decreased in the siFOXR2 group compared with the control group.
Figure 3.
Downregulation of FOXR2 inhibits CRC cell growth and metastasis in vivo. Tumor volume (A) and weight (B) were significantly decreased in SW480 cells, compared to the control group. (C) The number of lung metastasis was quantified and shown by each data point. n = 6; *p < 0.05.
To explore the role of FOXR2 in CRC metastasis, SW480 cells transfected with siFOXR2 or siNC were intravenously injected into the tails of nude mice. Four weeks later, all mice were sacrificed, and all main organs were checked for tumor metastasis. Downregulation of FOXR2 remarkably decreased metastatic foci in the lungs (Fig. 3C).
Downregulation of FOXR2 Inhibits the EMT Process in CRC Cells
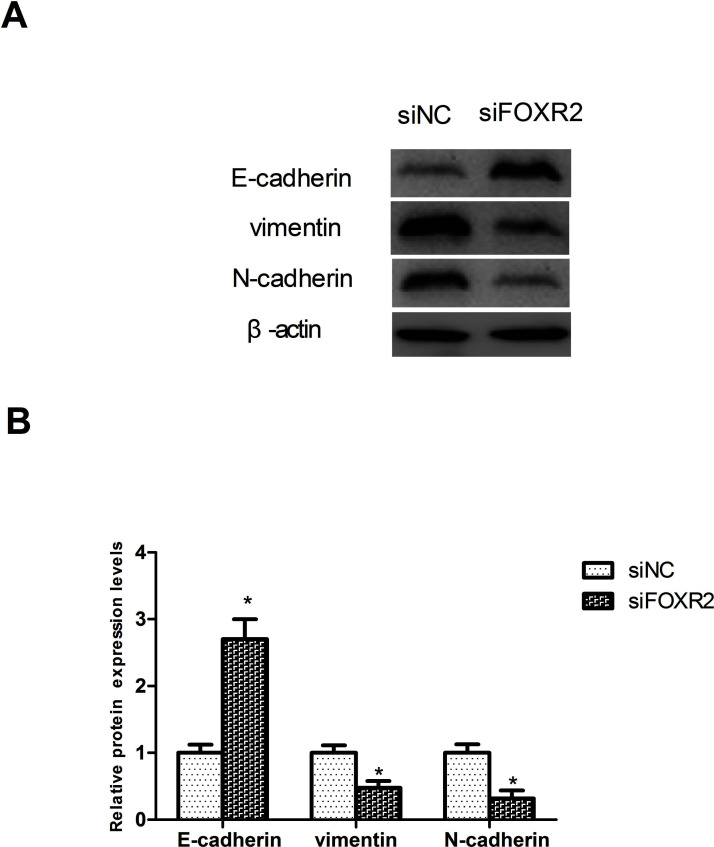
Involvement of EMT in cancer cell invasion and metastasis is well known23–25, so we tested the effect of FOXR2 downregulation on the EMT process by detecting the protein expression of EMT-associated markers in SW480 cells. Downregulation of FOXR2 significantly increased the protein expression of the epithelial marker E-cadherin and decreased that of the mesenchymal markers vimentin and N-cadherin in SW480 cells, compared to the control group (Fig. 4).
Figure 4.
Downregulation of FOXR2 inhibits the EMT process in CRC cells. (A) Western blot analysis was performed to detect the protein expression levels of E-cadherin, vimentin, and N-cadherin in SW480 cells. (B) Relative protein expression levels of E-cadherin, vimentin, and N-cadherin in SW480 cells were measured using analysis software. *p < 0.05.
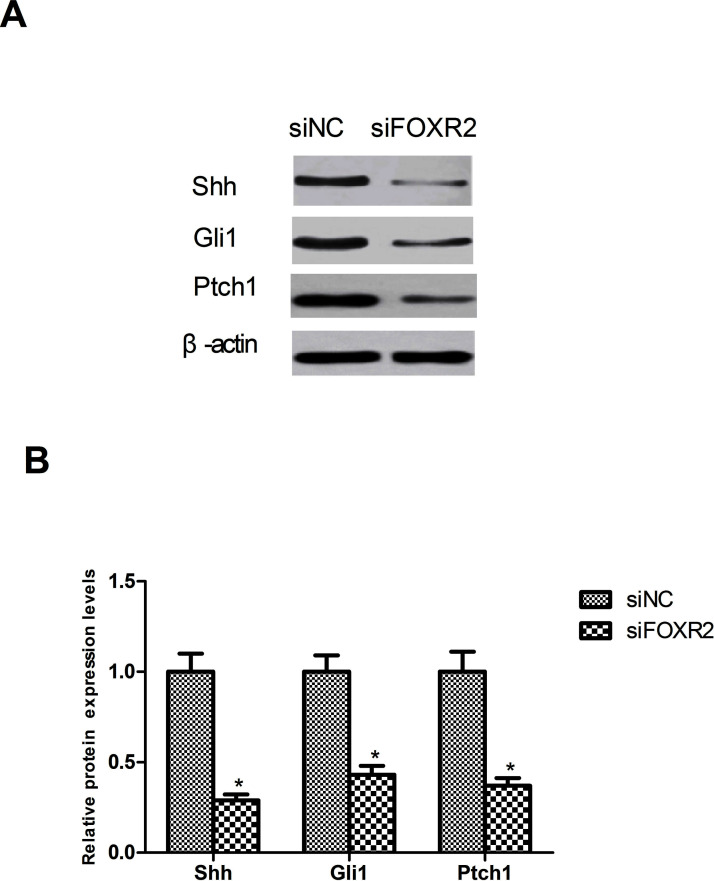
Downregulation of FOXR2 Inhibits the Activation of the Shh Signaling Pathway
It has been found that the Shh signaling pathway plays a key role in the pathogenesis of CRC26. Therefore, we tested the effect of FOXR2 downregulation on the Shh signaling pathway by detecting the protein expression levels of its several important components Shh, Gli1, and Ptch1 in SW480 cells. Downregulation of FOXR2 remarkably reduced the protein expression of Shh, Gli1, and Ptch1 in SW480 cells in comparison with the control group (Fig. 5).
Figure 5.
Downregulation of FOXR2 inhibits the activation of the Shh signaling pathway. (A) As shown by the Western blot assay, downregulation of FOXR2 remarkably decreased the protein expression of Shh, Gli1, and Ptch1 in SW480 cells. (B) Relative protein expression levels of Shh, Gli1, and Ptch1 in SW480 cells were measured using analysis software. *p < 0.05.
DISCUSSION
CRC has drawn worldwide attention for its increasing incidence and unacceptably high mortality2,3. The outcome for CRC patients remains poor due to metastasis or drug resistance, despite improvements in clinical practice and therapies11,12. Therefore, there is an urgent need to identify a novel therapeutic target and to better understand the underlying molecular mechanism.
The FOX gene family has frequently been studied and is reported to play an important role in cancer development27. In addition, many of the family members function as oncogenes in CRC. For example, FOXM1 was reported to promote CRC cell migration and invasion28. Similarly, Kaneda et al. found the tumor-promoting role of FOXQ1 in CRC29. However, another member of the FOX gene family, FOXR2, has been scarcely investigated in tumorigenesis. In this study, we explored the effect of FOXR2 on CRC cells for the first time. The results of our study showed that FOXR2 was upregulated in CRC tissues and cells, and downregulation of FOXR2 exerted an inhibitory effect on the proliferation and invasion of CRC cells. Similar results were found in hepatocellular cancer, breast cancer, and medulloblastoma20,21,30. All the evidence has strongly suggested FOXR2 as an oncogene that plays a significant role in CRC development, which was further proved by our functional studies.
Since proliferation and invasion are important biological features during tumor formation, we performed corresponding experiments to test the effect of FOXR2 on these biological processes. The MTT and Transwell assays indicated that downregulation of FOXR2 decreased CRC cell proliferation and invasion, respectively. To validate the conclusions drawn from our in vitro experiments, we designed xenograft models of CRC cells in nude mice. In the in vivo tumor growth and metastasis assays, CRC cells transfected with siFOXR2 were injected into nude mice. The assay results showed that downregulation of FOXR2 remarkably decreased tumor volume and weight as well as metastases, which were consistent with the results of our in vitro experiments. Increasing evidence has demonstrated the critical role of EMT in cancer progression, so we investigated the effect of FOXR2 on the EMT process in CRC cells by measuring the protein expression of EMT-associated markers. As expected, downregulation of FOXR2 significantly inhibited the EMT process in CRC cells by increasing the protein expression of the epithelial marker E-cadherin and decreasing that of the mesenchymal markers vimentin and N-cadherin.
Given that the Shh signaling pathway is strongly correlated with the pathogenesis of CRC26,31, we investigated the effect of FOXR2 downregulation on the Shh signaling pathway via detecting the protein expression levels of its several important components Shh, Gli1, and Ptch1 in CRC cells. The results showed that downregulation of FOXR2 remarkably reduced the protein expression of Shh, Gli1, and Ptch1 in CRC cells.
In conclusion, we demonstrated that FOXR2 was upregulated in CRC tissues and cells. Downregulation of FOXR2 significantly inhibited proliferation, invasion, and EMT of CRC cells. Furthermore, downregulation of FOXR2 remarkably reduced the protein expression of Shh, Gli1, and Ptch1 in CRC cells. These findings provide evidence for the role of FOXR2 as an oncogene in CRC development.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Compton CC. Colorectal carcinoma: Diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376–88. [DOI] [PubMed] [Google Scholar]
- 2. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer Statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 4. Lagra F, Karastergiou K, Delithanasis I, Koutsika E, Katsikas I, Papadopoulou-Zekeridou P. Obesity and colorectal cancer. Clin Colon Rectal Surg. 2011;24:229–43. [DOI] [PubMed] [Google Scholar]
- 5. Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–38. [DOI] [PubMed] [Google Scholar]
- 6. Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: Before and after diagnosis. World J Gastroenterol. 2009;15:3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, Negri E, Straif K, Romieu I, Vecchia CL. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann Oncol. 2011;22:1958–72. [DOI] [PubMed] [Google Scholar]
- 8. Perrigue MM, Kantor ED, Hastert TA, Patterson R, Potter JD, Neuhouser ML, White E. Eating frequency and risk of colorectal cancer. Cancer Causes Control 2013;24:2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 10. Hendon SE, Dipalma JA. U.S. practices for colon cancer screening. Keio J Med. 2005;54:179–83. [DOI] [PubMed] [Google Scholar]
- 11. Desantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. [DOI] [PubMed] [Google Scholar]
- 12. Krijger ID, Mekenkamp LJ, Punt CJ, Nagtegaal ID. MicroRNAs in colorectal cancer metastasis. J Pathol. 2011;224:438–47. [DOI] [PubMed] [Google Scholar]
- 13. Masuko K, Maki I, Hirokazu F, Hitoshi N, Masaru K. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. [DOI] [PubMed] [Google Scholar]
- 14. Myatt SS, Lam WF. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007;7:847–59. [DOI] [PubMed] [Google Scholar]
- 15. Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics 2010;4:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss MB, Abel EV, Dadpey N, Aplin AE. FOXD3 modulates migration through direct transcriptional repression of TWIST1 in melanoma. Mol Cancer Res. 2014;12:1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuda M, Kohashi K, Yamada Y, Maekawa A, Kinoshita Y, Nakatsura T, Iwamoto Y, Taguchi T, Oda Y. FOXM1 expression in rhabdomyosarcoma: A novel prognostic factor and therapeutic target. Tumor Biol. 2016;37:5213–23. [DOI] [PubMed] [Google Scholar]
- 19. Halasi M, Gartel AL. FOX(M1) news—It is cancer. Mol Cancer Ther. 2013;12:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song H, He W, Huang X, Zhang H, Tao H. High expression of FOXR2 in breast cancer correlates with poor prognosis. Tumor Biol. 2016;37:5991–7. [DOI] [PubMed] [Google Scholar]
- 21. Koso H, Tsuhako A, Lyons E, Ward JM, Rust AG, Adams DJ, Jenkins NA, Copeland NG, Watanabe S. Identification of FoxR2 as an oncogene in medulloblastoma. Cancer Res. 2014;74:2351–61. [DOI] [PubMed] [Google Scholar]
- 22. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 23. Jemal A, Bray F, Center MM, Ferlay JJ, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61: 69–90. [DOI] [PubMed] [Google Scholar]
- 24. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The number of metastatic lymph nodes: A promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg. 1998;187:597–603. [DOI] [PubMed] [Google Scholar]
- 25. Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, Mochizuki H. Evaluation of the new American Joint Committee on Cancer/International Union Against Cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer 199;86:553–8. [DOI] [PubMed] [Google Scholar]
- 26. Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, Conti M, Vaubourdolle M, Cugnenc PH, Loric S. Sonic Hedgehog–dependent proliferation in a series of patients with colorectal cancer. Surgery 2006;139:665–70. [DOI] [PubMed] [Google Scholar]
- 27. Carlsson P, Mahlapuu M. Forkhead transcription factors: Key players in development and metabolism. Dev Biol. 2001;250:1–23. [DOI] [PubMed] [Google Scholar]
- 28. Zheng Y, Guo J, Zhou J, Lu J, Chen Q, Zhang C, Chen Q, Koeffler HP, Tong Y. FoxM1 transactivates PTTG1 and promotes colorectal cancer cell migration and invasion. BMC Med Genomics 2015;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, Sakai K, Velasco MAD, Matsumoto K, Fujita Y. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res. 2010;70:2053–63. [DOI] [PubMed] [Google Scholar]
- 30. Xiao W, He B, Yong G, Li Y. FOXR2 contributes to cell proliferation and malignancy in human hepatocellular carcinoma. Tumour Biol. 2016;37:10459–67. [DOI] [PubMed] [Google Scholar]
- 31. Song J, Zhang J, Wang J, Wang J, Guo X, Dong W. β1 integrin mediates colorectal cancer cell proliferation and migration through regulation of the Hedgehog pathway. Tumour Biol. 2014;36:2013–21. [DOI] [PubMed] [Google Scholar]