Abstract
Hepatocellular carcinoma (HCC) is the most common cancer in the world. MicroRNAs (miRNAs) are a type of small noncoding RNA that can regulate the expression of target genes under physiological and pathophysiological conditions. Aberrant expression of microRNA-935 (miR-935) has been reported in cancer studies. However, its expression and mechanism in HCC remain unclear. In our study, we found that miR-935 was upregulated in liver cancer tissues and cells. Overexpression of miR-935 in liver cells promoted cell proliferation, tumorigenesis, and cell cycle progression, whereas inhibition of miR-935 reduced cell proliferation, tumorigenicity, and cell cycle progression. These changes in the properties of HCC cells were associated with upregulation of two well-known cellular G1/S transitional regulators: cyclin D1 and c-Myc. Additionally, we identified SOX7 as a direct target of miR-935. Overexpression of miR-935 inhibited SOX7 expression but promoted the levels of c-Myc and cyclin D1, which promotes cell proliferation and tumorigenesis; knockdown of miR-935 increased SOX7 level and inhibited c-Myc and cyclin D1 expression, whereas SOX7 silencing could promote cell proliferation, cell motility, and invasiveness in vitro. Our findings suggest that miR-935 represents a biomarker and a potential new target in HCC progression by suppressing SOX7 expression.
Key words: Liver cancer, MicroRNA-935 (miR-935), SOX7
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer mortality in the world1–3. The absence of routine screening means that most HCC cases are initially diagnosed at advanced stages, and patients die from tumor growth or liver failure4. The late onset of clinical symptoms accounts for the late diagnosis and poor prognosis; therefore, the identification of reliable biomarkers for HCC is especially important. Screening for new targets and drugs for HCC is one important mission of doctors and biologists.
MicroRNAs (miRNAs) are about 21 nt noncoding RNAs. They inhibit gene expression by directly binding to the 3′-UTR of genes and blocking mRNA translation and/or mediate mRNA degradation5. They have been demonstrated to play an important role in the development and progression of various cancers. For example, miR-221 promotes the self-renewal of breast cancer stem cells by suppressing ATXN16, and miR-221/222 contributes to the invasion and angiogenesis of glioma through targeting TIMP27. miRNAs have been found to regulate the progression of HCC. Recently, multiple miRNAs have been shown to be implicated in HCC progression, such as miR-1018, miR-126, miR-1439, miR-14510,11, miR-18512, miR-199b8, miR-22110, miR-22310, and miR-2249. miR-935 may be a prognostic biomarker of weight loss13; it also upregulates in CD133+ neural stem cells compared to medulloblastoma specimens14. However, its role in liver cancer has not been reported.
We first determined the miR-935 level in liver cancer cells and tissues and examined its role in the proliferation of liver cancer cell lines HepG2 and BEL7402 by overexpressing and downregulating its expression. We found that miR-935 was upregulated in liver cancer cells and tissues, and overexpression of miR-935 promoted cell proliferation and tumorigenesis in vitro and that knockdown of miR-935 reduced these effects. We found that sex-determining region Y box 7 (SOX7) was the direct target of miR-935. Simultaneous knockdown of SOX7 and miR-935 promoted cell proliferation and tumorigenesis in vitro, and miR-935 contributed to the cell proliferation of liver cancer through inhibiting SOX7.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human liver cancer cell lines BEL7402, HCCC-9810, Hep3B, HepG2, HuH7, MHCC97-L, MHCC97-H, and QGY-7703 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cell lines were cultivated in RPMI-1640 medium (Gibco, USA) supplied with 10% fetal bovine serum (FBS; Gibco) at 37°C in a humidified atmosphere containing 5% CO2. Immortalized normal liver epithelial cells (Chang liver) were maintained in RPMI-1640 medium (Gibco) supplied with 10% FBS (Gibco) at 37°C in a humidified atmosphere containing 5% CO2.
Tissue Samples and Patient Data
Tumor specimens and nontumorous specimens from 60 patients with liver cancer were obtained from the First Affiliated Hospital of Zhengzhou University between 2011 and 2015. Follow-up data were obtained from review of the patients’ medical records. None of the patients had received radiotherapy or chemotherapy before surgical resection. This study was conducted under the regulations of the Institutional Review Board of Zhengzhou Medical University. Informed consent was obtained prior to surgery from all enrolled patients.
RNA Extraction, Reverse Transcription, and Real-Time PCR
Total RNA from cultured cells was extracted using the TRIzol reagent (Takara, Japan) according to the manufacturer’s instructions. TaqMan microRNA assays (Applied Biosystems, Foster City, CA, USA) were used to determine the expression levels of miR-935 after reverse transcribing by sequence-specific primers (Applied Biosystems), and U6 small nuclear RNA was used as an internal control. Quantitation of miRNA was based on the threshold cycle (Ct), and relative expression was calculated as 2−[(Ct of miR-935) − (Ct of U6)] after normalization to the U6 small nuclear RNA. Three independent experiments were carried out to analyze the relative gene expression, and each sample was tested in triplicate.
Western Blot Analysis
Western blot was performed according to the method described previously. The following antibodies were used for analysis: anti-SOX7 (sc-20093; 1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and GAPDH (1:1,000; 10494-1-AP; Proteintech) served as the loading control.
miR-935 Mimics and Inhibitors
miRNA-935 mimic, miR-935 inhibitor, small interference RNA for SOX7 (SOX7-siRNA), and their negative control RNA and their negative control RNA oligonucleotides were purchased from GenePharma (Shanghai, P.R. China). The liver cancer cell line cells were transfected using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Scramble siRNA was used as the control. After 48 h, the cells were harvested for further analysis.
Cell Proliferation and Colony Formation Assay
Cells (2 × 103 cells per well) were incubated in 96-well culture plates in 100 μl of medium. CCK-8 (Dojindo Laboratories, Japan) was used according to the manufacturer’s instructions. Absorbance was measured at 450 nm 2 h after the addition of 10 μl of CCK-8 reagent per well to calculate the number of viable cells using a microplate. Cell viability was shown as the percentage of viable cells with vehicle-treated cells arbitrarily set as 100% viability. Cells (0.5 × 103) were plated into six-well plates and cultured for 10 days. Colonies were then fixed for 5 min with 10% formaldehyde and stained with 1.0% crystal violet for 30 s.
Flow Cytometry Analysis
Cells (0.5 × 104) were harvested by trypsinization, washed in ice-cold phosphate-buffered saline, and fixed in 80% ice-cold ethanol in phosphate-buffered saline. Before staining, cells were gently precipitated and resuspended in cold phosphate-buffered saline. Bovine pancreatic ribonuclease (Sigma-Aldrich, St. Louis, MO, USA) was added to a final concentration of 2 μg/ml, and cells were incubated at 37°C for 30 min, followed by incubation with 20 μg/ml propidium iodide (Sigma-Aldrich) for 20 min at room temperature. Cell samples (5 × 105) were then analyzed by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Migration and Invasion Assay
Cells were seeded into Transwell chambers (Corning, Corning, NY, USA) in the upper chambers in 200 μl of RMPI-1640 medium, and 500 μl of RMPI-1640 medium containing 10% FBS was added into the lower wells as the chemoattractant. Twenty-four hours later, the filters were stained with crystal violet. For invasion assay, chambers were coated with Matrigel (BD Biosciences). Cell migration and invasion were assessed by counting the number of cells that had penetrated through the filter. The experiments were repeated at least three times.
Polycarbonate filters (8-um pore size) were coated with Matrigel (BD Biosciences, Canada) at a concentration of 1 μg/ml and placed in 24-well cell culture plates. Then 1 × 105 cells per well in 100 μl of FBS-free media were seeded onto the upper part of the chamber, whereas its lower compartment was filled with media containing 10% FBS as a control. After 36 h at 37°C, cells that had invaded the Matrigel-coated membrane were fixed with 4% formaldehyde and stained using hematoxylin and eosin (H&E). Nonmigrated cells at the upper side of the filter were swiped off. A minimum of 10 fields per filter were counted.
Statistical Analysis
All statistical analyses were performed using the SPSS17.0 (SPSS Statistics, Chicago, IL, USA) software. Data were expressed as mean ± SD and analyzed using the Student’s t-test. Paired t-test was used for paired samples. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-935 Is Upregulated in Human HCC Tissues and Cell Lines
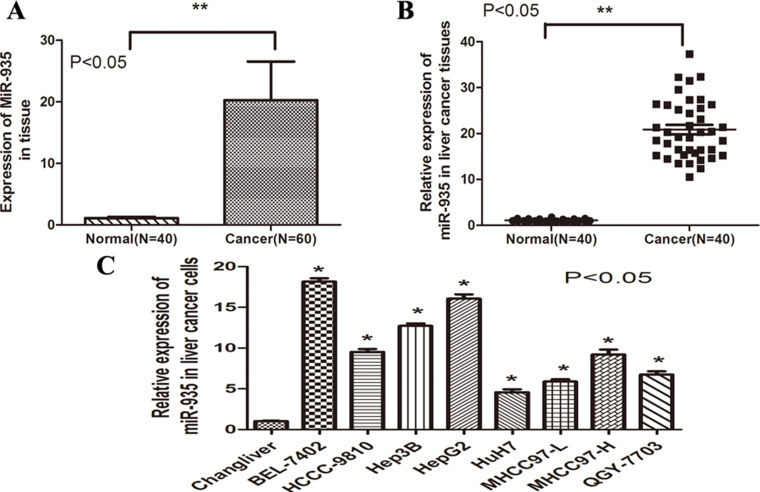
To determine the role of miR-935 in liver cancer, real-time (RT)-PCR was performed in 60 tumor and 40 corresponding nontumorous tissues. RT-PCR analysis revealed frequent upregulation of miR-935 expression in tumor tissues compared with the neighboring noncancerous tissues (p < 0.01) (Fig. 1A). In addition, 40 paired tumor tissues and corresponding neighboring noncancerous tissues were analyzed, showing significant upregulation of miR-935 in tumor tissues (p < 0.001) (Fig. 1B). Subsequently, RT-PCR demonstrated that miR-935 was overexpressed in eight HCC cell lines compared with the noncancer hepatocyte cell line Chang liver (p < 0.001) (Fig. 1C). Collectively, these results indicate that miR-935 is overexpressed in HCC cell lines and tissues.
Figure 1.
miR-935 was significantly upregulated in human HCC tissues and cell lines. (A) The expression of miR-935 in nontumorous tissues (Normal, n = 40) and liver cancer tissues (Cancer, n = 60) was determined by RT-PCR. (B) Expression of miR-935 in 40 representative liver cancer tissues and their corresponding nontumorous tissues from the same patients were analyzed side by side for comparison. (C) Expression of miR-935 in eight HCC cell lines compared with the noncancer hepatocyte cell line Chang liver.
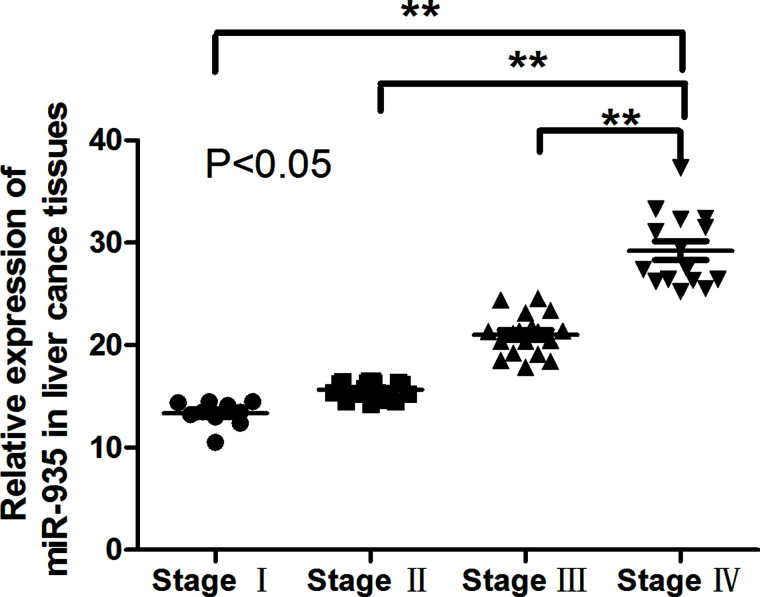
miR-935 Upexpression Is Related to Disease Progression
To investigate the prognostic role of miR-935 upregulation in liver cancer, patients were divided into high- and low-935 groups according to the median value of miR-935 expression in all 60 cases. Kaplan–Meier analysis revealed that the upregulation of miR-935 was significantly correlated with poorer outcome in liver cancer (Fig. 2). These results suggested that miR-935 might play an important role in liver cancer progression.
Figure 2.
miR-935 upexpression is related to disease progression. The Kaplan–Meier curve for overall survival rate of liver cancer patients according to the expression of miR-935.
Overexpression of miR-935 Promotes the Proliferation of HCC Cells
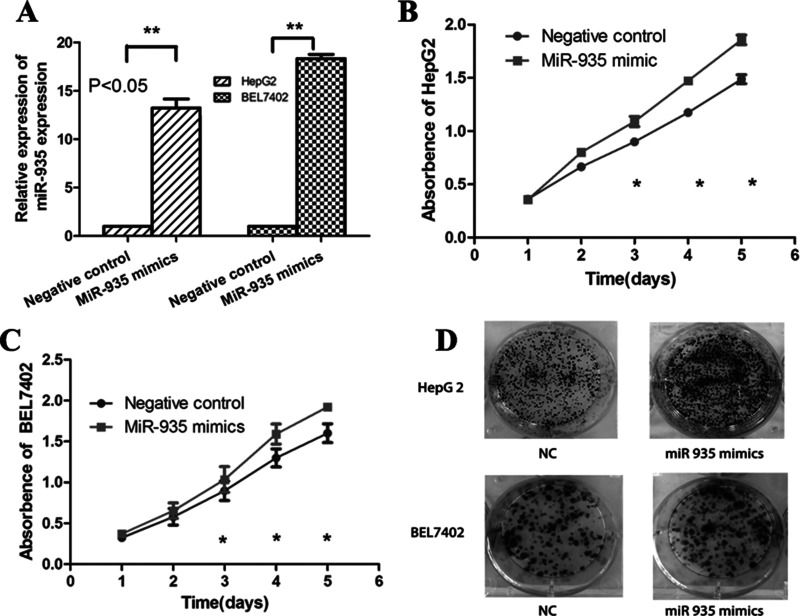
We transfected miR-935 mimic into liver cancer cell line HepG2 and BEL7402 to investigate the role of miR-935 (Fig. 3A). CCK-8 analysis showed that overexpression of miR-935 increased the cell proliferation rate (Fig. 3B and C). Colony formation analysis found that overexpression of miR-935 significantly promoted cell proliferation (Fig. 3D). Together, miR-935 overexpression promoted cell proliferation and tumorigenesis in vitro.
Figure 3.
Overexpression of miR-935 promotes the proliferation of HCC cells. (A) The expression of miR-935 in liver cancer cell HepG2 and BEL7402 after transfection of miR-935 mimic. (B, C) Cell proliferation was detected by the CCK-8 assay at 24, 48, 72, and 96 h after transfection with NC or miR-935 mimics in HepG2 and BEL7402. (D) Colony formation analysis of HepG2 and BEL7402 in response to the overexpression of miR-935 was performed.
Downregulation of miR-935 Inhibits Proliferation Through Inducing Cell Apoptosis In Vitro
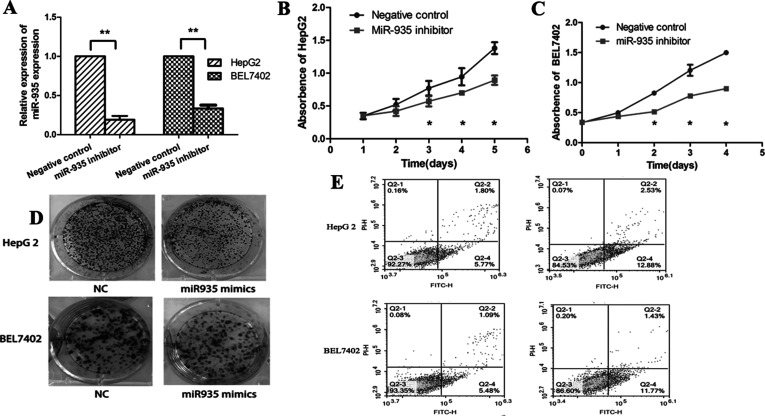
HepG2 and BEL7402 cells were successfully transfected with the miR-935 inhibitor (Fig. 4A). The CCK-8 assay demonstrated that inhibition of miR-935 significantly reduced the cellular growth of HepG2 and BEL7402 cells (Fig. 4B and C). Furthermore, downregulation of miR-935 inhibited HepG2 and BEL7402 cell proliferation in the colony formation assay and anchorage in the dependent growth ability assay (Fig. 4D). Moreover, we found that downregulation of miR-935 could promote cell apoptosis compared with control cells (Fig. 4E). These results suggest that downregulation of miR-935 reduced proliferation through promoting apoptosis in HCC cells.
Figure 4.
Downregulation of miR-935 inhibits proliferation and promotes apoptosis of HCC cells. (A) Expression of miR-935 in liver cancer cell lines HepG2 and BEL7402 after transfection with the miR-935 inhibitor. (B, C) Cell proliferation was detected by the CCK-8 assay at 24, 48, 72, and 96 h after transfection with NC or the miR-935 inhibitor in HepG2 and BEL7402. (D) Colony formation analysis of HepG2 and BEL7402 in response to downexpression of miR-935 was performed. (E) Apoptosis analysis of HepG2 and BEL7402 in response to downexpression of miR-935 was performed.
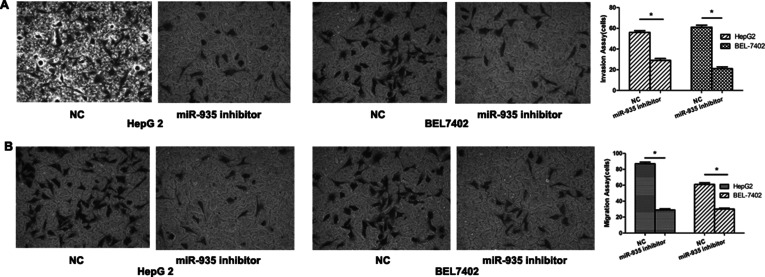
Downexpression of miR-935 Inhibited Cell Motility and Invasiveness In Vitro
An invasion assay was used to evaluate the effect of miR-935 on the metastatic potential of human liver cancer. BEL7402 and HepG2 were transfected with the miR-935 inhibitor and then analyzed for their metastatic potential using Transwell assays. Our results showed that the migration and invasion abilities of the miR-935 downexpression group were significantly reduced when compared with the control group (Fig. 5A and B). These results demonstrated that downregulation of miR-935 could inhibit the metastatic potential of human liver cancer.
Figure 5.
miR-935 downexpression suppressed cell motility and invasiveness in vitro. Migration and invasion assays were performed using Transwell chambers after transfection with NC or miR-935 mimics in HepG2 (A) and BEL7402 (B) cells.
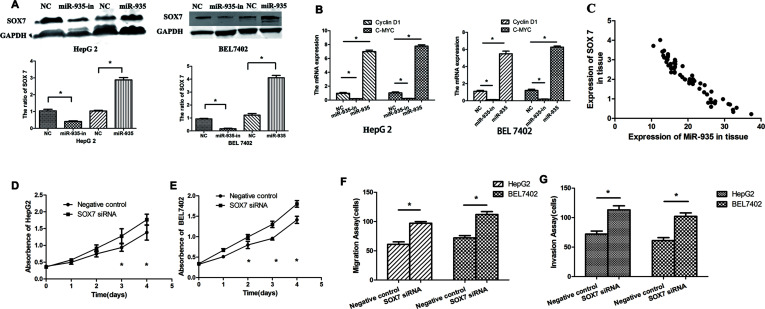
SOX7 Was a Direct and Functional Target of miR-935 in HCC
SOX7 was the theoretical target gene of miR-935, as determined by analysis using publicly available algorithms including TargetScan. Western blot analysis found that overexpression of miR-935 inhibited SOX7 expression, and knockdown of miR-935 increased SOX7 expression (Fig. 6A). Cyclin D1 and c-Myc are well-known oncogenes. RT-PCR analysis found that miR-935 knockdown inhibited cyclin D1 and c-Myc, and overexpression of miR-935 promoted cyclin D1 and c-Myc expression (Fig. 6B). Further analysis revealed that the expression of miR-935 is inversely correlated with the SOX7 mRNA level in human liver cancer (Fig. 6C). We found that SOX7 silencing could promote cell proliferation, motility, and invasiveness in vitro (Fig. 6D–G). These results showed that SOX7 has an opposite effect on cell proliferation and migration, in comparison with miR-935, indicating that it may be a functional target of miR-935 in human liver cancer cells.
Figure 6.
SOX7 is a direct and functional target of miR-935 in HCC. (A) Transfection of miR-935 mimics in liver cancer cells significantly inhibited SOX7 expression levels, as assessed by Western blot analysis. (B) Transfection of miR-935 mimics in liver cancer cells significantly promoted cyclin D1 and c-Myc mRNA expression levels, as assessed by RT-PCR analysis. (C) The correlation between the relative miR-935 expression and the relative SOX7 expression in liver cancer specimens was analyzed. Cell proliferation was detected by the CCK-8 assay at 0, 24, 48, 72, and 96 h after transfection with NC or SOX7 siRNA in HepG2 (D) and BEL7402 (E) cells. Transwell chambers were used to analyze the migration and invasion capabilities after transfection with NC or SOX7 siRNA in HepG2 (F) and BEL7402 (G) cells.
DISCUSSION
In this study, we found that miR-935 was upregulated in liver cancer tissues and cells, suggesting it might contribute to the development and progression of liver cancer. In addition, it was found that overexpression of miR-935 promoted cell proliferation and tumorigenesis in vitro, whereas knockdown of miR-935 inhibited cell proliferation and tumorigenesis in vitro. The tumor suppressor SOX7 is a direct target of miR-935, and knockdown of SOX7 in miR-935 inhibited cell promotion, proliferation, and tumorigenesis in vitro.
SOX7, together with SOX17 and SOX18, belongs to the SOX F subfamily15,16. It has been implicated in human cancers17, and its involvement in suppressing pathways such as Wnt/β-catenin signaling is highly activated in breast cancer18. Increasing lines of evidence have shown that SOX7 may disrupt the transcriptional function of the β-catenin–TCF/LEF (T-cell factor/lymphoid enhancer factor) complex and inhibit the activity of Wnt target genes, including cyclin D1, c-Myc, and COX-219. Several recent studies have demonstrated SOX7 to be a tumor suppressor through the Wnt/β-catenin signaling pathway in multiple cancers20,21. c-Myc and cyclin D1 are the downstream targets of the Wnt pathway, and cyclin D1 promotes the G1/S transition. c-Myc promotes cell cycle progression by multiple mechanisms. In our study, we found that miR-935 overexpression downregulated SOX7 protein expression and also increased c-Myc and cyclin D1 levels.
Taken together, this study demonstrates that miR-935 is markedly upregulated in HCC cells and clinical tissues compared with the matched adjacent noncancerous tissues from the same patients. Furthermore, SOX7 is a direct target gene of miR-935, and overexpression of miR-935 reduced the expression of SOX7 and inhibited the proliferation of HCC cells in vitro, whereas downregulation of miR-935 had the opposite effect. Further investigation is required to fully characterize the biological function of miR-935 and its clinical relevance in the development of HCC. Although the precise mechanisms are not yet fully understood, this study suggests that miR-935 may play an important role in regulating the proliferation of HCC cells and indicates that miR-935 may represent a potential therapeutic target for HCC.
ACKNOWLEDGMENTS
This study was supported by the Department of Hepatic Surgery and Institute of Clinical Medicine, The First Affiliated Hospital, Zhengzhou University. Author responsibilities: Xiaorui Liu and Jingjing Li made a significant contribution to the study and to the writing of the manuscript. Quancheng Kan contributed to the study and the statistical analysis. Zujiang Yu obtained the specimens. Juan Li and Ranran Sun contributed to the study.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Willatt JM, Francis IR, Novelli PM, Vellody R, Pandya A, Krishnamurthy VN. Interventional therapies for hepatocellular carcinoma. Cancer Imaging 2012;12:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS; Chen DS. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 2016;151:472–80. [DOI] [PubMed] [Google Scholar]
- 3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. [DOI] [PubMed] [Google Scholar]
- 4. Wu GG, Li WH, He WG, Jiang N, Zhang GX, Chen W, Yang HF, Liu QL, Huang YN, Zhang L, Zhang T, Zeng XC. Mir-184 post-transcriptionally regulates SOX7 expression and promotes cell proliferation in human hepatocellular carcinoma. PLoS One 2014;9:e88796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 6. Ke J, Zhao Z, Hong SH, Bai S, He Z, Malik F, Xu J, Zhou L, Chen W, Martin-Trevino R, Wu X, Lan P, Yi Y, Ginestier C, Ibarra I, Shang L, McDermott S, Luther T, Clouthier SG, Wicha MS, Liu S. Role of microRNA221 in regulating normal mammary epithelial hierarchy and breast cancer stem-like cells. Oncotarget 2015;6:3709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang F, Wang W, Zhou C, Xi W, Yuan L, Chen X, Li Y, Yang A, Zhang J, Wang T. MiR-221/222 promote human glioma cell invasion and angiogenesis by targeting TIMP2. Tumour Biol. 2015;36:3763–73. [DOI] [PubMed] [Google Scholar]
- 8. Wong CM, Wong CC, Lee JM, Fan DN, Au SL, Ng IO. Sequential alterations of microRNA expression in hepatocellular carcinoma development and venous metastasis. Hepatology 2012;55:1453–61. [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Liu S, Hu T, Liu S, He Y, Sun S. Upregulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 2009;50:490–9. [DOI] [PubMed] [Google Scholar]
- 10. Law PT, Ching AK, Chan AW, Wong QW, Wong CK, To KF, Wong N. MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis 2012;33:1134–41. [DOI] [PubMed] [Google Scholar]
- 11. Gao P, Wong CC, Tung EK, Lee JM, Wong CM, Ng IO. Deregulation of microRNA expression occurs early and accumulates in early stages of HBV-associated multistep hepatocarcinogenesis. J Hepatol. 2011;54:1177–84. [DOI] [PubMed] [Google Scholar]
- 12. Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, Tang ZY, Wang XW. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 2008;47:897–907. [DOI] [PubMed] [Google Scholar]
- 13. Milagro FI, Miranda J, Portillo MP, Fernandez-Quintela A, Campion J, Martinez JA. High-throughput sequencing of microRNAs in peripheral blood mononuclear cells: Identification of potential weight loss biomarkers. PLoS One 2013;8:e54319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genovesi LA, Carter KW, Gottardo NG, Giles KM, Dallas PB. Integrated analysis of miRNA and mRNA expression in childhood medulloblastoma compared with neural stem cells. PLoS One 2011;6:e23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005;434:843–50. [DOI] [PubMed] [Google Scholar]
- 16. Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003;423:448–52. [DOI] [PubMed] [Google Scholar]
- 17. Stovall DB, Cao P, Sui G. SOX7: From a developmental regulator to an emerging tumor suppressor. Histol Histopathol. 2014;29:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–80. [DOI] [PubMed] [Google Scholar]
- 19. Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget 2012;3:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 2003;424:797–801. [DOI] [PubMed] [Google Scholar]
- 21. Gautheron J, Luedde T. A novel player in inflammation and cancer: The deubiquitinase CYLD controls HCC development. J Hepatol. 2012;57:937–9. [DOI] [PubMed] [Google Scholar]