Abstract
Antagonists of inhibitors of apoptosis proteins (IAPs), alone or in combination with genotoxic therapeutics, have been shown to efficiently induce cell death in various solid tumors. The IAP antagonist birinapant is currently being tested in phase II clinical trials. We herein aimed to investigate the antitumor efficacy of dacarbazine in vitro, both as a single agent and in combination with birinapant, in melanoma cell lines. Covering clinically relevant drug concentration ranges, we conducted a total of 5,400 measurements in a panel of 12 human melanoma cell lines representing different stages of disease progression. Surprisingly, functionally relevant synergies or response potentiation in combination treatments was not observed, and only one cell line modestly responded to birinapant single treatment (approximately 16% cell death). Although we did not study the underlying resistance mechanisms or more complex in vivo scenarios in which dacarbazine/birinapant response synergies may still possibly manifest, our findings are nevertheless noteworthy because IAP antagonists were demonstrated to strongly enhance responses to DNA-damaging agents in cell lines of other cancer types under comparable experimental conditions in vitro.
Key words: Melanoma, Birinapant, Dacarbazine, Synergy, Cell death
INTRODUCTION
Apoptosis resistance significantly limits the efficacy of genotoxic anticancer drugs1. Inhibitors of apoptosis proteins (IAPs) such as X-linked IAP, ML-IAP, and cIAPs 1 and 2 are major repressors of apoptosis, either directly inhibiting apoptotic caspases or preventing the formation of apoptosis-inducing complexes2. The development of synthetic IAP antagonists therefore provides a novel opportunity to trigger cancer cell death upon single treatment or in combination with approved therapeutics and, as such, have sparked hope for improving treatment outcome in patients suffering from highly chemoresistant cancers3. As a single treatment, birinapant induces apoptosis through the rapid degradation of cIAPs, resulting in caspase 8 activation by autocrine TNF-α signaling or ripoptosome formation, while also suppressing prosurvival NF-κB signaling2,3. These responses, as well as the inhibition of XIAP, the major intracellular inhibitor of caspase 3, 7, and 9, are thought to give rise to cotreatment synergies with DNA-damaging agents3. Birinapant is tolerated with only modest side effects4 and has entered phase I/II trials as a single or combination treatment with chemotherapeutics in advanced or metastatic solid tumors and hematological cancers (e.g., NCT01188499, NCT01828346). However, whether birinapant sensitizes melanoma cells to DNA-alkylating agents such as dacarbazine has not yet been explored. Whereas novel targeted kinase inhibitors such as MEK and BRAF V600 mutation inhibitors and immunotherapeutics currently revolutionize first-line metastatic melanoma therapy5, dacarbazine and related agents (temozolomide, fotemustine, and mephalan) remain in frequent clinical use as second- or last-line treatment options or for the treatment of locoregionally spreading of advanced melanoma by isolated limb perfusion. Furthermore, dacarbazine remains the primary treatment option in poorly funded healthcare environments, despite low response rates and a lack of evidence for improved overall survival6. Prior to initiating costly translational studies and trials, it is therefore important to assess the preclinical efficacy of birinapant/dacarbazine combination treatments in melanoma and to identify whether synergies observed in cell line models of other cancers can be replicated.
MATERIALS AND METHODS
Cell Culture, Reagents, and Treatment Schedules
Twelve melanoma cell lines from progressing disease stages and diverse mutational backgrounds (Fig. 1) were purchased as authenticated stocks from the ATCC (Manassas, VA, USA; WM115, Sk-Mel-1, Sk-Mel-5, Malme-3M, MeWo, and Sk-Mel-2), the Wistar Institute (Philadelphia, PA, USA; WM35, WM3211, WM1366, WM1719C, and WM3060), or the DSMZ (Brunswick, Germany; Mel Juso) and cultured as described previously7. Cells were seeded into 96-well plates and treated with birinapant (Active Biochem, Maplewood, NJ, USA) and dacarbazine (Medac GmbH, Wedel, Germany) using 5 × 5 concentration–combination matrices, with the concentrations for birinapant ranging from 1 nM to 1 μM and for dacarbazine ranging from 1 μg/ml to 1 mg/ml. Drugs were applied simultaneously. Cells were treated for 24 or 48 h and subsequently stained with propidium iodide (1.3 μg/ml for 10 min) for cell death measurements.
Figure 1.
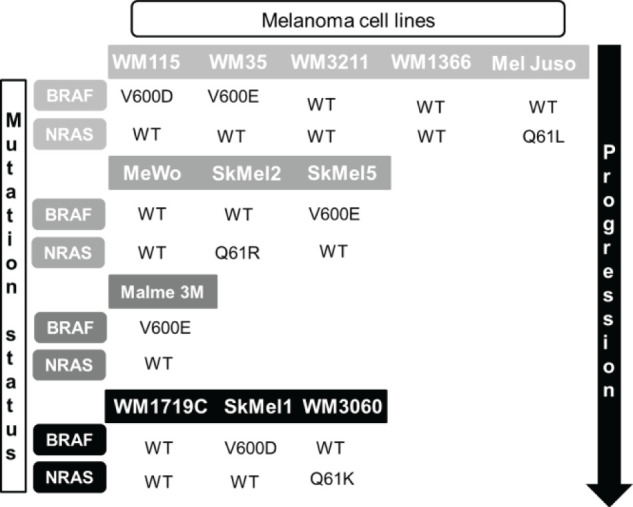
Overview of melanoma cell line panel and cell line mutation status.
High-Throughput Cell Death Measurements
Flow cytometric measurements of propidium iodide positivity were performed on a BD LSRII SORP HTS cytometer (BD, Franklin Lakes, NJ, USA). Data were stored in a .fcs file format and processed using Cyflogic software (CyFlo Ltd., Turku, Finland). All conditions were measured in parallel triplicates for every cell line, and all experiments were independently repeated (n = 3), so that a total of 5,400 samples were analyzed. Results between repeat experiments were highly reproducible (average SEM, <5%). To test for synergistic responses, combination index analysis was performed (CompuSyn software; ComboSyn Inc., Paramus, NJ, USA)8.
Luminex xMAP Technology Measurements
Measurement of GAPDH proteins was performed on a FLEXMAP 3D® instrument using bead-based ELISA-type assays (Luminex xMAP assay; Austin, TX, USA). Cells were seeded into 96-well plates and lysed after incubation with birinapant for up to 72 h.
RESULTS
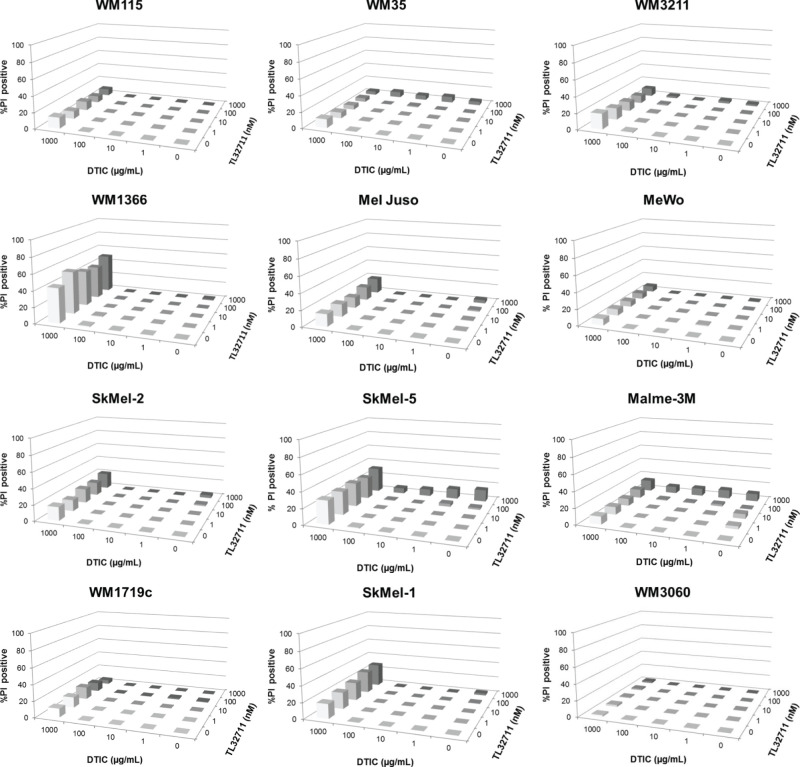
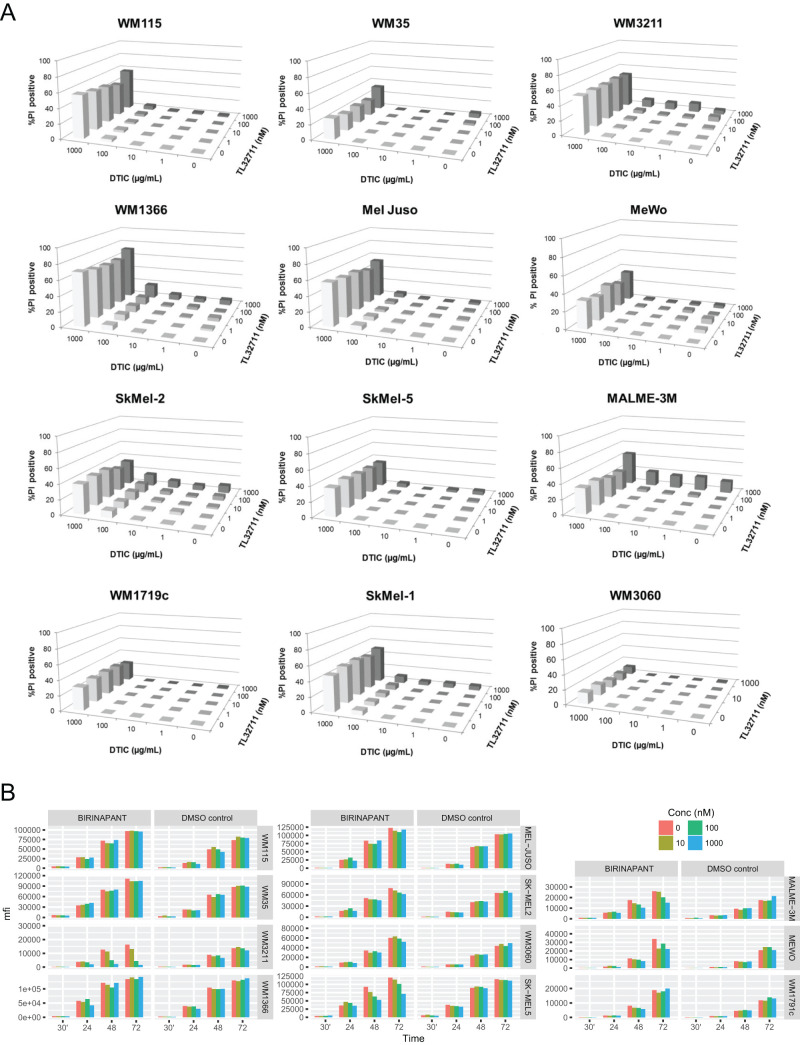
To obtain a comprehensive overview of the responsiveness and response heterogeneity to combinations of birinapant and dacarbazine, we employed semi-high throughput flow cytometry with propidium iodide-based cell death readouts. Twelve melanoma cell lines from progressing disease stages and diverse mutational backgrounds (Fig. 1) were treated with birinapant and dacarbazine using 5 × 5 concentration–combination matrices, with highest concentrations being 1 μM (birinapant) and 1 mg/ml (dacarbazine), respectively. Birinapant begins antagonizing IAPs at concentrations of 1 nM and in phase I studies reached serum concentrations from approximately 12 nM to 1.6 mM9–11. During systemic chemotherapy, dacarbazine serum concentrations can reach up to 29 μg/ml12, but considerably higher concentrations of DNA-alkylating therapeutics are achieved during isolated limb perfusion or infusion13. Twenty-four hours after the start of treatment, all cell lines remained highly resistant to single and combination treatments with birinapant and dacarbazine for concentration combinations of up to 100 nM and 100 μg/ml, respectively (<5% cell death) (Fig. 2). Dacarbazine at 1 mg/ml resulted in less than 20% cell death, with the exception of Sk-Mel-5 and WM1366 cells, where up to 50% cell death was detected (Fig. 2). No synergy was detected for any of the cell lines or treatment conditions. At 48 h, all cell lines remained highly resistant for concentration combinations of up to 100 nM birinapant and 100 μg/ml dacarbazine (Fig. 3A). A modest response to birinapant as a single agent and at the highest concentration was observed only in Malme-3M cells (16% cell death). Notable responses to dacarbazine were observed only when given at 1 mg/ml, and these responses were highly heterogeneous between cell lines. WM35 and WM3060 cells remained largely resistant (<20% cell death), whereas WM1366, WM3211, and SkMel1 cells presented with 60% to 80% cell death (Fig. 3A). Combination index analysis identified responses in Malme-3M cells (1 μM birinapant/1 mg/ml dacarbazine; CI = 0.658) and WM1366 cells (1 μM birinapant/100 μg/ml dacarbazine; CI = 0.398) as synergistic. However, because these combinations failed to notably enhance cell death over the dacarbazine-only response, these CI-defined synergies appear to largely lack relevance. Similarly, no notable response potentiation could be observed for any of the 25 concentration combinations analyzed in any of the other cell lines. We also analyzed whether cellular proliferation is affected by birinapant. To this end, we quantified GAPDH protein amounts as a common proliferation readout of ELISA-based Luminex xMAP technology measurements (Fig. 3B). With the exception of WM3211 cells, proliferation was not significantly affected. Since nonadherent Sk-Mel-1 cells could not be measured by this approach, we also conducted flow cytometric fixed-volume (300 μl) cell counts and also studied proliferation inhibition for dacarbazine treatments and birinapant/dacarbazine combinations (same conditions as in Fig. 3A). While impaired growth after treatment with dacarbazine could be observed for all cell lines, again no prominent synergies or potentiation could be identified in combination treatments with birinapant (not shown).
Figure 2.
Cell death responsiveness (propidium iodide positivity) of melanoma cell lines after single and combination treatment with birinapant and dacarbazine (24 h, 5 × 5 combination matrices). Bars represent mean values from three independent experiments, each run with triplicate samples. Error bars (typically <5% SEM) were omitted for clarity.
Figure 3.
(A) Cell death responsiveness (propidium iodide positivity) of melanoma cell lines after single and combination treatment with birinapant and dacarbazine (48 h, 5 × 5 combination matrices). Bars represent mean values from three independent experiments, each run with triplicate samples. Error bars (typically <5% SEM) were omitted for clarity. (B) Effect of birinapant on melanoma cell proliferation. GAPDH amounts were measured by ELISA-based Luminex technology and shown as median fluorescence intensity (mfi).
DISCUSSION
In this study, we set out to examine whether combinations of the DNA-alkylating drug dacarbazine and the IAP antagonist birinapant efficiently induce cell death in melanoma cell lines. Unexpectedly, we did not observe any notable synergies or response potentiation, despite having analyzed 12 cell lines from different disease stages and with diverse mutational backgrounds. Our results are surprising, because combinations of IAP antagonists and chemotherapeutics were reported to synergistically induce cell death in preclinical models of various other cancers, including acute myeloid leukemia, hepatocellular carcinoma, and ovarian and bladder cancers9,14,15.
We found that melanoma cells in vitro are resistant or poorly responsive to birinapant as a single agent. These findings confirm a previous study that likewise reported a high resistance of melanoma cells to birinapant, despite clear evidence of on-target activity in these cells16. While we did not investigate resistance mechanisms in our study, the lack of intrinsic ripoptosome formation upon cIAP depletion or the failure to produce and secrete TNF-α for autocrine apoptosis induction could contribute to limiting birinapant responsiveness2,3. Upon dacarbazine treatment, we observed impaired cell growth in all cell lines tested (not shown). However, growth arrest did not always translate into cell death. The variability in dacarbazine-induced cell death between melanoma cell lines might be attributable to the heterogeneous expression patterns of apoptosis regulators7. Our finding that the combination of birinapant and dacarbazine failed to cause notable response synergies in vitro could either point toward a fundamental melanoma-inherent resistance mechanism for this treatment regimen or, alternatively, indicates that stress responses to DNA-alkylating agents in general cannot be enhanced by IAP antagonists. However, the latter conclusion is in conflict with the finding that A172 glioma cells can respond synergistically to the combination of temozolomide, a DNA-alkylating agent related to dacarbazine, and BV6, a bivalent IAP antagonist with properties similar to birinapant17.
Even though our in vitro findings would call into question whether clinical studies for the combination of birinapant and dacarbazine are warranted in melanoma, the possibility of synergies manifesting in vivo cannot be excluded. As shown in other cancer models2,3, IAP antagonists can sensitize cancer cell lines to extrinsic apoptosis, induced, for example, by tumor necrosis factor receptor-1/-2 ligand TNF-α. Indeed, birinapant-induced sensitization to TNF-α has previously been demonstrated for melanoma cells in vitro, and since TNF-α is secreted into proinflammatory tumor microenvironments by invading macrophages, this may explain why 451Lu melanoma cells responded to birinapant single treatment in an in vivo mouse xenograft model16. Whether synergies between dacarbazine and birinapant can manifest within the complexity of an in vivo environment, where additional sensitizing factors such as TNF-α might be present, however, would require more comprehensive studies and go beyond the scope of our in vitro screening.
ACKNOWLEDGMENTS
The authors receive funding through the EU Horizon 2020 MEL-PLEX and FP7 IAPP SYS-MEL programs as well as through the Health Research Board Ireland (HRA-POR-2013-245).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 2. Kocab AJ, Duckett CS. Inhibitor of apoptosis proteins as intracellular signaling intermediates. FEBS J. 2016;283(2):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fulda S. Smac mimetics as IAP antagonists. Semin Cell Dev Biol. 2015;39:132–8. [DOI] [PubMed] [Google Scholar]
- 4. Amaravadi RK, Schilder RJ, Dy GK, Ma WW, Fetterly GJ, Weng DE, Graham MA, Burns JM, Chunduru SK, Condon SM, McKinlay MA, Adjei AA. Phase 1 study of the Smac mimetic TL32711 in adult subjects with advanced solid tumors and lymphoma to evaluate safety, pharmacokinetics, pharmacodynamics and antitumor activity. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research 2011;(2–6 Apr 2011; Orlando, Florida): Abstract LB-406. [Google Scholar]
- 5. Davey RJ, Westhuizen A, Bowden NA. Metastatic melanoma treatment: Combining old and new therapies. Crit Rev Oncol Hematol. 2016;98:242–53. [DOI] [PubMed] [Google Scholar]
- 6. Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: Thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21–34. [PubMed] [Google Scholar]
- 7. Passante E, Wurstle ML, Hellwig CT, Leverkus M, Rehm M. Systems analysis of apoptosis protein expression allows the case-specific prediction of cell death responsiveness of melanoma cells. Cell Death Differ. 2013;20(11):1521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. [DOI] [PubMed] [Google Scholar]
- 9. Benetatos CA, Mitsuuchi Y, Burns JM, Neiman EM, Condon SM, Yu G, Seipel ME, Kapoor GS, Laporte MG, Rippin SR, Deng Y, Hendi MS, Tirunahari PK, Lee YH, Haimowitz T, Alexander MD, Graham MA, Weng D, Shi Y, McKinlay MA, Chunduru SK. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Mol Cancer Ther. 2014;13(4):867–79. [DOI] [PubMed] [Google Scholar]
- 10. Fetterly GJ, Liu B, Senzer NN, Amaravadi RK, Schilder RJ, Martin LP, LoRusso P, Papadopoulos KP, Adjei AA, Zagst PD, McKinlay MM, Weng DE, Graham M. Clinical pharmacokinetics of the Smac-mimetic birinapant (TL32711) as a single agent and in combination with multiple chemotherapy regimens. J Clin Oncol 2012;30(Suppl):abstr 3029. [Google Scholar]
- 11. Amaravadi RK, Senzer NN, Martin LP, Schilder RJ, LoRusso P, Papadopoulos KP, Weng DE, Graham M, Adjei AA. A phase I study of birinapant (TL32711) combined with multiple chemotherapies evaluating tolerability and clinical activity for solid tumor patients. J Clin Oncol. 2013;31(Suppl):abstr 2504. [Google Scholar]
- 12. Joukhadar C, Klein N, Mader RM, Schrolnberger C, Rizovski B, Heere-Ress E, Pehamberger H, Strauchmann N, Jansen B, Muller M. Penetration of dacarbazine and its active metabolite 5-aminoimidazole-4-carboxamide into cutaneous metastases of human malignant melanoma. Cancer 2001;92(8):2190–6. [DOI] [PubMed] [Google Scholar]
- 13. Rashid OM, Sloot S, Zager JS. Regional therapy in metastatic melanoma: An update on minimally invasive intraarterial isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2014;10(10):1355–64. [DOI] [PubMed] [Google Scholar]
- 14. Carter BZ, Mak PY, Mak DH, Shi Y, Qiu Y, Bogenberger JM, Mu H, Tibes R, Yao H, Coombes KR, Jacamo RO, McQueen T, Kornblau SM, Andreeff M. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014;106(2):djt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian A, Wilson GS, Lie S, Wu G, Hu Z, Hebbard L, Duan W, George J, Qiao L. Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer Lett. 2014;351(2):232–41. [DOI] [PubMed] [Google Scholar]
- 16. Krepler C, Chunduru SK, Halloran MB, He X, Xiao M, Vultur A, Villanueva J, Mitsuuchi Y, Neiman EM, Benetatos C, Nathanson KL, Amaravadi RK, Pehamberger H, McKinlay M, Herlyn M. The novel SMAC mimetic birinapant exhibits potent activity against human melanoma cells. Clin Cancer Res. 2013;19(7):1784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K, Vucic D, Debatin KM, Fulda S. Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-kappaB-dependent manner. Oncogene 2013;32(8):988–97. [DOI] [PubMed] [Google Scholar]