Abstract
Antiretroviral drugs used for the treatment of human immunodeficiency virus (HIV) have proven to be effective even against cancer. Drawing from this background, the aim of our research project was to evaluate the effects of anti-HIV drugs that belong to the nucleoside and nucleotide reverse transcriptase inhibitor [NRTI; abacavir (ABC) and tenofovir (TDF)], nonnucleoside reverse transcriptase inhibitor [NNRTI; efavirenz (EFV) and etravirine (ETR)], and protease inhibitor [PI; darunavir (DRV)] categories on ovarian adenocarcinoma cell line SKOV-3. Using FACS analysis, we observed that treatment with NRTIs and NNRTIs showed a block in the G0/G1 phase. In particular, ETR displayed a relevant block in the progression of the G0/G1 phase of the cell cycle compared with the other examined drugs, and it also induced differentiation of SKOV-3 cells. In contrast, FACS analysis demonstrated that ABC and the PI inhibitor DRV showed no effect on the proliferation of cancer cells. DAPI (4′,6-diamidino-2-phenylindole) staining demonstrated that cells treated with NNRTIs (EFV and ETR) presented more DNA damage compared with other treatments. Immunoblotting analysis demonstrated that TDF, EFV, and ETR were able to obtain a reduction in the expression of cyclin D1 and Rb hypophosphorylation, and an increase in p21 concentration. Finally, we observed that ETR also induced differentiation, as demonstrated by Western blot, with high levels of E-cadherin expression. Therefore, our study provides additional evidence supporting the in vitro cytotoxic effects of ETR and EFV. Furthermore, it promotes the hypothesis for their potential use as therapeutic agents in ovarian cancer.
Key words: Cell cycle, Ovarian cancer, Antiretroviral drugs, Antineoplastic effect
INTRODUCTION
HIV infection is characterized by an inherently increased risk for blood and solid organ malignancies. The use of highly active antiretroviral therapy (HAART) has resulted in a decrease in HIV viral load and, consequently, in a substantial reduction of the progression of HIV infection to acquired immune deficiency syndrome (AIDS), as well as a reduction in opportunistic infections, hospitalizations, and death1.
Interestingly, recent observations have underlined a decreasing incidence of neoplastic lesions in patients in antiretroviral therapy, such as Kaposi’s sarcoma and non-Hodgkin’s lymphoma2–5, supporting the possible potential antineoplastic impact of HAART.
Experimental studies have demonstrated a direct antineoplastic effect from antiretroviral drugs1. Ikezoe et al. reported that some protease inhibitors (PIs) are able to induce growth arrest and apoptosis in multiple myeloma cells in vitro6, whereas Jiang et al. described cell cycle arrest and apoptosis from nelfinavir (NLF) in melanoma cells7. In addition, it is demonstrated that efavirenz (EFV) and nevirapine (NVP) reversibly downregulated cell proliferation and induced significant cell differentiation in primary human renal cell carcinoma8. In a multicenter phase II trial, Houédé et al. observed the efficacy of EFV in patients with metastatic castration-resistant prostate cancer9. The exploratory analysis showed nonprogression of prostate-specific antigen (PSA) with higher plasma concentrations of this drug9.
Our research group previously described the ability of the PIs amprenavir (APV) and indinavir (IDV) to inhibit the migration of human hepatocarcinoma cells in vitro and the growth of xenografts in vivo10–12. Therefore, it is very suggestive to consider the hypothesis that some HIV drugs, which were independently developed for the inhibition of specific retroviral enzymes, could represent a potential reservoir for anticancer drugs13.
However, there is very little data on the differential antiproliferative effects of anti-HIV drugs on ovarian cancer. Several lines of evidence have confirmed the association between high levels of endogenous reverse transcriptase (RT) and the phenotype of the tumor cell. The gene expression that codes for RT is generally repressed in differentiated nonpathological tissues; instead, it is present in the germ cells of breast, embryonic, and tumor tissues2.
We can distinguish two major categories of drugs that inhibit the action of RT: enzyme nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs and NtRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs). NRTIs and NtRTIs, such as abacavir (ABC) and tenofovir (TDF), are analogs of the natural substrate (dNTP), and, competing with its binding site (dTTP), they act as chain terminators. They are incorporated by inverse transcriptase in viral DNA chains in growth, blocking further development14. NNRTIs, such as EFV and etravirine (ETR), are molecules that are chemically different from the natural substrate that blocks the enzyme polymerase activity interacting with it in a noncompetitive way (allosteric site)3,4. Instead, PIs such as darunavir (DRV) block an enzyme called protease that is involved in HIV reproduction15. When the enzyme is blocked, the virus cannot reproduce normally, and the rate of multiplication is slowed down.
Cell cycle control is regulated by specific check points at the G1/S phase and at the G2/M transition. The activities and interactions of several genes, by different family members, have the ability to regulate cell cycle checkpoints, cell proliferation, and the differentiation processes16.
The aim of our study was to evaluate the effects of various classes of antiretroviral drugs on the proliferation and differentiation of ovarian cancer cells in vitro by evaluating their influence on the cell cycle machine.
MATERIALS AND METHODS
Cells and Cell Cultures
The SKOV-3 human ovarian carcinoma cell line was purchased from the ATCC (Rockville, MD, USA) and cultivated, according to the recommendation of the supplier, in RPMI-1640 medium supplemented with 10% fetal bovine solution (FBS), 1% penicillin/streptomycin, and 1% l-glutamine at 37°C in humidified air containing 5% CO2. The medium, FBS, and antibiotics were purchased from Gibco (Life Technologies, Milan, Italy) for passaging; cells were detached with trypsin/EDTA and subsequently replated. The cells were seeded into six-well plates at a concentration of 5 × 104 cells/well. Once they reached confluence of 90%, plated SKOV-3 cells were starved and then treated with the chosen drugs (ABC, TDF, EFV, ETR, and DRV).
Chemicals and Treatments
EFV, TDF, and ABC were purchased from Sequoia Research Product Ltd. (Pangbourne, UK). ETR was obtained from Janssen Pharmaceutical N.V. Belgium. DRV, EFV, DRV, TDF, and ETR were dissolved in dimethyl sulfoxide (DMSO), and ABC was dissolved in H2O. All agents tested were diluted into culture medium at a final concentration of 20 mM for 48 h. A preliminary dose–response curve was performed to determine the highest concentration of the drugs not toxic for the cells.
Flow Cytometry for Detection of Cell Cycle
As previously described,17 after 48 h, treated cells were collected by mild trypsination, washed in PBS, and fixed in 70% ethanol at 4°C. Cells were collected and resuspended in 500 ml of a hypotonic buffer (0.1%Triton X-100, 0.1% sodium citrate, 50 mg/ml propidium iodide, and 100 mg/ml RNase). The cells were then analyzed using a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA, USA), and the percentages of G1, S, G2/M, and sub-G1 (apoptotic cells) populations were calculated, respectively. All the experiments were performed three times18.
Immunofluorescence Expression of DAPI
DAPI is a fluorescent stain that binds strongly to A-T-rich regions in DNA. It is used extensively in fluorescence microscopy. DAPI can pass through an intact cell membrane and can be used to stain both live and fixed cells, although it passes through the membrane less efficiently in live cells, and therefore the effectiveness of the stain is lower. As previously described17, cells were placed on coverslips at a density of 5 × 105 cells/well in DMEM and treated with various drugs. At the time of the experiment, old medium was removed, and cells were washed in PBS and fixed with 4% paraformaldehyde for 8 min at 4°C followed by washing three times with 1× PBS. Cells were incubated with 0.1% Triton X-100 for 10 min and with DAPI (1:1,000 dilution in blocking buffer) for 15 min and mounted using 50% glycerol. Signals were visualized at a wavelength of 350/460 nm (excitation/emission) using a fluorescence microscope (model IX71; Olympus, Japan).
Immunoblotting
Immunoblotting was performed as previously described19–21. Briefly, the cells were lysed in 100 μl of RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min in ice. Lysates were centrifuged at 14,000 × g for 10 min at 4°C. Total cell protein extracts were normalized for concentration by the Bradford assay (Bio-Rad Laboratories Segrate, Milan, Italy), and 20 μg of proteins was separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Membranes were incubated with cyclin D1 mouse monoclonal antibody (1:500; Santa Cruz Biotechnology), RBP1 goat polyclonal antibody (1:1,000; Santa Cruz Biotechnology), p21 rabbit polyclonal antibody (1:500; Santa Cruz Biotechnology), and E-cadherin (1:500; Santa Cruz Biotechnology). Antibodies were detected using anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ, USA) and visualized with the ECL detection system (Amersham Biosciences) according to the manufacturer’s instructions. Each membrane was probed with the monoclonal anti-actin antibody (Fitzgerald Industries International, Acton, MA, USA) to estimate equal protein loading. The quantification of membranes was made using ImageJ software.
Statistical Analysis
Where applicable, numerical results were expressed as the mean ± standard error of the mean (SEM) of three independent experiments. Statistical differences were considered if the value was p < 0.05, p < 0.01, or p < 0.001 as determined by ANOVA followed by Student’s t-test. Data are expressed as the percentage and SEM.
RESULTS
The ovarian adenocarcinoma cell line SKOV-3 was treated with five different drugs (ABC, TDF, EFV, ETR, and DRV) at concentrations between 5 and 20 μM for 24 and 48 h (data not shown) in order to evaluate the influence of anti-HIV drugs on the differentiation and proliferation of the cells.
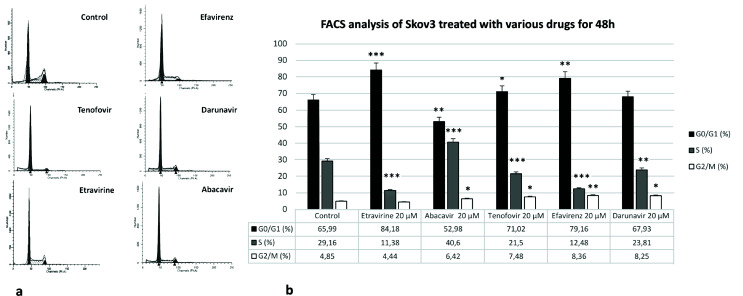
Samples analyzed by FACS assays showed that, with regard to different concentrations and time, the concentration of 20 μM for 48 h was the most suitable for studying the effects of these anti-HIV drugs (Fig. 1a). The results obtained on SKOV-3 cells that were treated with antiretroviral drugs (20 μM) for 48 h are shown in Figure 1b.
Figure 1.
(a) FACS assays of SKOV-3 cells treated with five different anti-HIV drugs [abacavir (ABC), tenofovir (TDF), efavirenz (EFV), etravirine (ETR), and darunavir (DRV)] (20 μM) for 48 h. (b) Graphic representation of data obtained with five different anti-HIV drugs (ABC, TDF, EFV, ETR, and DRV) (20 μM for 48 h). All data are presented as the means ± SEM of three independent experiments.
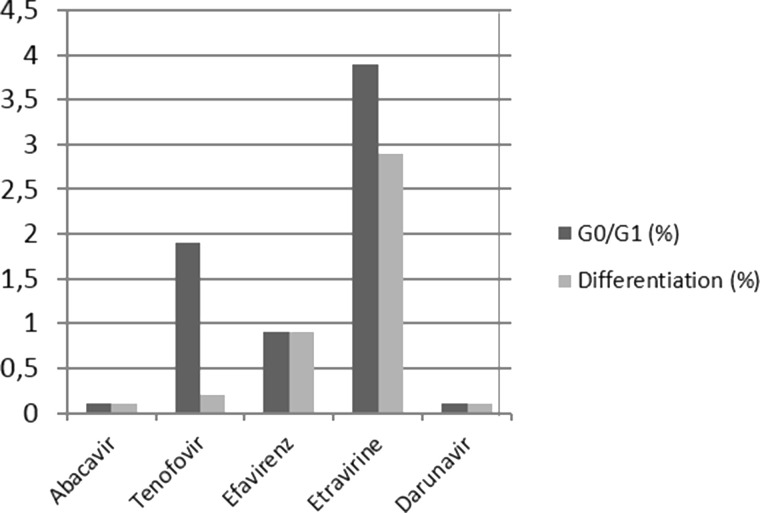
After TDF treatment, cells showed a delay in the G0/G1 phase of cell cycle progression. Treatment with EFV resulted in a block in the progression of the G0/G1 phase of the cell cycle. After ETR treatment, cells exhibited a relevant block in the progression of the G0/G1 phase of the cell cycle. This blocking of the G0/G1 phase in the cell cycle was higher when compared to EFV. Moreover, ETR induced differentiation. Finally, neither ABC nor DRV showed any particular modulation on SKOV-3 cells (Fig. 2).
Figure 2.
Graphic representation of the percentage distribution of cells in the G0/G1 phase and differentiation after treatment with drugs.
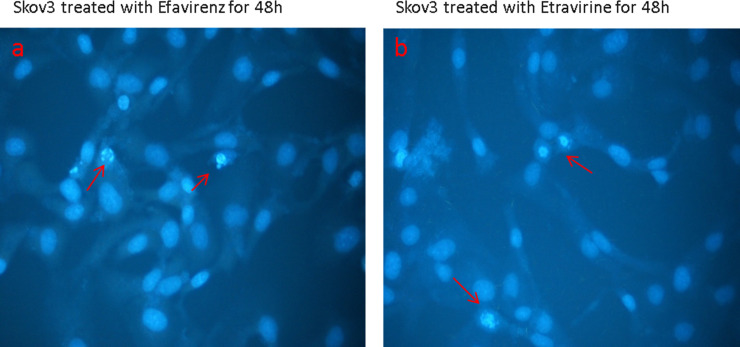
Drawing from the data obtained, we used DAPI staining to evaluate whether treatment with different drugs could cause nuclear damage. DNA labeling allows a morphological evaluation of normal or apoptotic nuclei. In apoptosis, there is chromatin clump condensation, and with DAPI, there are spots with much more intense coloring.
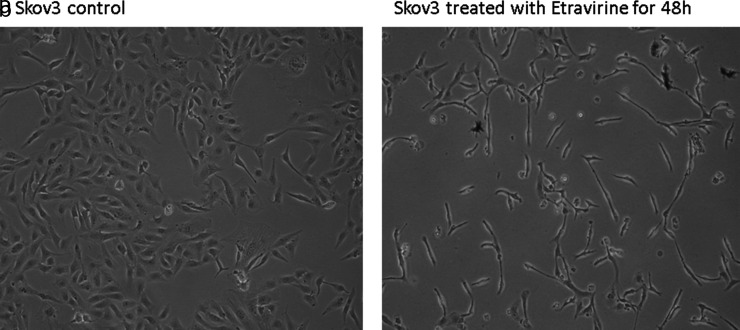
Our results pointed out that there was significant DNA damage in cells treated with EFV (Fig. 3a) and an increased condensation in cells treated with ETR (Fig. 3b). Finally, a morphological observation of the treated cells showed that only the cells treated with ETR appeared to be flattened with fusiform extensions protruding from the cell periphery (Fig. 4).
Figure 3.
DAPI assay on SKOV-3 cells treated for 48 h with 20 μM efavirenz (a) and etravirine (b).
Figure 4.
Morphological analysis of SKOV-3 cells treated with etravirine (a) compared with untreated cells (b).
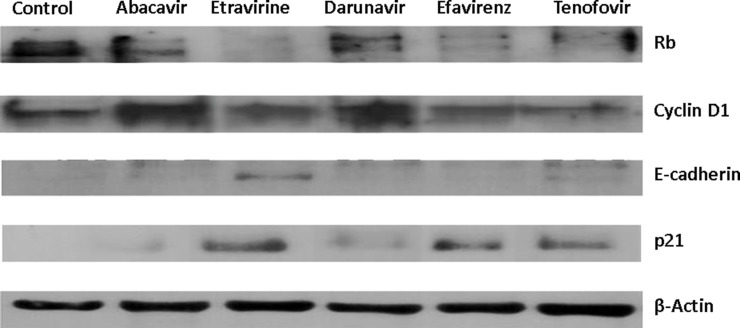
As the final step, we decided to evaluate whether a modulation of proteins involved in the cell cycle and differentiation was present when using different treatments. Immunoblotting demonstrated that, especially with TDF, EFV, and ETR, there was a reduction in the expression of cyclin D1 and Rb hypophosphorylation and an increase in p21 concentration. Only the SKOV-3 cells treated with ETR exhibited high levels of E-cadherin expression (Fig. 5).
Figure 5.
Effects of antiretroviral drugs (20 μM) on the cell cycle of SKOV-3 treated for 48 h. Data obtained by Western blot analyzing cyclin D1, Rb, p21, and E-cadherin.
DISCUSSION
An estimated 200,000 new cases of ovarian cancer occur per year in the world, with about 60,000 cases per year occurring in the European Union22. Ovarian cancer accounts for about 3% of the tumors in women. This kind of carcinoma often remains undiagnosed until it reaches a large size or spreads to the pelvis. In many patients, at the moment of the diagnosis, the tumor is not limited to the ovaries23.
Statistical data indicate that one third of the women who are subjected to chemotherapy for ovarian cancer fail to achieve clinical remission, and approximately 50% of patients who do achieve clinical remission in the first course of chemotherapy eventually have relapse of their disease24. Both of these categories of patients have a less than 5-year survival rate, indicating the need to develop novel chemotherapeutic drugs that could find their use either as single therapy or in combination with other existing drugs25.
Treatment of an ovarian adenocarcinoma cell line with five different drugs (ABC, TDF, EFV, ETR, and DRV) demonstrated that these drugs have different effects on the cell line.
ABC and DRV did not show any particular modulation on SKOV-3 carcinoma cells. This result is not consistent with a previous report demonstrating that ABC significantly reduced cell growth, inducing a delay in the S phase progression in prostate cancer cells23. A similar effect on cell cycle alteration and senescence induction was previously reported by Rossi et al. in medulloblastoma ABC-treated cells26. That group observed that ABC inhibits cell growth and induces differentiation of human medulloblastoma cell lines through the downregulation of telomerase activity followed by a dramatic increase in cell death27. Instead, other drugs of the same class of DRV, such as fosamprenavir (FPV), lopinavir (LPV), and IDV, have been reported to have direct and indirect cytotoxicity as well as the ability to stop the cell cycle in 60 tumor cell lines28. In particular, several anecdotal reports and some retrospective studies describe the efficacy of PIs of the first generation on Kaposi sarcoma29, and different experimental studies in various oncologic contest demonstrate the direct effect only of first-generation antiretroviral agents—saquinavir (SQV), IDV, NLF, ritonavir (RTV), and APV—on angiogenic and inflammatory processes, cell invasion, and the promotion of apoptosis10–12,30–32. There are no data about the potential antineoplastic and antiangiogenic activities of PIs of the new generation33.
In our experimental subset, TDF was not able to induce differentiation, while in literature it has been demonstrated that it induces differentiation at least in mesenchymal stem cells of the rhesus monkey34. Treatment of SKOV-3 cells with EFV showed blocking in the progression of the G0/G1 phase of the cell cycle, while ETR treatment resulted in a noticeable block in the progression of the G0/G1 phase of the cell cycle and was able to induce differentiation. This blocking activity in the G0/G1 phase of cell cycle was higher when compared to EFV.
These data are consistent with previous reports showing the cytotoxicity of NNRTIs on tumor cell lines, promoting the hypothesis of using EFV either as a protective drug against the development of precancerous lesions or in patients who have already developed cancer35. In the ovarian adenocarcinoma cell line treated with EFV, no apoptotic or necrotic effects were observed36, while it was demonstrated that EFV induced differentiation on breast37 and kidney cancer cells38. This effect also occurred in melanoma and prostate cancer cells39. Moreover, the phenomena of apoptosis and necrosis have been observed in tumor cell lines of glioblastoma, colorectal and pancreatic cancers, and T-cell acute leukemia40.
All of these observations lead to the hypothesis that NNRTIs, such as EFV and ETR, cause the appearance of a low proliferation-differentiated phenotype rather than senescence41. Recently, it has been demonstrated that ETR administration increases calcium deposit collagen type I production in a model of bone differentiation as a result of Wnt3a mRNA overexpression, and upregulation of collagen type I expression, being the only drug able to increase the expression of p21 cdk inhibitor as a further marker of terminal differentiation42.
From the literature, it is evident that ETV constitutes a valuable option for concomitant use with chemotherapy for Hodgkin’s lymphoma due to its moderate effect on inducing drug-metabolizing enzymes43. The use of PIs is generally limited, as they may inhibit the metabolism of co-administrated cancer drugs and thereby increase their toxicity. Conversely, first-generation NNRTIs (i.e., EFV, NVP) may induce metabolism and potentially reduce the efficacy of cancer drugs. Nevertheless, no clinical interactions (decreased efficacy or increased toxicity of the antineoplastic agent) have been described in the literature for those NNRTIs. In particular, ETV constitutes a good option for intensifying raltegravir-based regimens without compromising the efficacy of chemotherapy. ETV has been reported to be a weak inducer of cytochrome P450 (CYP) 3A and a weak inhibitor of P-glycoprotein. The antineoplastic agents (i.e., bleomycin and doxorubicin) undergo mainly non-CYP-mediated metabolism and thus are unlikely to interact with ETV. Procarbazine is activated by CYP2B6, which is not affected by ETV. Etoposide is only partially metabolized by CYP3A4, thus limiting the effect of ETR-mediated CYP3A induction43.
In summary, we observed that drugs belonging to the NRTI, NtRTI, and NNRTI categories have some suggestive effects on the ovarian cancer cell line SKOV-3, compared to previous reports on different neoplastic cell lines. In addition, PI DRV seems not to have an effect on SKOV-3 cells, in contrast with other reports on first- and second-generation PIs that have been reported to have antitumoral features. Further studies are required to confirm these suggestions in other comparative settings.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Pia Furno and Dr. Rosalina Perna for editorial assistance and are grateful to Maria Giuseppa Sellitto for technical help. Dr. Iolanda Agliata is supported by the University of Molise. The study was partially supported by the University of Molise and by Università degli Studi della Campania “L. Vanvitelli”. This article does not contain any studies with human participants or animals performed by any of the authors. Author contributions: A.P., C.S., A.L., and I.A. helped to set up the protocols and carried out the experiments; M.A.C. and V.S. gave advice on the work and helped in the interpretation of the data; V.E. and G.G. contributed to references update and final editing of the manuscript; L.C. planned the study design, supervised all the work, and wrote the manuscript together with A.D.L.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Yarchoan R, Tosato G, Little RF. Therapy insight: AIDS-related malignancies—The influence of antiviral therapy on pathogenesis and management. Nat Clin Pract Oncol. 2005;2(8):406–15. [DOI] [PubMed] [Google Scholar]
- 2. Van Marck VL, Bracke ME. Epithelial-mesenchymal transitions in human cancer. In: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013. [Google Scholar]
- 3. Iyidogan P, Anderson KS. Current perspectives on HIV-1 antiretroviral drug resistance. Viruses 2014;6(10):4095–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li A, Ouyang Y, Wang Z, Cao Y, Liu X, Ran L, Li C, Li L, Zhang L, Qiao K, Xu W, Huang Y, Zhang Z, Tian C, Liu Z, Jiang S, Shao Y, Du Y, Ma L, Wang X, Liu J. Novel pyridinone derivatives as non-nucleoside reverse transcriptase inhibitors (NNRTIs) with high potency against NNRTI-resistant HIV-1 strains. J Med Chem. 2013;56(9):3593–608. [DOI] [PubMed] [Google Scholar]
- 5. Jones JL, Hanson DL, Dworkin MS, Ward JW, Jaffe HW. Effect of antiretroviral therapy on recent trends in selected cancers among HIV-infected persons. J Acquir Immune Defic Syndr. 1999;21(Suppl 1):S11–7. [PubMed] [Google Scholar]
- 6. Ikezoe T, Saito T, Bandobashi K, Yang Y, Koeffler HP, Taguchi H. HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Mol Cancer Ther. 2004;3(4):473–9. [PubMed] [Google Scholar]
- 7. Jiang W, Mikochik PJ, Ra JH, Lei H, Flaherty KT, Winkler JD, Spitz FR. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67(3):1221–7. [DOI] [PubMed] [Google Scholar]
- 8. Landriscina M, Altamura SA, Roca L, Gigante M, Piscazzi A, Cavalcanti E, Costantino E, Barone C, Cignarelli M, Gesualdo L, Ranieri E. Reverse transcriptase inhibitors induce cell differentiation and enhance the immunogenic phenotype in human renal clear-cell carcinoma. Int J Cancer 2008;122(12):2842–50. [DOI] [PubMed] [Google Scholar]
- 9. Houédé N, Pulido M, Mourey L, Joly F, Ferrero JM, Bellera C, Priou F, Lalet C, Laroche-Clary A, Raffin MC, Ichas F, Puech A, Piazza PV. A phase II trial evaluating the efficacy and safety of efavirenz in metastatic castration-resistant prostate cancer. Oncologist 2014;19(12):1227–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spugnini EP, Esposito V, Groeger AM, Cassandro R, Onori N, Chirianni A, Baldi A. Effects of indinavir in a preliminary phase I study on dogs with stage III slenic hemangiosarcoma. In Vivo 2006;20(1):125–7. [PubMed] [Google Scholar]
- 11. Esposito V, Palescandolo E, Spugnini EP, Montesarchio V, De Luca A, Cardillo I, Cortese G, Baldi A, Chirianni A. Evaluation of antitumoral properties of the protease inhibitor indinavir in a murine model of hepatocarcinoma. Clin Cancer Res. 2006;12(8):2634–9. [DOI] [PubMed] [Google Scholar]
- 12. Esposito V, Verdina A, Manente L, Spugnini EP, Viglietti R, Parrella R. Amprenavir inhibits the migration in human hepatocarcinoma cells and the growth of xenografts. J Cell Physiol. 2013;228(3):640–5. [DOI] [PubMed] [Google Scholar]
- 13. Reza Sadaie M, Mayner R, Doniger J. A novel approach to develop anti-HIV drugs: Adapting non-nucleoside anticancer chemotherapeutics. Antiviral Res. 2004;61(1):1–18. [DOI] [PubMed] [Google Scholar]
- 14. Brüning A, Burger P, Gingelmaier A, Mylonas I. The HIV reverse transcriptase inhibitor tenofovir induces cell cycle arrest in human cancer cells. Invest New Drugs 2012;30(4):1389–95. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi H, Takamune N, Nirasawa T, Aoki M, Morishita Y, Das D, Koh Y, Ghosh AK, Misumi S, Mitsuya H. Dimerization of HIV-1 protease occurs through two steps relating to the mechanism of protease dimerization inhibition by darunavir. Proc Natl Acad Sci USA 2014;111(33):12234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Falco M, De Luca A. Cell cycle as a target of antineoplastic drugs. Curr Pharm Des. 2010;16(12):1417–26. [DOI] [PubMed] [Google Scholar]
- 17. Mazzarella G, Perna A, Marano A, Lucariello A, Aufiero VR, Melina R, Guerra G, Taccone FS, Iaquinto G, De Luca A. Pathogenic role of associated adherent-invasive Escherichia coli in Crohn’s disease. J Cell Physiol. 2017;232(10):2860–8. [DOI] [PubMed] [Google Scholar]
- 18. Kundu M, Sharma S, De Luca A, Giordano A, Rappaport J, Khalili K, Amini S. HIV-1 Tat elongation the G1 phase and indirectly promotes HIV-1 gene expression in cells of glial origin. J Biol Chem. 1998;273(14):8130–6. [DOI] [PubMed] [Google Scholar]
- 19. Esposito V, Perna A, Lucariello A, Carleo MA, Viglietti R, Sangiovanni V, Coppola N, Guerra G, De Luca A, Chirianni A. Different impact of antiretroviral drugs on bone differentiation in an in vitro model. J Cell Biochem. 2015;116(10):2188–94. [DOI] [PubMed] [Google Scholar]
- 20. Esposito V, Manente L, Lucariello A, Perna A, Viglietti R, Gargiulo M, Parrella R, Parrella G, Baldi A, De Luca A, Chirianni A. Role of FAP48 in HIV-associated lipodystrophy. J Cell Biochem. 2012;113(11):3446–54. [DOI] [PubMed] [Google Scholar]
- 21. Esposito V, Manente L, Perna A, Gargiulo M, Viglietti R, Sangiovanni V, Doula N, Liuzzi G, Baldi A, De Luca A, Chirianni A. Role of NEDD8 in HIV-associated lipodystrophy. Differentiation 2009;77(2):148–53. [DOI] [PubMed] [Google Scholar]
- 22. Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival—A review of the epidemiological literature. J Ovarian Res. 2009;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol. 1998;25(3):255–64. [PubMed] [Google Scholar]
- 24. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61(3):183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar S, Bryant S, Chamala S, Qazi A, Seward S, Pal J. Ritonavir blocks AKT signaling, activates apoptosis and inhibits migration and invasion in ovarian cancer cells. Mol Cancer 2009;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi A, Russo G, Puca A, La Montagna R, Caputo M, Mattioli E. The antiretroviral nucleoside analogue Abacavir reduces cell growth and promotes differentiation of human medulloblastoma cells. Int J Cancer 2009;125(1):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlini F, Ridolfi B, Molinari A, Parisi C, Bozzuto G, Toccacieli L, Formisano G, De Orsi D, Paradisi S, Grober OM, Ravo M, Weisz A, Arcieri R, Vella S, Gaudi S. The reverse transcription inhibitor abacavir shows anticancer activity in prostate cancer cell lines. PLoS One 2010;5(12):e14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egistelli L, Chichiarelli S, Gaucci E, Eufemi M, Schininà ME, Giorgi A, Lascu I, Turano C, Giartosio A, Cervoni L. IFI16 and NM23 bind to a common DNA fragment both in the P53 and the c-MYC gene promoters. J Cell Biochem. 2009;106(4):666–72. [DOI] [PubMed] [Google Scholar]
- 29. Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, Hollander MC, Kawabata S, Tsokos M, Figg WD, Steeg PS, Dennis PA. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13(17):5183–94. [DOI] [PubMed] [Google Scholar]
- 30. Aversa SM, Cattelan AM, Salvagno L, Crivellari G, Banna G, Trevenzoli M, Chiarion-Sileni V, Monfardini S. Treatments of AIDS-related Kaposi’s sarcoma. Crit Rev Oncol Hematol. 2005;53(3):253–65. [DOI] [PubMed] [Google Scholar]
- 31. Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, Palladino C, Leone P, Bugarini R, Malavasi L, Cafaro A, Falchi M, Valdembri D, Rezza G, Bussolino F, Monini P, Ensoli B. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8(3):225–32. [DOI] [PubMed] [Google Scholar]
- 32. Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS Jr, Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: Inhibition of Kaposi sarcoma. Blood 2002;99(10):3771–9. [DOI] [PubMed] [Google Scholar]
- 33. Gantt S, Casper C, Ambinder RF. Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr Opin Oncol. 2013;25(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee CC, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev. 2006;15(2):209–20. [DOI] [PubMed] [Google Scholar]
- 35. Hecht M, Harrer T, Büttner M, Schwegler M, Erber S, Fietkau R, Distel LV. Cytotoxic effect of efavirenz is selective against cancer cells and associated with the cannabinoid system. AIDS 2013;27(13):2031–40. [DOI] [PubMed] [Google Scholar]
- 36. McLean K, Sorenson D.R. NA, Daudi S, Liu JR. The HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cells. Gynecol Oncol. 2009;112(3):623–30. [DOI] [PubMed] [Google Scholar]
- 37. Patnala R, Lee SH, Dahlstrom JE, Ohms S, Chen L, Dheen ST, Rangasamy D. Inhibition of LINE-1 retrotransposon-encoded reverse transcriptase modulates the expression of cell differentiation genes in breast cancer cells. Breast Cancer Res Treat. 2014;143(2):239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pittoggi C, Martis G, Mastrangeli G, Mastrangeli B, Spadafora C. In vitro evidence for a new therapeutic approach in renal cell carcinoma. Int Braz J Urol. 2008;34(4):492–502. [DOI] [PubMed] [Google Scholar]
- 39. Sciamanna I, Landriscina M, Pittoggi C, Quirino M, Mearelli C, Beraldi R, Mattei E, Serafino A, Cassano A, Sinibaldi-Vallebona P, Garaci E, Barone C, Spadafora C. Inhibition of endogenous reverse transcriptase antagonizes human tumor growth. Oncogene 2005;24(24):3923–31. [DOI] [PubMed] [Google Scholar]
- 40. Su Z, Yang Z, Xie L, Dewitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Diff. 2016;23(5):748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esposito V, Perna A, Lucariello A, Carleo MA, Viglietti R, Sangiovanni V, Coppola N, Guerra G, De Luca A, Chirianni A. Different impact of antiretroviral drugs on bone differentiation in an in vitro model. J Cell Biochem. 2015;116(10):2188–94. [DOI] [PubMed] [Google Scholar]
- 42. Kurz M, Stoeckle M, Krasniqi F, Battegay M, Marzolini C. Etravirine: A good option for concomitant use with chemotherapy for Hodgkin’s lymphoma. Int J STD AIDS 2015;26(3):212–4. [DOI] [PubMed] [Google Scholar]
- 43. Kurz M, Stoeckle M, Krasniqi F, Battegay M, Marzolini C. Etravirine: A good option for concomitant use with chemotherapy for Hodgkin’s lymphoma. Int J STD AIDS 2015;26(3):212–4. [DOI] [PubMed] [Google Scholar]