Abstract
Gastric cancer (GC) is one of the most common cancers and the second leading cause of cancer deaths in the world. Many factors have been reported regarding the progression and development of GC. In this study, we aimed to investigate the correlation of 3-phosphoinositide dependent protein kinase-1 (PDK-1) with cell viability, migration, and invasion of GC. The expression of PDK-1 was measured in different GC cell lines. Thereafter, the expression of PDK-1 was interfered by small hairpin RNA (shRNA) and then incubated with or without the inhibitor of nuclear factor-κB (NF-κB) pyrrolidine dithiocarbamate (PDTC). We then investigated the effects of PDK-1 aberrant expression on GC cell viability, migration, invasion, and the epithelial–mesenchymal transition (EMT) progress. The results showed that PDK-1 was highly expressed in GC cells, and PDK-1 promoted cell viability, migration, invasion, and EMT in GC. Moreover, we confirmed that PDK-1 activated the phosphatidylinositol 3-hydroxy kinase (PI3K)/AKT and NF-κB signaling pathways. However, administration of PDTC reversed the effects of overexpression of PDK-1 on cell migration and invasion. All these findings suggest that PDK-1 may be involved in progression of GC and could be a new therapeutic target for this disease.
Key words: 3-Phosphoinositide dependent protein kinase-1 (PDK-1), Gastric cancer, Migration, Invasion, Nuclear factor-κB (NF-κB)
INTRODUCTION
Gastric cancer (GC) is one of the most common malignant tumors of the digestive system worldwide, mainly epithelial malignant tumors, and has been reported to be the second leading cause of cancer-related deaths worldwide1–3. Adenocarcinoma accounted for 95% in malignant tumors of the stomach, of which invasion and metastasis of cancer cells are the main cause of GC-related death, with a 5-year survival rate below 30%4,5. Cancer of the stomach can be divided into early GC and advanced GC, although the clinical symptoms are vague and nonspecific6,7. The lack of mass screening programs means that most of the patients have been diagnosed with advanced diseases, and more patients are choosing surgery8. Although there has been considerable progress in therapies, diagnosis, and mechanism research, the incidence and mortality rates in patients with GC have still been high over the years9,10. Insight into the pathogenesis and molecular mechanisms responsible for the progress and metastasis of GC remains urgently needed.
3-Phosphoinositide dependent protein kinase-1 (PDK-1), also named PDPK-1, is a kind of protein encoded by the human PDPK gene, which is also an important kinase that plays a crucial role in physiological processes associated with cell metabolism, growth, proliferation, and survival11,12. Changes in the expression and activity of PDK-1 have also been reported to be linked to human disease, including cancer13,14. The objective of our study was to evaluate the correlation of PDK-1 expression with cell proliferation, invasion, and migration in GC cell lines, as well as its underlying mechanism.
We mainly studied the role and mechanism of PDK- 1 on GC cell migration and the epithelial–mesenchymal transition (EMT) process. First, qRT-PCR and Western blot were constructed to detect the expression of PDK-1 level in GC cell lines, and we found that PDK-1 was highly expressed in GC. Next, silencing and overexpression of PDK-1 expression were performed to further elucidate the role of PDK-1 in GC by small hairpin RNA (shRNA). Cell counting kit-8 (CCK-8), wound healing, and cell invasion assays were then used to investigate the cell viability, migration, and invasion in GC in vitro. All of our efforts will provide a theoretical basis and new insights into the treatment of GC.
MATERIALS AND METHODS
Cell Culture
The human gastric epithelial cell line GES-1 and human GC lines MKN45, MKN28, and SGC7901 were purchased from the American Type Cell Culture Collection (ATCC; Manassas, VA, USA). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), which was supplemented with 20 mM HEPES, 10% heat-inactivated fetal bovine serum (FBS2), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml), at 37°C with 5% CO2 15.
Plasmid Transfection and Treatment
In brief, for PDK-1 stable silencing, pLKO.1 lentiviral vectors carrying PDK-1 targeting shRNA called shPDK-1 was used. For the negative control group, a vector leading the expression of a scrambled nontargeting shRNA, called shScr, was used. pLV-puro lentiviral vector was used for PDK-1 construct expression. An empty vector was used as a negative control16. Moreover, a nuclear factor-κB (NF-κB) inhibitor, pyrrolidine dithiocarbamate (PDTC; 0.1 μmol/L; Sigma-Aldrich, St. Louis, MO, USA), was incubated in the presence or absence of plasmid transfection17.
CCK-8 Assay
SGC7901 cells were transfected by the plasmids or shRNAs for 48 h after being seeded into 96-well plates with special time. Cell proliferation was assessed by a CCK-8 (Dojindo Molecular Technologies, Gaithersburg, MD, USA). CCK-8 solution was added to the culture medium, and cultures were incubated for 1 h at 37°C in humidified 95% air and 5% CO2 atmosphere. Absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Wound Healing Assay
SGC7901 cells were cultured in 60-mm dishes until confluence. Cell wounds were created by scratching cell sheets with a sterile 200-μl pipette tip after 3 h of pretreatment with 50 μM mytomicin C. An inverted microscope (Leica, Germany) was used to take pictures of a specific position on the scratched areas every 24 h. Wound widths were measured, and relative wound widths were calculated.
Invasion Assay
The invasion behavior of SGC7901 cells was determined using 24-well Millicell Hanging Cell Culture inserts with 8-mm PET membranes (Millipore, Bedford, MA, USA). Human cardiac fibroblasts (5.0 × 104) in 200 μl of serum-free DMEM were plated onto BD BioCoat™ Matrigel™ Invasion Chambers (8-μm pore size polycarbonate filters; BD Biosciences, San Jose, CA, USA) after the cells were treated for their indicated condition, while complete medium containing 10% FBS was added to the lower chamber. After processing the invasion chambers for 48 h (37°C, 5% CO2) in accordance with the manufacturer’s protocol, the noninvading cells were removed with a cotton swab, and the invading cells were fixed in 100% methanol and then stained with crystal violet solution and counted microscopically.
qRT-PCR
Total RNA was isolated using RNAiso Plus Reagent according to the manufacturer’s instructions. Total RNA (500 ng) was reversely transcribed to cDNA using PrimeScript™ RT Master Mix (Perfect Real Time). Real-time PCR was performed on the Bio-Rad CFX 96 Real-time PCR system using SYBR® Premix Ex TaqTM II (TliRnaseH Plus) and specific primers. The mRNA level of each gene was normalized to GAPDH with the 2ΔΔCT method using Bio-Rad CFX Manager V1.1.308.1111 software.
Western Blot
The protein used for Western blotting was extracted using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, P.R. China) supplemented with protease inhibitors (Roche, Guangzhou, P.R. China). The proteins were quantified using the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The Western blot system was established using a Bio-Rad Bis-Tris Gel system according to the manufacturer’s instructions. The primary antibody was prepared in a 5% blocking buffer at a dilution of 1:1,000. Primary antibodies were incubated with the membrane at 4°C overnight, followed by washing and incubation with the secondary antibody marked by horseradish peroxidase for 1 h at room temperature. After rinsing, the polyvinylidene difluoride (PVDF) membrane carrying blots and antibodies was transferred into the Bio-Rad ChemiDoc™ XRS system, and then 200 μl of Immobilon Western Chemiluminescent HRP Substrate (Millipore) was added to cover the membrane surface. The signals were captured, and the intensity of the bands was quantified using the Image Lab™ Software (Bio-Rad, Shanghai, P.R. China).
Statistical Analysis
All experiments were repeated three times. The results of multiple experiments were presented as the mean ± SD. Statistical analyses were performed using the GraphPad statistical software. The p values were calculated using a one-way analysis of variance (ANOVA) method. A value of p < 0.05 was considered to indicate a statistically significant result.
RESULTS
PDK-1 Was Highly Expressed in GC Cell Lines
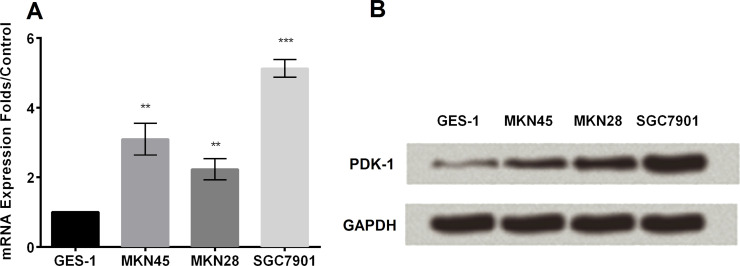
We used qRT-PCR and Western blot to detect the expression of the PDK-1 level of multiple GC cell lines, including GES-1, MKN45, MKN28, and SGC7901 (Fig. 1A and B). Obviously, PDK-1 was highly expressed in GC cells in both mRNA and protein levels (p < 0.05).
Figure 1.
The expression level of 3-phosphoinositide-dependent protein kinase-1 (PDK-1). (A) The expression level of PDK-1 was analyzed by RT-PCR. (B) The expression level of PDK-1 was analyzed by Western blot. **p < 0.01, ***p < 0.001.
Overexpression and Knockdown of PDK-1
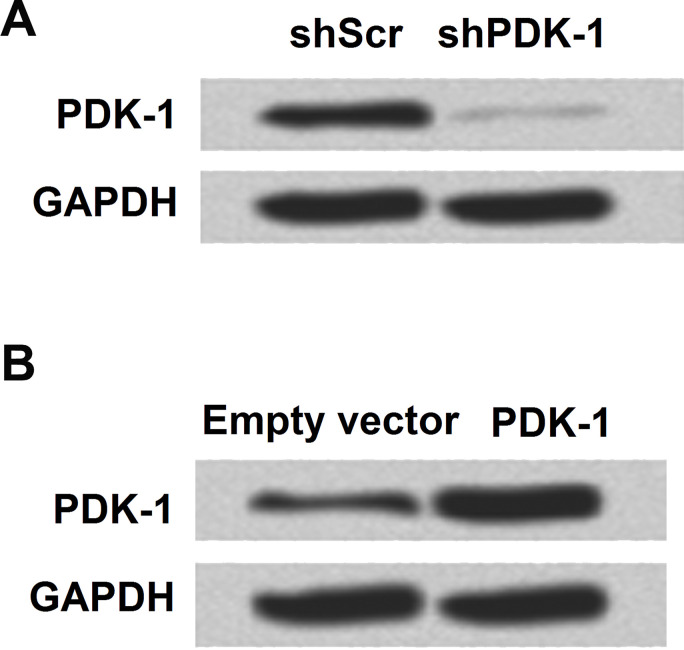
In the following study, we tried to use different plasmids to promote or inhibit the expression of PDK-1 and then analyzed PDK-1 expression in the protein level. PDK-1 was successfully overexpressed or downregulated by plasmid transfection (Fig. 2A and B).
Figure 2.
Overexpression and knockdown of PDK-1 in SGC7901. (A) Knockdown of PDK-1 and expression level were analyzed by Western blot. (B) The expression level of PDK-1 was analyzed by Western blot after being overexpressed.
PDK-1 Promotes Cell Viability, Migration, and Invasion
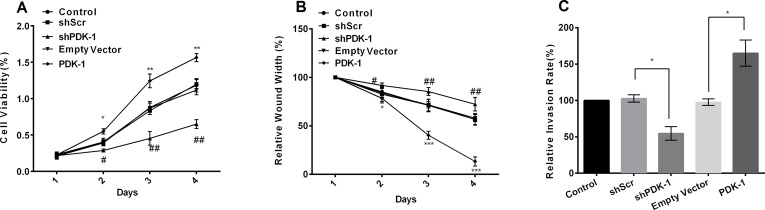
We used SGC7901 for the following research. CCK-8, wound healing, and cell invasion assays were applied to analyze the expression of PDK-1 on the GC cell progress. Overexpression of PDK-1 significantly promoted cell viability at days 2, 3, and 4, while downregulation of PDK-1 dramatically inhibited cell viability (p < 0.05 or p < 0.01) (Fig. 3A). Moreover, with the results of wound width, we observed that overexpression of PDK-1 significantly promoted cell migration at days 2, 3, and 4, while downregulation of PDK-1 inhibited cell migration (p < 0.05, p < 0.01, or p < 0.001) (Fig. 3B). PDK-1 promoted cell invasion in SGC7901 cells (p < 0.05) (Fig. 3C).
Figure 3.
Correlation of PDK-1 expression with cell viability, migration, and invasion. (A) Knockdown of PDK-1 inhibited cell viability. (B) Knockdown of PDK-1 inhibited cell migration. (C) Knockdown of PDK-1 inhibited cell invasion. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the empty vector; #p < 0.05 and ##p < 0.01 compared to shScr.
PDK-1 Promotes EMT in GC
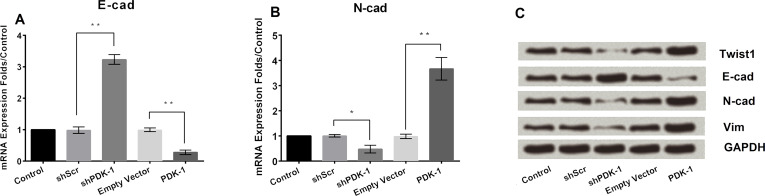
In the following study, we aimed to investigate the role of PDK-1 in the EMT progress. We monitored the EMT progress of SGC7901. E-cad and N-cad expressions were detected by mRNA level (Fig. 4A). Results revealed that PDK-1 inhibited E-cad expression and promoted N-cad expression (p < 0.05). Finally, the marker genes of EMT including Twist1, N-cad, Vim, and E-cad were detected in the protein level. PDK-1 promoted expression of Twist1, N-cad, and Vim, and downregulated E-cad expression (Fig. 4B).
Figure 4.
Effects of PDK-1 on epithelial–mesenchymal transition (EMT). (A) Overexpression of PDK-1 inhibited expression of E-cad and promoted expression of N-cad in mRNA level. (B) Overexpression of PDK-1 promoted Twist1, N-cad, and Vim expression and inhibited E-cad expression in protein level. *p < 0.05, **p < 0.01.
PDK-1 Activates Phosphatidylinositol 3-Hydroxy Kinase (PI3K)/AKT and Nuclear Factor-κB (NF-κB) Signaling Pathways
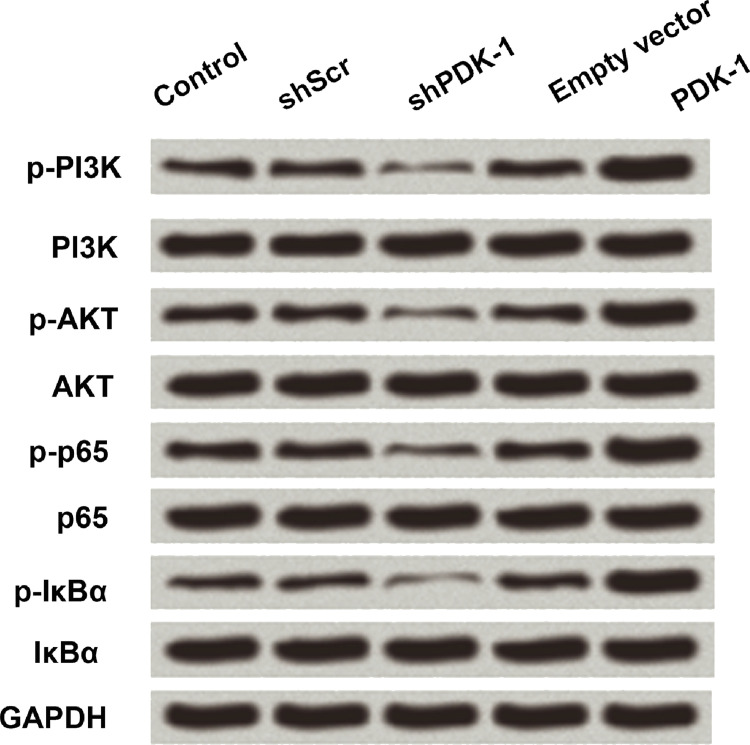
PI3K/AKT and NF-κB play an important role in the EMT progress. For this work, we aimed to confirm the signaling pathway of PDK-1 in the GC progress. We tried to detect the expression of PI3K/AKT- and NF-κB-related genes after PDK-1 aberrant expression. Overexpression of PDK-1 markedly increased the levels of p-PI3K, p-AKT, p-p65, and p-IKBα, while suppression of PDK-1 showed contrary results (Fig. 5). The results demonstrated that PDK-1 activated the PI3K/AKT and NF-κB signaling pathways.
Figure 5.
Effects of PDK-1 on the PI3K/AKT and NF-κB pathways in SGC7901. PDK-1 activated the expression of marker gene in phosphatidylinositol 3-hydroxy kinase (PI3K)/AKT and nuclear factor κB (NF-κB) pathway by Western blot.
PDK-1 Promotes Cell Migration and Cell Invasion by Activating the NF-κB Signaling Pathway
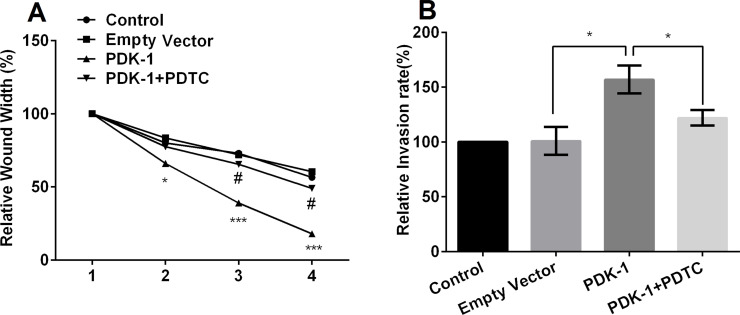
To further explore whether PDK-1 promotes cell migration and invasion by activating the NF-κB signaling pathway, we used one of the chemical inhibitors of NF-κB, PDTC (0.1 μmol/L), and then again studied the effect on migration and invasion. The results demonstrated that application of PDTC reversed the effects of overexpression of PDK-1 on cell migration and invasion (p < 0.05) (Fig. 6A and B), indicating that PDK-1 promoted cell migration and invasion by activating the PI3K/AKT and NF-κB signaling pathways.
Figure 6.
Effects of pyrrolidine dithiocarbamate (PDTC) on overexpressed PDK-1-induced cell migration and invasion. (A) Administration of PDTC reversed the effects of overexpression of PDK-1 on cell migration, and (B) administration of PDTC reversed the effects of overexpression of PDK-1 on cell invasion. *p < 0.05 and ***p < 0.001 compared to the empty vector; #p < 0.05 compared to the PDK-1 group.
DISCUSSION
GC is an important healthcare problem, representing the second leading cause of death from malignant disease worldwide. It is among the most frequent malignant tumors in East Asian countries18,19. PDK-1 is a master kinase regulating phosphorylation of AKT and cell survival, which was examined in human gastric epithelial cells20. Moreover, PDK-1 has been reported to participate in many biological progresses, including tumors. For example, in the study of Lin et al., PDK-1 is involved in the translocation of glucose transporter 4 to the plasma membrane in diabetic hearts and contributes to diabetic cardiomyopathy21. Moreover, the investigation of Misra and Pizzo demonstrated that PDK-1, Raptor, and mTOR coimmunoprecipitate and are involved in prostate cancer22.
EMT has been documented during embryonic development, tissue fibrosis in vitro, and in human specimens23–25. Moreover, EMT has been recognized as a critical factor for the spread of many kinds of cancer cells. EMT participates in molecular and cellular alterations when epithelial cells switch in differentiation, which generates mesenchymal-like cells with newly acquired migratory and invasive properties25–29. The study of Chen et al. investigated the expression of CNTN-1 and its underlying mechanism of metastasis mediated by EMT in GC and finally found that CNTN-1 was closely related to GC metastasis, and its functions seemed to be important in the migration and invasion of GC cells via EMT30.
However, the role of PDK-1 and the relationship of PDK-1 to EMT in GC progress are far from clear. So in this present study we preliminarily investigated the effect of PDK-1 on GC cell proliferation, migration, and invasion, and the regulation and mechanism of EMT in GC. First, we found that PDK-1 was shown to have a higher expression in GC cells than in normal cells, suggesting it may play a role in the development of GC. By upregulation and knockout of PDK-1, we searched for the role of PDK-1 in GC growth, and the results showed that PDK-1 promoted GC cell proliferation, migration, and invasion, and promoted the EMT process in GC cells. Moreover, Western blot was used to detect the marker gene expression during the PI3K/AKT and the NF-κB signaling pathways. The results confirmed that PDK-1 activated the PI3K/AKT and NF-κB signaling pathways. We further explored whether PDK-1 promoted cell migration and invasion by activation of the NF-κB signaling pathway. The chemical inhibitor of NF-κB, PDTC (0.1 μmol/L), was incubated with the cells. We observed that administration of PDTC reversed the effects of overexpression of PDK-1 on the cell migration and invasion, indicating that PDK-1 promoted cell viability and migration by activating the NF-κB signaling pathway. Taken together, our findings suggest that PDK-1 may be involved in the progression of GC and could be a new therapeutic target for this disease.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, Riegert-Johnson D, Foretova L, Silva EM, Digianni L. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu H, Hua Y, Zheng X, Shen Z, Luo H, Tao X, Wang Z. Effect of coffee consumption on the risk of gastric cancer: A systematic review and meta-analysis of prospective cohort studies. PLoS One 2015;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Subhash VV, Mei SY, Tan WL, Yong WP. Strategies and advancements in harnessing the immune system for gastric cancer immunotherapy. J Immunol Res. 2014;2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng L, Li D, Xiang X, Tong L, Qi M, Pu J, Huang K, Tong Q. Methyl jasmonate abolishes the migration, invasion and angiogenesis of gastric cancer cells through down-regulation of matrix metalloproteinase 14. BMC Cancer 2013;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang W, Tong JH, Chan AW, Lung RW, Chau SL, Wong QW, Wong N, Yu J, Cheng AS, To KF. Stathmin1 plays oncogenic role and is a target of microRNA-223 in gastric cancer. PLoS One 2012;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: Current status and future perspectives. Surg Endosc. 1999;13:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tinoco RC, Tinoco AC, Elkadre LJ, Sueth DM, Conde LM. Laparoscopic gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech. 2009;30:384–7. [DOI] [PubMed] [Google Scholar]
- 8. Li G, Hu Y, Hao L. Current status of randomized controlled trials for laparoscopic gastric surgery for gastric cancer in China. Asian J Endosc Surg. 2015;8:263–7. [DOI] [PubMed] [Google Scholar]
- 9. Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, Weitz J, Rahbari NN. Free intraperitoneal tumor cells and outcome in gastric cancer patients: A systematic review and meta-analysis. Oncotarget 2015;6:35564–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang WM, Meng QB, Yu JC, Ma ZQ, Li ZT. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol. 2015;21:5934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arsenic R. Immunohistochemical analysis of PDK1 expression in breast cancer. Diagn Pathol. 2014;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fyffe C, Falasca M. 3-Phosphoinositide-dependent protein kinase-1 as an emerging target in the management of breast cancer. Cancer Manag Res. 2013;5:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gagliardi PA, Di BL, Orso F, Seano G, Sessa R, Taverna D, Bussolino F, Primo L. 3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner. Neoplasia 2012;14:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jian B, Yang S, Chaudry IH, Raju R. Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury in a rat model. Mol Med. 2014;20:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou CH, Lin FL, Hou SM, Liu JF. Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol Cancer 2014;13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gagliardi PA, Di BL, Puliafito A, Seano G, Sessa R, Chianale F, Leung T, Bussolino F, Primo L. PDK1-mediated activation of MRCKα regulates directional cell migration and lamellipodia retraction. J Cell Biol. 2014;206:415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS, Lin JT, Inoue H, Chen GH. Helicobacter pylori promote gastric cancer cells invasion through a NF-kappaB and COX-2-mediated pathway. World J Gastroenterol. 2005;11:3197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mori F, Canu V, Lorenzon L, Garofalo A, Blandino G, Strano S. Cancer gastric chemoprevention: Isolation of gastric tumor-initiating cells. Methods Mol Biol. 2016;1379:129–37. [DOI] [PubMed] [Google Scholar]
- 19. Li D, Xu CY, Cui RJ, Tang JB, Sun H, Yang ZK, Bu JY, Lin P, Huang N, Du YD. DNA methylation inhibitor, decitabine, promotes MGC803 gastric cancer cell migration and invasion via the upregulation of NEDD4-1. Mol Med Rep. 2015;12:1043–4. [DOI] [PubMed] [Google Scholar]
- 20. King CC, Obonyo M. Helicobacter pylori modulates host cell survival regulation through the serine-threonine kinase, 3-phosphoinositide dependent kinase 1 (PDK-1). BMC Microbiol. 2015;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin G, Brownsey RW, Macleod KM. Complex regulation of PKCβ2 and PDK-1/AKT by ROCK2 in diabetic heart. PLoS One 2014;9:e86520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Misra UK, Pizzo SV. Activated α2-macroglobulin binding to cell surface GRP78 induces T-loop phosphorylation of Akt1 by PDK1 in association with Raptor. PLoS One 2014;9:e88373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan ZX, Chen YH, Xu S, Qin HY, Zhang C, Zhao H, Xu DX. Calcitriol inhibits bleomycin-induced early pulmonary inflammatory response and epithelial-mesenchymal transition in mice. Toxicol Lett. 2016;240:161–71 [DOI] [PubMed] [Google Scholar]
- 24. Valcourt U, Carthy J, Okita Y, Alcaraz L, Kato M, Thuault S, Bartholin L, Moustakas A. Analysis of epithelial-mesenchymal transition induced by transforming growth factor β. Methods Mol Biol. 2016;1344:147–81. [DOI] [PubMed] [Google Scholar]
- 25. Busaranon K, Plaimee P, Sritularak B, Chanvorachote P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J Nat Med. 2016;70:18–27. [DOI] [PubMed] [Google Scholar]
- 26. Preca BT, Bajdak K, Mock K, Sundararajan V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz S. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer 2015;137:2566–77. [DOI] [PubMed] [Google Scholar]
- 27. Hu D, Zhou J, Wang F, Shi H, Li Y, Li B. HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo. Arch Gynecol Obstet. 2015;292:1345–54. [DOI] [PubMed] [Google Scholar]
- 28. Qiao N, Wang S, Hu L. Retinoblastoma-binding protein 2 induces epithelial-mesenchymal transition in esophageal squamous cancer cells. Biotechnol Lett. 2015;37:2365–70. [DOI] [PubMed] [Google Scholar]
- 29. Shiota M, Itsumi M, Takeuchi A, Imada K, Yokomizo A, Kuruma H, Inokuchi J, Tatsugami K, Uchiumi T, Oda Y. Crosstalk between epithelial-mesenchymal transition and castration resistance mediated by Twist1/AR signaling in prostate cancer. Endocr Relat Cancer 2015;22(6):899–900. [DOI] [PubMed] [Google Scholar]
- 30. Chen DH, Yu JW, Wu JG, Wang SL, Jiang BJ. Significances of contactin-1 expression in human gastric cancer and knockdown of contactin-1 expression inhibits invasion and metastasis of MKN45 gastric cancer cells. J Cancer Res Clin Oncol. 2015;141:2109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]