Abstract
A-kinase anchor protein 4 (AKAP4), a member of the A-kinase anchor family of proteins, plays a role in tumor development and progression. However, its expression pattern and function in human thyroid cancer remain obscure. Here we examined AKAP4 expression in thyroid cancer cell lines as well as the effects of AKAP4 on the proliferation and metastasis of thyroid cancer cells. We also explored the molecular mechanism by which AKAP4 mediates the metastatic potential of thyroid cancer cells. Our results revealed that the transcript and protein levels of AKAP4 were significantly upregulated in thyroid cancer cell lines. In vitro experiments showed that knockdown of AKAP4 significantly inhibited the proliferation, migration, invasion, and epithelial–mesenchymal transition (EMT) process in thyroid cancer cells. Additionally, knockdown of AKAP4 greatly decreased the protein expression of Shh as well as Smo, Ptc, and Gli-1 in ACT-1 cells. Finally, the in vivo nude mice model confirmed that knockdown of AKAP4 attenuated tumor growth. In conclusion, our findings demonstrated that knockdown of AKAP4 inhibited proliferation and metastasis, likely through suppressing the Shh signaling pathway, in thyroid cancer cells. Thus, AKAP4 may act as a potential therapeutic target for human thyroid cancer.
Key words: A-kinase anchor protein 4 (AKAP4), Thyroid cancer, Invasion, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Thyroid cancer is one of the most malignant cancers of the endocrine system. Its incidence has been rising steadily during the past decades1. Anaplastic thyroid cancer accounts for only 2% of thyroid cancer2. Despite considerable progress in treatment strategies, including surgery and a combination of chemotherapy and radiation, there has been no significant improvement in the survival rate of anaplastic thyroid cancer patients3–5. Therefore, elucidation of the molecular mechanisms underlying anaplastic thyroid cancer tumorigenesis is critical to determine individual treatments for thyroid cancer.
A-kinase anchor protein 4 (AKAP4) is a member of the A-kinase anchor family of proteins that bind the protein kinase A (PKA) regulatory subunit and functions to anchor PKA to specific cellular locations6. Previous studies demonstrated that AKAP4 plays a role in tumor development and progression in a variety of cancers, including esophageal, ovarian, breast, and lung7–10. A study by Jagadish et al. reported that the expression of AKAP4 was highly expressed in all colon cancer cells. Downregulation of AKAP4 inhibited the growth, migration, and invasion, and increased apoptosis and senescence of colorectal cancer cells, as well as attenuated tumor growth in a human xenograft mouse model11. However, its expression pattern and function in human thyroid cancer remain obscure. Here we examined AKAP4 expression in thyroid cancer cell lines. Additionally, the effects of AKAP4 on the proliferation and metastasis of thyroid cancer cells were examined. Finally, we explored the molecular mechanism by which AKAP4 mediates the metastatic potential of thyroid cancer cells. Our results suggest that AKAP4 is involved in the carcinogenesis and development of thyroid cancer and may serve as a potential therapeutic target for the treatment of thyroid cancer.
MATERIALS AND METHODS
Cell Culture
Human anaplastic thyroid cancer cell lines (ACT-1, FRO, and CAL62) and the normal thyroid epithelial cell-derived cell line HTori-3 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 1% glutamine, and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA). All cells were cultured at 37°C with 5% CO2 in an incubator (Life Technologies, Baltimore, MD, USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was isolated from thyroid cancer cells using TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions. RNA (5 μg) was reverse transcribed to cDNA using a SimpliAmp Thermal Cycler (Applied Biosystems, Foster City, CA, USA). RT-PCR was performed on Bio-Rad iQ5 Quantitative PCR System (Takara Bio Inc., Otsu, Japan). The primers of each gene were shown as follows: AKAP4, 5′-GGGTGTGTGCAAGGTAGATCTCT-3′ (forward) and 5′-CACATCGACAAAGCATATCACTTTC-3′ (reverse); β-actin, 5′-GAGGCACTCTTCCAGCCTTC-3′ (forward) and 5′-GGATGTCCACGTCACACTTC-3′ (reverse). The relative mRNA levels were normalized to β-actin and determined by calculating the comparative Ct method.
Western Blot Analysis
The cells were homogenized and lysed with RIPA lysis buffer (Invitrogen). The protein lysates (40 μg/lane) were subjected to 12% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in TBST solution containing 5% skimmed milk powder at room temperature for 1 h, and then incubated with primary antibodies (dilution: 1:1,000) targeting AKAP4, E-cadherin, N-cadherin, Sonic hedgehog (Shh), Smoothened (Smo), Ptc, Gli-1, and GAPDH (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG secondary antibodies (Santa Cruz Biotechnology) for 1 h. Antigen–antibody complexes were visualized using chemiluminescence (ECL) detection system (Amersham, Little Chalfont, UK).
RNA Interference and Transfection
The short hairpin RNA against AKAP4 (sh-AKAP4) and its negative control (sh-NC) were synthesized by RiBo Biotech (GuangZhou RiBo Biotech, P.R. China). For in vitro transfection, ACT-1 cells were seeded into each well of 24-well microplates, grown for 24 h to reach 50% confluence, and transfected with sh-AKAP4 or sh-NC using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Cell Proliferation Assay
Infected cells at a density of 1 × 104 cells/well were seeded into 96-well plates and incubated for 4 days. Cell proliferation was detected using a water-soluble tetrazolium salt assay using cell counting kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. The absorbance at 570 nm was determined using a Dynex microplate reader.
Cell Migration and Invasion Assays
For the Transwell migration assay, infected cells at a density of 5 × 104 cells per chamber were seeded into the upper chambers, and 600 μl of 10% FBS-containing medium was placed in the lower chamber as a chemoattractant. After 24 h of culture, cells on the underside of the filter were fixed with 10% formalin, stained with 0.1% crystal violet, examined by light microscope, and counted using a high-power microscope. For the Transwell invasion assay, the upper chamber was precoated with 1 mg/ml of Matrigel (BD Biosciences, San Jose, CA, USA)or 3 h at 37°C to form a basement membrane. Then the assay was performed as described above for the Transwell assay.
Nude Mouse Xenograft Model
Four-week-old male BALB/c nude mice (18–20 g) were purchased from the Laboratory Animal of Huaihe Hospital of Henan University (P.R. China). Animal procedures were carried out according to a protocol approved by the Institutional Animal Care and Use Committee at Huaihe Hospital of Henan University. Infected ACT-1 cells (5 × 106) in 0.2 ml of PBS were injected subcutaneously into the flanks of mice (n = 6 per group). Tumor volumes were measured with calipers every 1 week and calculated according to the following formula: V = L × W 2/2. After 4 weeks, mice were sacrificed, and the tumor was resected and weighed.
Statistical Analysis
All results are reported as means ± SD. Statistical analysis involved Student’s t-test for the comparison of two groups or one-way ANOVA for multiple comparisons. A value of p < 0.05 was considered significantly different.
RESULTS
AKAP4 Was Highly Expressed in Thyroid Cancer Cell Lines
We initially analyzed endogenous AKAP4 expression in three human anaplastic thyroid cancer cell lines. Compared with HTori-3 cells, the mRNA expression levels of AKAP4 were significantly increased in ACT-1, FRO, and CAL62 cells (Fig. 1A). Western blotting showed similar results (Fig. 1B).
Figure 1.
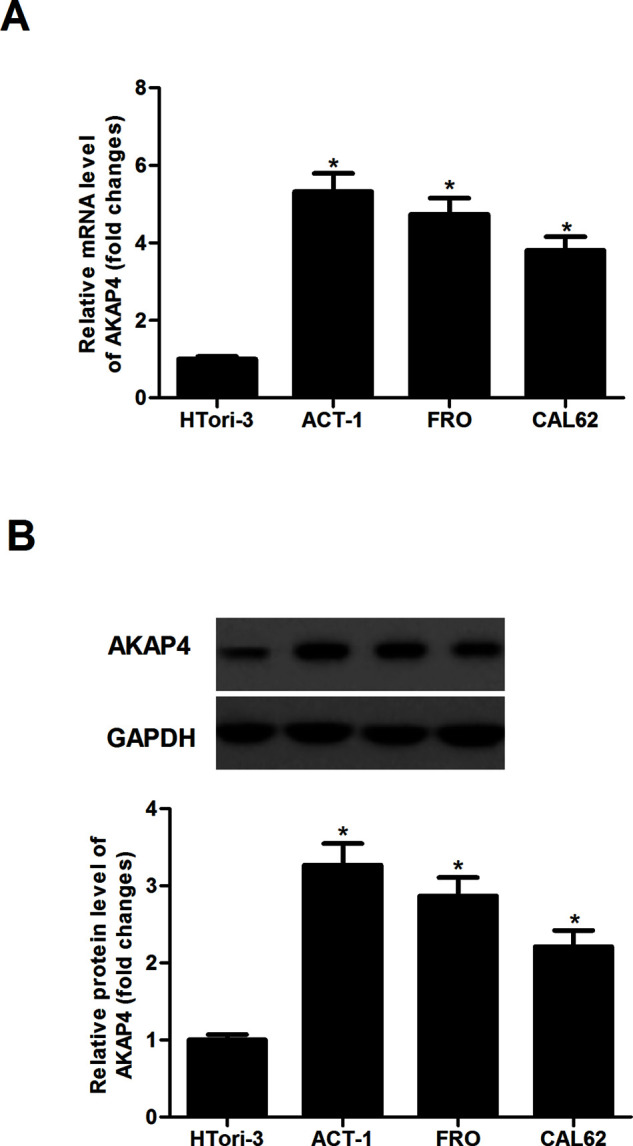
AKAP4 was highly expressed in thyroid cancer cell lines. (A) qRT-PCR analysis was performed to detect the mRNA expression of AKAP4 in three human thyroid cancer cell lines. (B) Western blot analysis was performed to detect the protein expression of AKAP4 in three human thyroid cancer cell lines. Columns represent the means ± SD. *p < 0.05 versus sh-NC group.
Silencing of AKAP4 Inhibited the Proliferation of Thyroid Cancer Cells
To explore the role of AKAP4 in thyroid cancer, AKAP4 was downregulated by shRNA against AKAP4 in ACT-1 cells. The results of the Western blot analysis indicated that the protein expression of AKAP4 was significantly decreased in ACT-1 cells transfected with sh-AKAP4 (Fig. 2A). We then tested the effect of sh-AKAP4 on thyroid cancer cell proliferation. The results showed that silencing of AKAP4 significantly inhibited the proliferation of ACT-1 cells, exhibiting a time-dependent manner (Fig. 2B).
Figure 2.
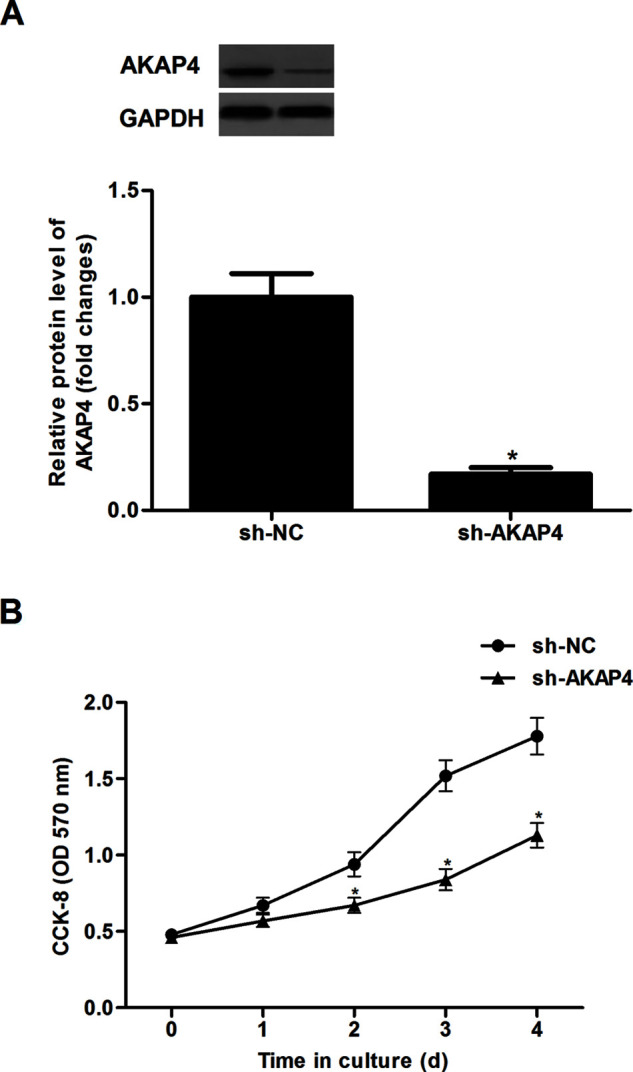
Silencing of AKAP4 inhibited the proliferation of thyroid cancer cells. ACT-1 cells were infected with sh-AKAP4 or si-NC for 24 h, respectively. (A) The protein expression of AKAP4 was measured by Western blot. (B) Cell proliferation was evaluated using the CCK-8 assay. Columns represent the means ± SD. *p < 0.05 versus sh-NC group.
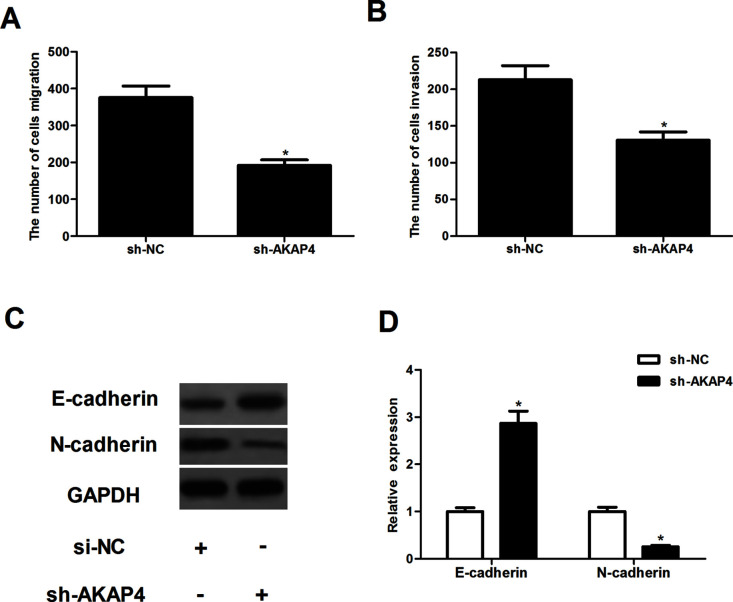
Silencing of AKAP4 Inhibited Cell Migration and Invasion
To examine the effects of AKAP4 on thyroid cancer cell migration and invasion, the Transwell migration and invasion assays were performed in thyroid cancer cells. Compared with the sh-NC group, the number of cells that migrated was significantly reduced in ACT-1 cells transfected with sh-AKAP4 (Fig. 3A). Transwell invasion indicated that knockdown of AKAP4 obviously repressed the invasiveness of ACT-1 cells (Fig. 3B). Furthermore, to investigate the role played by AKAP4 during EMT, we investigated the effect of AKAP4 on the expression levels of EMT marker proteins. The results of the Western blotting analysis demonstrated that knockdown of AKAP4 significantly upregulated the expression of E-cadherin and downregulated the expression of N-cadherin in ACT-1 cells, compared to sh-NC-transfected cells (Fig. 3C).
Figure 3.
Silencing of AKAP4 inhibited cell migration and invasion. ACT-1 cells were infected with sh-AKAP4 or si-NC for 24 h, respectively. (A, B) Cell migration and invasion were measured using the Transwell migration and invasion assays, respectively. (C) The expression of E-cadherin and N-cadherin was measured by Western blot. (D) The average band intensities of E-cadherin and N-cadherin normalized to GAPDH. Columns represent the means ± SD. *p < 0.05 versus sh-NC group.
Silencing of AKAP4 Inhibited the Activation of Shh Signaling Pathway in Thyroid Cancer Cells
To further investigate the underlying molecular mechanism of AKAP4 in regulating thyroid cancer growth and metastasis, we analyzed the effect of AKAP4 on Shh pathway activation in ACT-1 cells. Knockdown of AKAP4 greatly decreased the protein expression of Shh, Smo, Ptc, and Gli-1 in ACT-1 cells, compared with the sh-NC group (Fig. 4).
Figure 4.
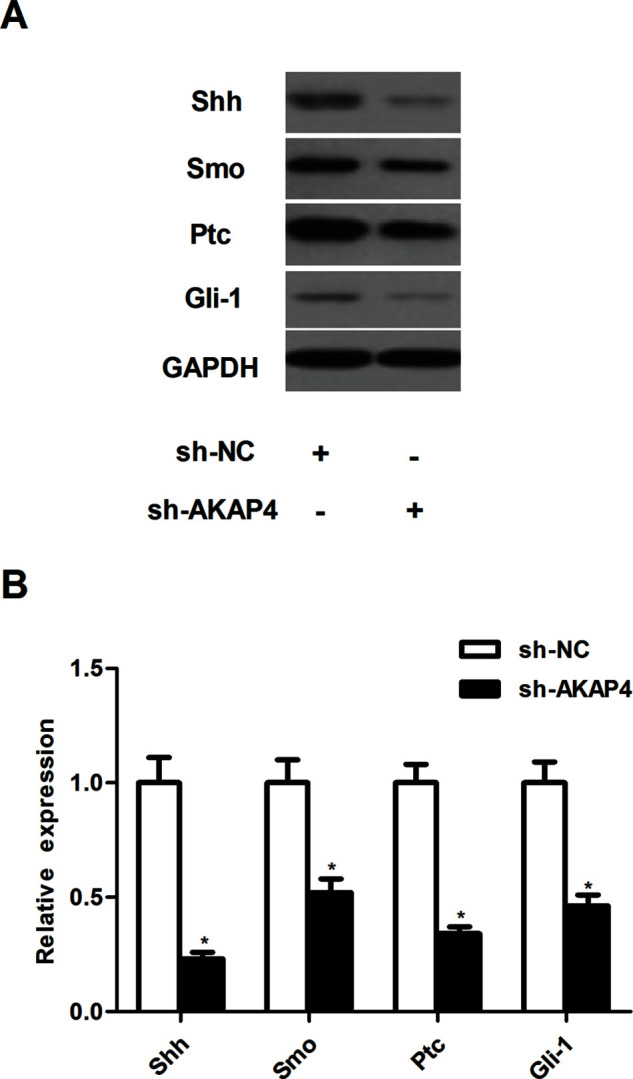
Silencing of AKAP4 inhibited the activation of Sonic hedgehog signaling pathway in thyroid cancer cells. ACT-1 cells were infected with sh-AKAP4 or si-NC for 24 h, respectively. (A) The expression of Shh, Smo, Ptc, and Gli-1 was measured by Western blot. (B) The average band intensities of Shh, Smo, Ptc, and Gli-1 normalized to GAPDH. Columns represent the means ± SD. *p < 0.05 versus sh-NC group.
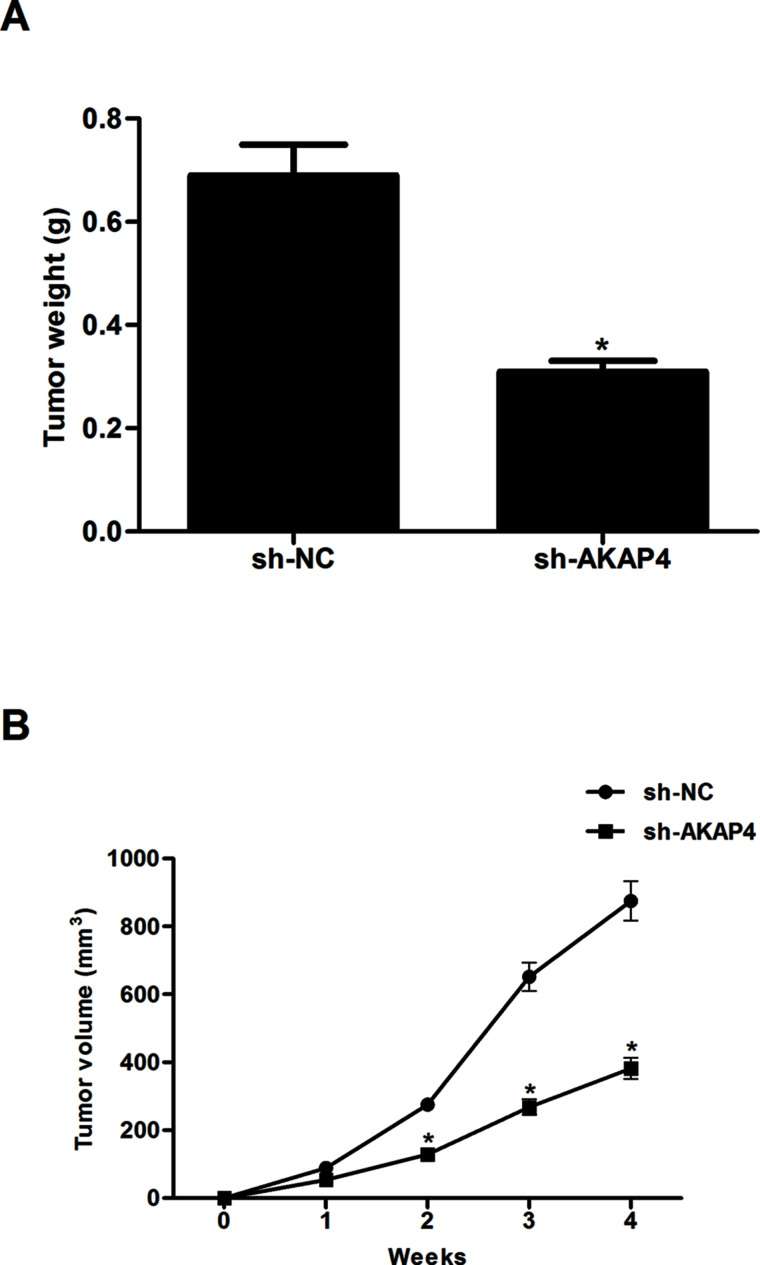
Silencing of AKAP4 Attenuated Xenografted Tumor Growth in Nude Mice
In order to further test the role of AKAP4 in thyroid cancer in vivo, we established a subcutaneous xenograft tumor model in nude mice. The volume of tumors derived from the sh-AKAP4-transfected group was markedly smaller than that of controls (Fig. 5A). In addition, compared with the sh-NC group, knockdown of AKAP4 sharply reduced the mean weight of tumors (Fig. 5B).
Figure 5.
Silencing of AKAP4 attenuated xenografted tumor growth in nude mice. Infected ACT-1 cells (5 × 106) in 0.2 ml of PBS were injected subcutaneously into the flanks of mice. (A) Tumor growths were observed continuously for 4 weeks. (B) After 4 weeks, mice were sacrificed, and the tumor was resected and weighed. Columns represent the means ± SD. *p < 0.05 versus sh-NC group.
DISCUSSION
In this study, our work first revealed that the transcript and protein levels of AKAP4 were significantly upregulated in thyroid cancer cell lines. Based on in vitro assays, we found that knockdown of AKAP4 significantly inhibited the proliferation, migration, invasion, and EMT process in thyroid cancer cells. Additionally, knockdown of AKAP4 greatly decreased the protein expression of Shh, Smo, Ptc, and Gli-1 in ACT-1 cells. Finally, the in vivo nude mice model confirmed that knockdown of AKAP4 attenuated tumor growth.
AKAP4 has been suggested to correlate with the development of different types of carcinoma. Li and his team showed that AKAP4 mRNA and protein levels were obviously upregulated in esophageal cancer tissues, silencing AKAP4 inhibited cell growth in vitro and suppressing tumor growth in a xenograft model7. Li et al. reported that, compared to adjacent noncancerous tissues, AKAP4 was highly expressed in lung adenocarcinoma tissues12. In accordance with previous studies, our experiment results showed that AKAP4 expression was significantly upregulated in thyroid cancer cell lines, and knockdown of AKAP4 significantly inhibited the proliferation of thyroid cancer cells and attenuated the tumor growth in vivo. These data suggest that AKAP4 is involved in the carcinogenesis of thyroid cancer.
EMT is a core process during thyroid cancer cell invasion13. During the EMT process, epithelial cells lose their cell polarity and cell–cell junctions and gain mesenchymal properties of migration and invasion14. Reduction or loss of E-cadherin expression is one of the well-established hallmarks of EMT15. Furthermore, Saini et al. confirmed that gene silencing of AKAP4 suppressed the migration and invasion abilities of cervical cancer cells16. In this study, we observed that knockdown of AKAP4 significantly inhibited thyroid cancer cell migration and invasion. Moreover, knockdown of AKAP4 resulted in increased E-cadherin expression and reduced expression of N-cadherin in thyroid cancer cells. Our data suggest that AKAP4 might promote EMT-associated tumor invasion and metastasis in thyroid cancer.
The Shh signaling pathway plays an important role in tumor cell proliferation, metastasis, and tumorigenesis17–19. It is highly activated in thyroid neoplasms, and activation of the Hh pathway resulted in increased expression of key Hh signaling components, Smo and Gli-119,20. Gli-1, a transcriptional factor of the Hedgehog pathway, can directly bind the E-cadherin promoter and induce its expression21,22. In addition, knockdown of Shh or Gli-1 led to decreased cell motility and invasiveness in thyroid cancer cells23. Thus, the Shh signaling pathway may be a potential therapeutic target for thyroid cancer treatment. In the present study, we found that knockdown of AKAP4 greatly decreased the protein expression of Shh, Smo, Ptc, and Gli-1 in ACT-1 cells. All of these data imply that knockdown of AKAP4 inhibits proliferation and metastasis, likely through interfering the Shh signaling pathway in thyroid cancer cells.
In conclusion, our findings highlight the importance of AKAP4 in thyroid cancer development and progression in vitro and in vivo. Knockdown of AKAP4 inhibited proliferation and metastasis, likely through suppressing the Shh signaling pathway in thyroid cancer cells. Thus, AKAP4 may act as a potential therapeutic target for human thyroid cancer.
ACKNOWLEDGMENTS
This research was funded by the Project of Technology Department of Henan Province of China (Grant No. 162102310076) and the Project of Henan University of China (Grant No. 2009YBGG005).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Sherman SI, Angelos P, Ball DW, Beenken SW, Byrd D, Clark OH, Daniels GH, Dilawari RA, Ehya H, Farrar WB, Gagel RF, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey J, Olson JA Jr, Ridge JA, Shah JP, Sisson JC, Tuttle RM, Urist MM; National Comprehensive Cancer Network Thyroid Carcinoma Panel. Thyroid carcinoma. J Natl Compr Canc Netw. 2007;5:568–621. [DOI] [PubMed] [Google Scholar]
- 2. Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control 2006;13:119–28. [DOI] [PubMed] [Google Scholar]
- 3. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr Relat Cancer 2009;16:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green LD, Mack L, Pasieka JL. Anaplastic thyroid cancer and primary thyroid lymphoma: A review of these rare thyroid malignancies. J Surg Oncol. 2006;94:725–36. [DOI] [PubMed] [Google Scholar]
- 5. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol. 2010;22:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yanqiu H, Hongshi Y, Pask AJ, O’Brien DA, Geoff S, Renfree MB. A-kinase anchoring protein 4 has a conserved role in mammalian spermatogenesis. Reproduction 2009;137:645–53. [DOI] [PubMed] [Google Scholar]
- 7. Li S, Qin X, Li Y, Guo A, Ma L, Jiao F, Chai S. AKAP4 mediated tumor malignancy in esophageal cancer. Am J Transl Res. 2016;8:597–605. [PMC free article] [PubMed] [Google Scholar]
- 8. Jagadish N, Parashar D, Gupta N, Agarwal S, Sharma A, Fatima R, Suri V, Kumar R, Gupta A, Lohiya NK. A novel cancer testis antigen target A-kinase anchor protein (AKAP4) for the early diagnosis and immunotherapy of colon cancer. Oncoimmunology 2016;5:e1078965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saini S, Jagadish N, Gupta A, Bhatnagar A, Suri A. A novel cancer testis antigen, A-kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS One 2013;8:e57095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gumireddy K, Li A, Chang DH, Liu Q, Kossenkov AV, Yan J, Korst RJ, Nam BT, Xu H, Zhang L. AKAP4 is a circulating biomarker for non-small cell lung cancer. Oncotarget 2015;6:17637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jagadish N, Parashar D, Gupta N, Agarwal S, Purohit S, Kumar V, Sharma A, Fatima R, Topno AP, Shaha C. A-kinase anchor protein 4 (AKAP4) a promising therapeutic target of colorectal cancer. J Exp Clin Canc Res. 2015;34:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li HM, Kang G, Yu XY, Zhuang Y, Ping X. Expression and clinical significance of A-kinase anchor protein 4 in lung adenocarcinoma tissue. Thorac Cancer 2015;7:273–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie J, Fan Y, Zhang X. Molecular mechanisms in differentiated thyroid cancer. Front Biosci. 2016;21:119–29. [DOI] [PubMed] [Google Scholar]
- 14. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. J Oral Maxil Pathol. 2009;139:871–90. [DOI] [PubMed] [Google Scholar]
- 15. Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saini S, Agarwal S, Sinha A, Verma A, Parashar D, Gupta N, Ansari AS, Lohiya NK, Jagadish N, Suri A. Gene silencing of A-kinase anchor protein 4 inhibits cervical cancer growth in vitro and in vivo. Cancer Gene Ther. 2013;20:413–20. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Ding H, Rao G, Arora S, Saclarides CP, Esparaz J, Gattuso P, Solorzano CC, Prinz RA. Activation of the sonic hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation. Endocr Relat Cancer 2012;19:621–32. [DOI] [PubMed] [Google Scholar]
- 18. Dong W, Cui J, Tian X, He L, Wang Z, Zhang P, Zhang H. Aberrant sonic hedgehog signaling pathway and STAT3 activation in papillary thyroid cancer. Int J Clin Exp Med. 2014;7:1786–93. [PMC free article] [PubMed] [Google Scholar]
- 19. Bohinc B, Michelotti G, Diehl AM. Hedgehog signaling in human medullary thyroid carcinoma: A novel signaling pathway. Thyroid 2013;23:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinterseher U, Wunderlich A, Roth S, Ramaswamy A, Bartsch DK, Hauptmann S, Greene BH, Fendrich V, Hoffmann S. Expression of hedgehog signalling pathway in anaplastic thyroid cancer. Endocrine 2014;45:439–47. [DOI] [PubMed] [Google Scholar]
- 21. Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: Mediators of oncogenic hedgehog signalling. Eur J Cancer 2006;42:437–45. [DOI] [PubMed] [Google Scholar]
- 22. Chun HW, Hong R. Significance of the hedgehog pathway-associated proteins Gli-1 and Gli-2 and the epithelial-mesenchymal transition-associated proteins Twist and E-cadherin in hepatocellular carcinoma. Oncol Lett. 2016;12:1753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williamson AJ, Doscas ME, Ye J, Heiden KB, Xing M, Li Y, Prinz RA, Xu X. The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met. Oncotarget 2016;7:10472–85. [DOI] [PMC free article] [PubMed] [Google Scholar]