Abstract
Protease serine S1 family member 8 (PRSS8), a membrane-anchored serine protease, has been reported to be involved in the development of several human cancers. However, the role of PRSS8 in non-small cell lung cancer (NSCLC) pathogenesis remains unclear. The objective of this study was to investigate PRSS8 expression, biological function, and its related molecular mechanism in NSCLC. Our results showed that PRSS8 was expressed in a low amount in NSCLC cell lines. Ectopic expression of PRSS8 inhibited tumor growth in vitro and in vivo. Furthermore, ectopic expression of PRSS8 inhibited the migration and invasion of NSCLC cells. It also suppressed the EMT process in A549 cells. Mechanistically, we found that the ectopic expression of PRSS8 downregulated the protein expression levels of p-JAK1, p-JAK2, and p-STAT3 in A549 cells. Taken together, our study showed that PRSS8 plays an important role in the growth and metastasis of NSCLC. Thus, PRSS8 may be a novel therapeutic target for NSCLC.
Key words: Non-small cell lung cancer (NSCLC), Protease serine S1 family member 8 (PRSS8), Proliferation, Metastasis
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths in the world, and the incidence and mortality have been increasing rapidly in recent years. Non-small cell lung cancer (NSCLC) accounts for approximately 75%–80% of all lung cancers1. Despite the improvement in diagnosis and treatment, the 5-year survival rate of patients in advanced stages of NSCLC remains poor because of metastasis2–4. Thus, the identification of molecular mechanisms critical for driving NSCLC progression is urgently needed.
Serine proteases are proteolytic enzymes that are involved in the regulation of a range of physiological and pathological processes5–7. Protease serine S1 family member 8 (PRSS8), a membrane-anchored serine protease, was found in various mammalian tissues like semen, the prostate gland, and skin8. It is essential for epithelial barrier formation and homeostasis9. In addition, aberrant expression of PRSS8 is associated with many cancer types such as breast, bladder, gastric, and ovarian10–12. Downregulation of PRSS8 expression has been shown in colorectal adenocarcinomas, and knockdown of PRSS8 promoted cell proliferation in vitro and cancer cell growth in nude mice13. A study by Bao et al. reported that the expression of PRSS8 was significantly downregulated in esophageal squamous cell carcinomas (ESCCs), and the downregulation of PRSS8 was closely related to shorter survival time14. However, the role of PRSS8 in NSCLC pathogenesis remains unclear. The objective of this study was to investigate PRSS8 expression, biological function, and its related molecular mechanism in NSCLC. Our results demonstrated that PRSS8 was expressed in a low amount in NSCLC cell lines, and PRSS8 inhibited tumor growth in vitro and in vivo in human NSCLC.
MATERIALS AND METHODS
Cell Culture
Three human NSCLC cell lines (A549, PC9, and NCI-H1993) and a normal human bronchial epithelial cell line (BEAS-2B) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in DMEM (Gibco, Grand Island, NY, USA) in addition to 10% fetal bovine serum (FBS), 100 mg/ml streptomycin, and 100 U/ml penicillin at 37°C with 5% CO2 in an incubator (Life Technologies, Baltimore, MD, USA).
Construction of PRSS8-Expressing Vectors
For overexpression of PRSS8, the open reading frame of PRSS8 was amplified from human mRNA and was cloned into the pc-DNA3.1 vector (Invitrogen, Carlsbad, CA, USA). An empty pcDNA3.1 vector was used as a control and named “mock.” For transfection, cells were cultured to 80% confluence and transfected with PRSS8 or mock using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Quantitative Real-Time Polymerase Chain Reaction (qRT -PCR)
Total RNA was isolated from cultured cell lines using TRIzol reagent (Invitrogen), and complementary DNA (cDNA) was synthesized using the EasyScript First-Strand cDNA Synthesis SuperMix Kit (Invitrogen). All qRT-PCR tests were performed by the ABI StepOnePlus Real-Time PCR-System (Applied Biosystems, Foster City, CA, USA; Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. PCR amplification was performed using the following primers: PRSS8, 5′-AGAGGACATGGTGTGTGCTG-3′ (sense) and 5′-GAGGCTGGAGTTCTGTCACC-3′ (antisense); β-actin, 5′-TTAGTTGCGTTACACCCTTTC-3′ (sense) and 5′-ACCTTCACCGTTCCAGTTT-3′ (antisense). The data obtained were calculated by 2−ΔΔCt and treated for statistical analysis as described previously15.
Western Blot Analysis
NSCLC cells were washed with 1× PBS and lysed in RIPA buffer. The supernatant was collected following centrifugation at 10,000 × g for 10 min. Equal amounts of proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose filter membranes. The membranes were blocked with 5% nonfat milk at room temperature for 1 h and subsequently incubated with primary antibodies against PRSS8, E-cadherin, N-cadherin, p-JAK1, JAK1, p-JAK2, JAK2, p-STAT3, STAT3, and GAPDH (Santa Cruz Technology, Danvers, MA, USA) at 4°C overnight. The next day, membranes were washed three times for 5 min with TBST and incubated for 1 h with the corresponding secondary antibodies (Santa Cruz). After three 5-min washes with TBST, the signal was developed using the standard ECL (Pierce, Rockford, IL, USA). Densitometric scans were quantified using ImageJ software.
Cell Proliferation Assay and Xenograft Model
Cell proliferation was detected using the WST-1 assay according to the manufacturer’s instructions. Briefly, 2 × 103 cells were seeded into 96-well plates and cultured for 24, 48, 72, and 96 h, respectively. At each time point, cells were incubated according to the manufacturer’s protocol with the WST-1 labeling mixture for 2 h. The wavelength of 490 nm was measured using a spectrophotometer (Bio-Rad, Hercules, CA, USA).
For the in vivo tumorigenicity assay, 5 × 106 PRSS8 cells and control cells were injected subcutaneously into the left and right dorsal flanks of 4-week-old female BALB/c-nu mice (n = 6 per group). Tumor formation was monitored every 5 days by measuring the tumor size with a caliper. The tumor volume was then calculated using the following formula: V = (L × W 2)/2. Twenty days later, the animals were sacrificed, and the tumors were excised and weighed. All animal experiments were approved by the Animal Care Committee of Huaihe Hospital of Henan University (P.R. China) and were performed in accordance with institutional guidelines.
Migration and Invasion Assays
A cell migration assay was performed using Transwell insert chambers (Corning, New York, NY, USA). Infected cells (1 × 105 cells/well) were suspended in 200 μl of serum-free medium and seeded into the upper chamber of a Transwell insert; 600 μl of 10% FBS DMEM was added to the lower chamber. After incubation for 24 h under suitable conditions, the cells on the lower surface of the inserts were fixed and stained with 0.1% crystal violet, and six random fields for each insert were counted under a microscope. For the cell invasion assay, the process was analogous to the cell migration assay, except that the membranes were smeared with Matrigel (BD Biosciences, San Jose, CA, USA).
Statistical Analysis
Statistical analysis was performed using the SPSS 19.0 software (SPSS, Chicago, IL, USA). The results are expressed as the mean ± SD, and differences between groups were analyzed using the Student’s t-test. Values of p < 0.05 were considered statistically significant.
RESULTS
PRSS8 Was Expressed in a Low Amount in NSCLC Cell Lines
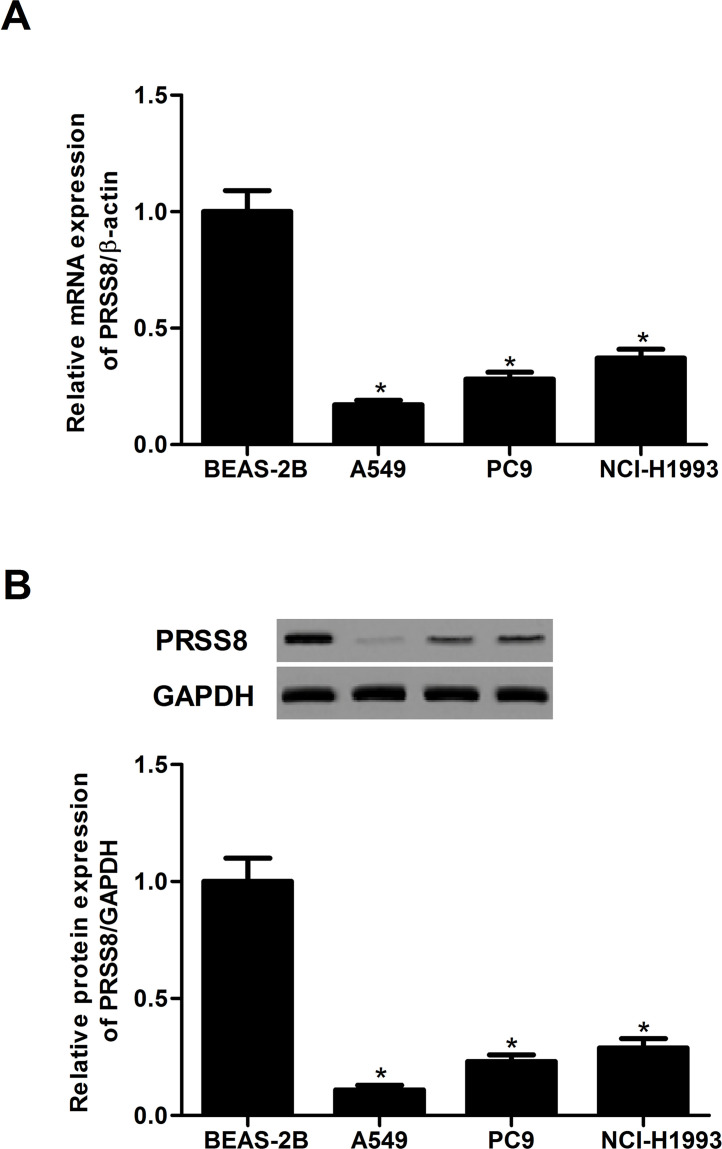
We first measured the expression level of PRSS8 in a panel of NSCLC cell lines including A549, PC9, and NCI-H1993. The mRNA levels of PRSS8 in human NSCLC cell lines were obviously lower than those in the normal human bronchial epithelial cell line (BEAS-2B) (Fig. 1A). Western blot analysis showed that the protein expression levels of PRSS8 were significantly downregulated in human NSCLC cell lines (Fig. 1B).
Figure 1.
PRSS8 was expressed in a low amount in NSCLC cell lines. (A) mRNA expression of PRSS8 in human NSCLC cell lines was analyzed by qRT-PCR. (B) Protein expression of PRSS8 in human NSCLC cell lines was analyzed by Western blot analysis. Experiments were performed in triplicate. *p < 0.05 versus BEAS-2B.
Ectopic Expression of PRSS8 Inhibited Tumor Growth In Vitro and In Vivo
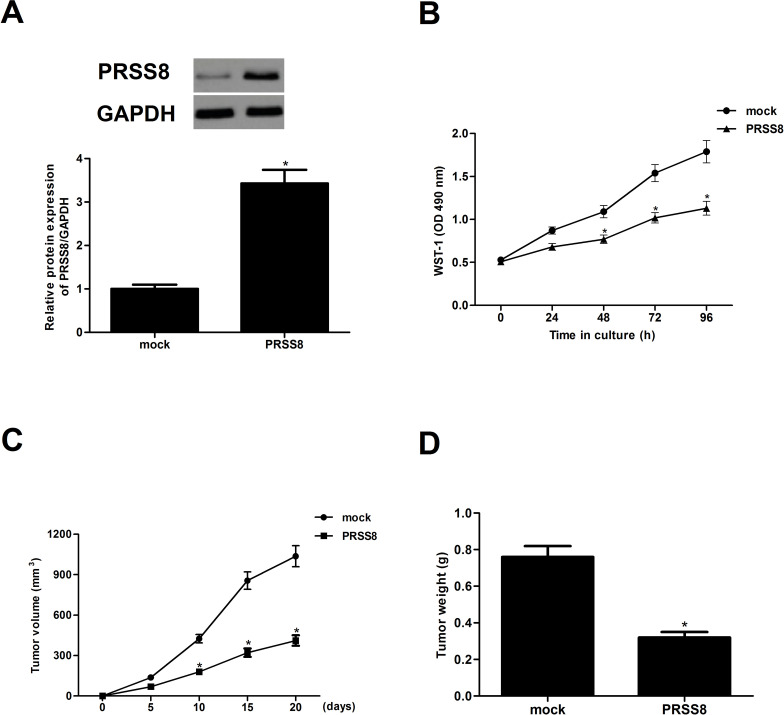
We generated a PRSS8-expressing vector in order to investigate the effect of PRSS8 on tumor growth in vitro and in vivo. Both the protein and mRNA levels of PRSS8 were dramatically increased in A549 cells after transfection with PRSS8 (Fig. 2A). In addition, results of the WST-1 assay demonstrated that ectopic expression of PRSS8 significantly inhibited the proliferation of A549 cells (Fig. 2B), compared with the mock group. To further confirm the role of PRSS8 in NSCLC in vivo, we constructed xenograft implanted human NSCLC in nude mice. The results indicated that mean subcutaneous tumor size was lower in the PRSS8-treated group than in the mock group over time (Fig. 2C). Ectopic expression of PRSS8 greatly reduced the tumor weight in a xenograft model (Fig. 2D).
Figure 2.
Ectopic expression of PRSS8 inhibited tumor growth in vitro and in vivo. A549 cells were transfected with PRSS8 or mock for 24 h. (A) The corresponding transfection efficiency was detected by Western blot and qRT-PCR. (B) Cell proliferation was evaluated using the WST-1 assay. PRSS8 cells (5 × 106) and control cells were injected subcutaneously into the left and right dorsal flanks of 4-week-old female BALB/c-nu mice. (C) Tumor volume was monitored every 5 days. (D) After 20 days, the animals were sacrificed, and the tumors were excised and weighed. Experiments were performed in triplicate. *p < 0.05 versus mock group.
Ectopic Expression of PRSS8 Inhibited the Migration and Invasion of NSCLC Cells
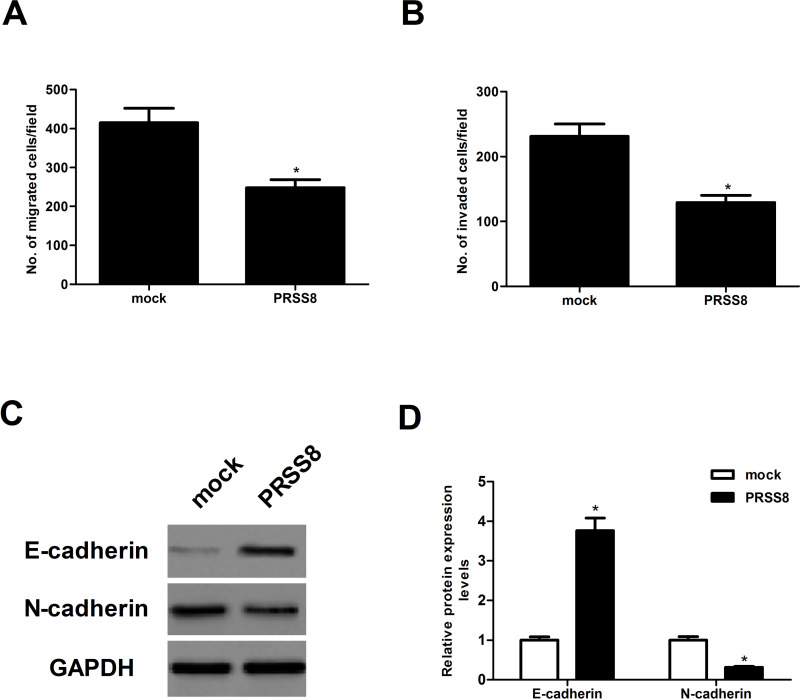
To detect the effect of PRSS8 on NSCLC cell migration, a Transwell migration assay was performed in A549 cells transfected with PRSS8. We found that ectopic expression of PRSS8 sharply reduced the number of A549 cells that migrated into the lower chamber, with a 40.3% reduction in A549 cells (Fig. 3A). Results of the Matrigel invasion assay indicated that ectopic expression of PRSS8 significantly inhibited the invasion of A549 cells (Fig. 3B). In order to examine whether PRSS8 inhibits the EMT process in NSCLC cells, A549 cells were transfected with PRSS8 or mock for 24 h. E-cadherin and N-cadherin expression levels were detected using Western bolt analysis. We found that the expression of E-cadherin was significantly upregulated, while the expression of N-cadherin was downregulated in A549 cells transfected with PRSS8, compared with the control cells (Fig. 3C and D).
Figure 3.
Ectopic expression of PRSS8 inhibited the migration and invasion of NSCLC cells. A549 cells were transfected with PRSS8 or mock for 24 h. (A) Cell migration was analyzed by Transwell assay. (B) Cell invasion was determined by Matrigel invasion assay. (C) Western blot assay was performed to detect the expression of N-cadherin and E-cadherin. (D) Graphic presentation of the relative abundance of N-cadherin and E-cadherin proteins. Experiments were performed in triplicate. *p < 0.05 versus mock group.
Ectopic Expression of PRSS8 Inhibited Activation of the JAK/STAT3 Signaling Pathway in NSCLC Cells
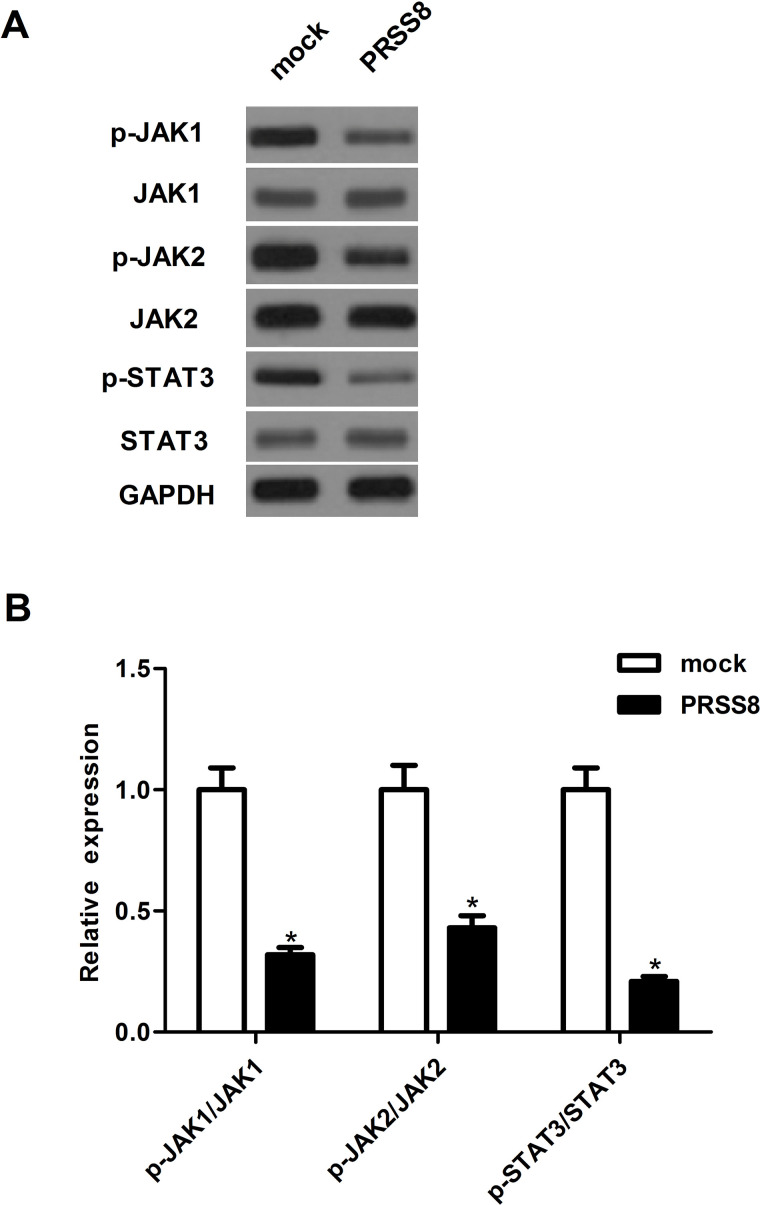
To investigate the molecular mechanism by which PRSS8 inhibited NSCLC cell proliferation and metastasis in A549 cells, we examined the effects of PRSS8 on JAK/STAT3 activation. Western blot analysis indicated that, compared with the mock group, ectopic expression of PRSS8 significantly downregulated the active tyrosine-phosphorylated forms of JAK1 and JAK2 and suppressed the expression of phosphorylation of the downstream JAK substrate STAT3 in A549 cells (Fig. 4).
Figure 4.
Ectopic expression of PRSS8 inhibited the activation of the JAK/STAT3 signaling pathway in NSCLC cells. A549 cells were transfected with PRSS8 or mock for 24 h. (A) Western blot assays were performed to detect the expression of p-JAK1, JAK1, p-JAK2, JAK2, p-STAT3, and STAT3. (B) Graphic presentation of the ratio of p-JAK1/JAK1, p-JAK2/JAK2, and p-STAT3/STAT3. Experiments were performed in triplicate. *p < 0.05 versus mock group.
DISCUSSION
In the present study, we showed that PRSS8 was expressed in a low amount in NSCLC cell lines. Ectopic expression of PRSS8 inhibited tumor growth in vitro and in vivo. Furthermore, ectopic expression of PRSS8 inhibited the migration and invasion of NSCLC cells and suppressed the EMT process in A549 cells. Mechanistically, we found that ectopic expression of PRSS8 downregulated the protein expression levels of p-JAK1, p-JAK2, and p-STAT3 in A549 cells.
Previous studies found that PRSS8 was involved in tumor progression and development. A study by Tamir et al. reported that the expression of PRSS8 was greatly upregulated in ovarian cancer tissues, compared with normal or benign ovarian lesions12. In contrast, PRSS8 expression was lower in high-grade transitional cell carcinomas and cell lines than that of normal human urothelium and in a normal human urothelial cell line10. These findings suggest that PRSS8 performs anoncogenic or a tumor suppressing function depending on the cell type or context. In this study, we observed that the expression levels of PRSS8 in both mRNA and protein were significantly downregulated in human NSCLC cell lines. Ectopic expression of PRSS8 inhibited tumor growth in vitro and in vivo. These data imply that PRSS8 may function as a tumor suppressor in the development and progression of NSCLC.
There is substantial evidence that EMT is crucial for the initiation of the metastatic process in NSCLC16–18. EMT is defined by the loss of epithelial characteristics and the acquisition of a motile, invasive, and migratory mesenchymal phenotype. Downregulation of E-cadherin, an epithelial marker, is a hallmark of EMT19,20. In addition, it has been reported that the loss of PRSS8 expression was related to reduced E-cadherin expression and a loss of epithelial morphology in transitional cell carcinoma cells10. Similarly, in this study, we observed that ectopic expression of PRSS8 inhibited the migration and invasion of NSCLC cells, as well as upregulated the expression of the epithelial marker E-cadherin and decreased the expression of the mesenchymal marker N-cadherin in A549 cells. These results suggest that PRSS8 is able to regulate the migration and invasion of NSCLC cells by suppressing the EMT process.
The JAK/STAT3 signaling pathway plays a critical role in the development and progression of various human cancers, including NSCLC21–23. The JAK family of proteins includes JAK1, JAK2, JAK3, and tyrosine kinase2. It was reported that JAK1 activated STAT3 activity in lung cancer cell lines24. STAT3 is persistently activated in 50% of lung adenocarcinomas and lung cancer-derived cell lines25, and activated STAT3 resulted in transmodulation of downstream target genes that are involved in cell proliferation, survival, angiogenesis, and metastasis25,26. Thus, inhibition of the JAK/STAT3 pathway may offer a novel targeted therapeutic approach for NSCLC. For example, two novel small-molecule STAT3 inhibitors (C188-9 and piperlongumine) reduced the levels of pSTAT3 and blocked tumor growth in nude mice bearing A549 tumor xenografts27. In line with these results, we found that the ectopic expression of PRSS8 downregulated the protein expression levels of p-JAK1, p-JAK2, and p-STAT3 in A549 cells. These results suggest that PRSS8 inhibited tumor growth in vitro and in vivo in human NSCLC cells, at least partially, through modulating the JAK/STAT3 signaling pathway.
In summary, our results showed that PRSS8 plays an important role in the growth and metastasis of NSCLC. PRSS8 inhibited tumor growth in vitro and in vivo in human NSCLC cells, at least partially, through modulating the JAK/STAT3 signaling pathway. Thus, PRSS8 may be a novel therapeutic target for the treatment of NSCLC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002;287:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aebi S, Davidson T, Gruber G, Cardoso F. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii27–39. [DOI] [PubMed] [Google Scholar]
- 3. Vansteenkiste J. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi89–98. [DOI] [PubMed] [Google Scholar]
- 4. Ramalingam, Suresh S, Dahlberg, Suzanne E, Langer, Gray, Robert, Belani, Chandra P. Outcomes for elderly advanced stage non-small cell lung cancer (NSCLC) patients (pts) treated with bevacizumab (B) in combination with carboplatin (C) and paclitaxel (P): Analysis of Eastern Cooperative Oncology Group (ECOG) 4599 study: PD3-3-5. J Thorac Oncol. 2007;2:S468. [Google Scholar]
- 5. Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102:4501–24. [DOI] [PubMed] [Google Scholar]
- 6. Wu Q. Type II transmembrane serine proteases. Curr Top Dev Biol. 2003;54:167–206. [DOI] [PubMed] [Google Scholar]
- 7. Ovaere P, Lippens SP, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009;34:453–63. [DOI] [PubMed] [Google Scholar]
- 8. Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois CN, Vandewalle A, Rossier B, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–34. [DOI] [PubMed] [Google Scholar]
- 9. Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LM, Verity NJ, Chai KX. Loss of prostasin (PRSS8) in human bladder transitional cell carcinoma cell lines is associated with epithelial-mesenchymal transition (EMT). BMC Cancer 2009;9:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakashita K, Mimori K, Tanaka F, Tahara K, Inoue H, Sawada T, Ohira M, Hirakawa K, Mori M. Clinical significance of low expression of Prostasin mRNA in human gastric cancer. J Surg Oncol. 2008;98:559–64. [DOI] [PubMed] [Google Scholar]
- 12. Tamir A, Gangadharan A, Balwani S, Tanaka T, Patel U, Hassan A, Benke S, Agas A, D’Agostino J, Shin D. The serine protease prostasin (PRSS8) is a potential biomarker for early detection of ovarian cancer. J Ovarian Res. 2016;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao Y, Li K, Guo Y, Wang Q, Li Z, Yang Y, Chen Z, Wang J, Zhao W, Zhang H. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget 2016;7:26780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bao Y, Wang Q, Guo Y, Chen Z, Li K, Yang Y, Zhang H, Dong H, Shen K, Yang W. PRSS8 methylation and its significance in esophageal squamous cell carcinoma. Oncotarget 2016;7:28540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Chen Z. Knockdown of CUL4B suppresses the proliferation and invasion in non-small cell lung cancer cells. Oncol Res. 2016;24:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Attar-Schneider O, Drucker L, Gottfried M. Migration and epithelial-to-mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI. Lab Invest. 2016;96:1004–15. [DOI] [PubMed] [Google Scholar]
- 18. Kucuksayan H, Ozes ON, Akca H. Downregulation of SATB2 is critical for induction of epithelial-to-mesenchymal transition and invasion of NSCLC cells. Lung Cancer 2016;98:122–9. [DOI] [PubMed] [Google Scholar]
- 19. Sánchez-Tilló E, Liu Y, Barrios OD, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu F, Dai C, Fu Y, Loo JFC, Xia D, Gao SP, Ma Z, Chen Z. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget 2016;7:9462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun MF, An XK, Pan LH, Zhang SL. Downregulation of leptin inhibits growth and induces apoptosis of lung cancer cells via the Notch and JAK/STAT3 signaling pathways. Biol Open 2016;5:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Y, Hong Y, Xu YJ, Liu P, Guo DH, Chen Y. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC. Apoptosis 2014;19:1627–36. [DOI] [PubMed] [Google Scholar]
- 24. Song L, Rawal B, Nemeth JA, Haura EB. JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther. 2011;10:481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–94. [DOI] [PubMed] [Google Scholar]
- 26. Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao YX, Pestell RG, Albanese C, Darnell JE. Stat3 as an oncogene (vol 98, pg 295, 1998). Cell 1999;98:295–303. [DOI] [PubMed] [Google Scholar]
- 27. Lewis KM, Bharadwaj U, Eckols TK, Kolosov M, Kasembeli MM, Fridley C, Siller R, Tweardy DJ. Small-molecule targeting of signal transducer and activator of transcription (STAT) 3 to treat non-small cell lung cancer. Lung Cancer 2015;90:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]