Abstract
CD147 is a transmembrane protein that can induce the expression and activity of matrix metalloproteinases (MMPs). Expression of CD147 has been shown to potentiate cell migration, invasion, and metastasis of cancer. In this study, the critical role of CD147 in metastasis was elucidated using CD147-overexpressing cholangiocarcinoma (CCA) cells in vitro and in vivo. The molecular mechanism, demonstrated herein, supported the hypothesis that metastasis increased in CD147-overexpressing cells. Five CD147-overexpressing clones (Ex-CD147) were established from a low CD147-expressing CCA cell line, KKU-055, using lentivirus containing pReceiver-Lenti-CD147. The metastatic capability was determined using the tail vein injection mouse model and an in vitro 3D invasion assay. Liver colonization was assessed using anti-HLA class I immunohistochemistry. Adhesion abilities, cytoskeletal arrangements, MMP activities, the expressions of adhesion molecules, and epithelial–mesenchymal transitional markers were analyzed. All Ex-CD147 clones exhibited a high CD147 expression and high liver colonization in the tail vein-injected mouse model, whereas parental cells lacked this ability. Ex-CD147 clones exhibited metastatic phenotypes (i.e., an increase in F-actin rearrangement) and cell invasion and a decrease in cell adhesion. The molecular mechanisms were shown to be via the induction of MMP-2 activity and enhancement of epithelial–mesenchymal transitions. An increase in mesenchymal markers Slug, vimentin, and N-cadherin, and a decrease in epithelial markers E-cadherin and claudin-1, together with suppression of the adhesion molecule ICAM-1, were observed in the Ex-CD147 clones. Moreover, suppression of CD147 expression using siCD147 in two CCA cell lines with high CD147 expression significantly decreased cell migration and invasion of these CCA cells. These findings emphasize the essential role of CD147 in CCA metastasis and suggest CD147 as a promising target for the effective treatment of CCA.
Key words: Extracellular matrix metalloproteinase inducer (EMMPRIN), Cell invasion, Metastasis, Epithelial–mesenchymal transition (EMT), Matrix metalloproteinase (MMP)
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignancy of the bile duct epithelium. It is a rare cancer worldwide, but the incidence is high in Southeast Asia, especially in northeast Thailand1. Infection with liver fluke, Opisthorchis viverrini, is one of the known risk factors in this area2. CCA is a slow-growing but highly metastatic tumor. It is difficult to diagnose CCA in an early stage as it has no specific symptoms, and hence the majority of patients are diagnosed at an advanced stage with a poor prognosis. Similar to many cancers, most CCA patients die of cancer metastasis. Thus, understanding the molecular mechanism of metastasis in CCA might provide an innovative strategy for better treatment of CCA.
The molecular mechanism associated with the metastasis of CCA has been studied intensively in the past decade. Methionine aminopeptidase-2 and its substrate cyclophilin A were found to be increased in CCA tissues and are related to the shorter survival of patients3,4. Suppression of cyclophilin A expression inhibited the growth of CCA cells in vitro and in vivo. In addition, neutralizing CD147, a known receptor of cyclophilin A with a specific antibody, could diminish the proliferation-promoting action of cyclophilin A in CCA cell lines5. This collective evidence suggested the significant roles of CD147 in CCA progression.
CD147, or extracellular matrix metalloproteinase inducer (EMMPRIN), is an integral membrane glycoprotein of an immunoglobulin superfamily that normally expresses on the cell surfaces of epithelial cells, fibroblasts, and T lymphocytes6,7. In physiological conditions, CD147 plays a role in immune response and tissue remodeling8,9 with a well-known function of inducing matrix metalloproteinase (MMP) expression10,11. An increase in CD147 expression was observed and correlated with lymph node metastases and shorter survival of cancer patients12,13.
However, information regarding CD147 in CCA is limited. The expression of CD147 was increased in CCA tissues and is related to a shorter survival of patients11. Data from serial analysis of gene expression (SAGE) indicated the higher expression of CD147 in patient CCA tissues than that of normal adjacent tissues14 (http://www.cgap.nci.nih.gov/sage). The role of CD147 in CCA progression at the molecular mechanism level, however, remains unknown. This study aimed to reveal the role of CD147 on tumor progression of CCA in vitro and in vivo. Overexpression of CD147 in low CD147-expressing CCA cells promotes aggressive phenotypes with high metastatic activity. The downstream signals underlying CD147 action were revealed. In addition, suppression of CD147 expression in the high CD147-expressing CCA cells significantly decreased their migration and invasion abilities. These findings, for the first time, demonstrate the essential role of CD147 in CCA metastasis and suggest CD147 as a promising target for an effective treatment of CCA.
MATERIALS AND METHODS
Cell Culture
Four CCA cell lines, KKU-055, KKU-100, KKU-213, and KKU-214, were established as previously described15. Cells were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank, Osaka, Japan. All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator at 37°C with 5% CO2.
Establishment of CD147-Overexpressing Cells
pReceiver-CD147-Lv122 containing the full-length open reading frame (ORF) of CD147 was purchased from GeneCopoeia (Rockville, MD, USA). pReceiver-CD147-Lv122 was cotransfected with pCMVR8.74 and pMD2.G into 293T cells16. pCMVR8.74 (Addgene plasmid #22036) and pMD2.G (Addgene plasmid #12259) were gifts from Professor Didier Trono, School of Life Sciences, Swiss Institutes of Technology, Lausanne, Switzerland. Viral supernatants were collected, centrifuged at 2,000 × g at 4°C for 5 min, and transduced to the KKU-055 cells using polybrene (Sigma-Aldrich, St. Louis, MO, USA). CD147-overexpressing cells (Ex-CD147) were selected using 0.5 μg/ml puromycin (Sigma-Aldrich). Single clones were selected using a sterile clonal cylinder17. The levels of CD147 expression were monitored using flow cytometry analysis and Western blotting analysis.
In Vivo Mouse Model for Analysis of Metastatic Capability
CCA cells (5 × 105 cells/100 μl of complete medium) were intravenously injected via the tail vein of Balb/c Rag-2/Jak3 double deficient (Balb/c RJ) mice as previously described18,19. Mice were housed in the animal research facility according to institutional guidelines, with 12-h light and food and water ad libitum. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at Kumamoto University. Mouse behavior was observed every day, and body weights were monitored twice a week. Mice were euthanized on day 24 after cancer cell injection, and lungs and livers were removed. Liver nodules were counted by gross inspection. Tissues were fixed and embedded in paraffin according to the standard procedures. Anti-HLA class I A, B, C (Hokudo, Suporo, Hokaido, Japan) was used for immunohistochemistry staining as the standard marker for human cells.
Immunofluorescence Staining of F-Actin
Cells (3 × 104 cells) were cultured on a sterile coverslip and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature (RT) and washed once with PBS. After cells were permeabilized with 0.5% Triton X-100 for 5 min at RT and washed twice with PBS, cells were incubated with 100 nM rhodamine phalloidin (Cytoskeleton, Inc., Denver, CO, USA) for 30 min in the dark at RT. The nuclei were counterstained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA). The fluorescent signals were analyzed under a fluorescence microscope (Bio-Zero BZ-8000; Keyence, Osaka, Japan).
Cell Adhesion Assay
The adhesion assay was performed as previously reported20. Briefly, cells at a density of 1 × 105 cells/ml were incubated in a 96-well plate for 12 h. The unbound cells were then discarded, and the adhered cells were washed once with PBS and cultured further in 100 μl of DMEM and 0.5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich). After 3 h of incubation, 100 μl of acidified isopropanol was added to dissolve the crystals. Absorption values at 595 nm were determined with an ELISA plate reader (Multiskan; Thermo Electron Vantaa, Finland).
Quantitative Real-Time PCR
Total RNA was extracted using RNAiso Plus reagent (Takara Bio Inc., Ohtsu, Japan), and cDNA was prepared using the PrimeScript RT-PCR kit (Takara Bio Inc.). Quantitative RT-PCR analysis was performed using the Applied Biosystems Step One Real-Time PCR System (Life Technologies, Foster City, CA, USA). The mRNA level of ICAM-1 and E-cadherin were normalized with Ct of GAPDH and calculated as (ΔCt = Cttarget − CtGAPDH), and 2−ΔΔCt was used to calculate the fold change. The oligonucleotide primers of E-cadherin, ICAM-1, and GADPH used were previously reported21.
Flow Cytometry Analysis
Cells (1.5 × 105) were stained with indicated antibodies purchased from BioLegend (San Diego, CA, USA); CD147 conjugated with allophycocyanin (APC; clone HIM6), E-cadherin (CD324) conjugated with APC (clone 67A4), ICAM-1 (CD54) conjugated with phycoerythrin (PE; clone31625X), or the corresponding isotype control antibodies for 30 min, on ice in the dark. Cells were analyzed using an LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo software version 9.7.6 (Tree Star, San Carlos, CA, USA).
3D Invasion Assay
The three-dimensional (3D) invasion assay, or Matrigel evasion assay, was performed as previously described22. Briefly, cells (1 × 104 cells) were suspended in 6 mg/ml Matrigel (Corning Inc., Corning, NY, USA) in a serum-free DMEM, dropped into a six-well plate, and maintained for 1 h at 37°C. The complete medium was then added into the well. Evasion ability was determined on day 6 after dropping the Matrigel into the wells and then measuring the distance of cell movement from the drops (two drops/cell). Images were taken using a microscope (Bio-Zero BZ-8000; Keyence). Two independent experiments were performed.
Gelatin Zymography Assay
Conditioned medium was collected from cells cultured in serum-free DMEM for 24 h. Debris cells were removed by centrifugation at 2,000 × g, 4°C for 5 min, and supernatant was collected. MMP-2 and MMP-9 activities were determined using the gelatin zymography assay as described previously23. Gels were stained with 0.5% Coomassie brilliant blue G-250 for 30 min and rehydrated in 2% acetic acid overnight at RT. The stained bands were scanned using Image Quant LAS 400 (GE Healthcare, Little Chalfont, UK) and analyzed by Image Quant TL (GE healthcare).
Protein Extraction and Western Blot Analysis
Cell lysate, SDS-polyacrylamide gel electrophoresis, and Western blots were performed as previously described24. The sources of antibodies were as indicated: CD147 (ab666; Abcam, Cambridge, UK) and HSP70/HSC73 (1B5; ENZO Life Science, Plymouth Meeting, PA, USA). The following antibodies were from Cell Signaling Technology (Danvers, MA, USA): vimentin (D21H3), N-cadherin (D4R1H) XP, claudin-1 (D5H1D) XP, Slug (C19G7), E-cadherin (24E10), horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, and HRP-conjugated anti-mouse IgG.
Suppression of CD147 Expression Using Specific siRNA
Suppression of CD147 in KKU-213 and KKU-214 cell lines was performed using si-RNA specific to CD147. Sequences of siCD147 were 5′-GUC GUC AGA ACA CAU CAA C-3′ (sense) and 5′-GUU GAU GUG UUC UGA CGA C-3′ (antisense) (Integrated DNA Technologies Pte. Ltd., Singapore). CCA cell lines (2 × 105 cells/well) were cultured in a six-well plate for 24 h and then transfected with 100 pmol of siCD147 using 2 μg/ml of Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to the recommendations of the manufacturer. Six hours later, medium was removed and cells were cultured in 10% FBS in DMEM for the subsequent experiments. Cells treated with siControl (Negative Control siRNA; 1027310; Qiagen, Valencia, CA, USA) were used as the experimental control.
Cell Proliferation, Migration, and Invasion Assays
Cell proliferation was measured using the MTT proliferation assay (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s guidelines. Migration and invasion were analyzed using the Boyden chamber assay with Transwell cell culture inserts (8.0-μm pore size; Corning Inc.) as previously described25. siCD147-treated or control cells (4 × 104 cells/well) were allowed to migrate or invade for 9 h for KKU-213 and 24 h for KKU-214. Experiments were performed in duplicate, and at least two independent experiments were done.
Statistical Analyses
The results are presented as the mean ± standard error of the mean (SEM) as indicated in the figure legends. Statistical significance was determined by the Student’s t-test. A value of p < 0.05 was set as the level of statistical significance. All of the statistical analyses were performed using SPSS 17.0 (IBM Corporation, Somers, NY, USA).
RESULTS
Establishment of Stably Overexpressed CD147 KKU-055 Cells
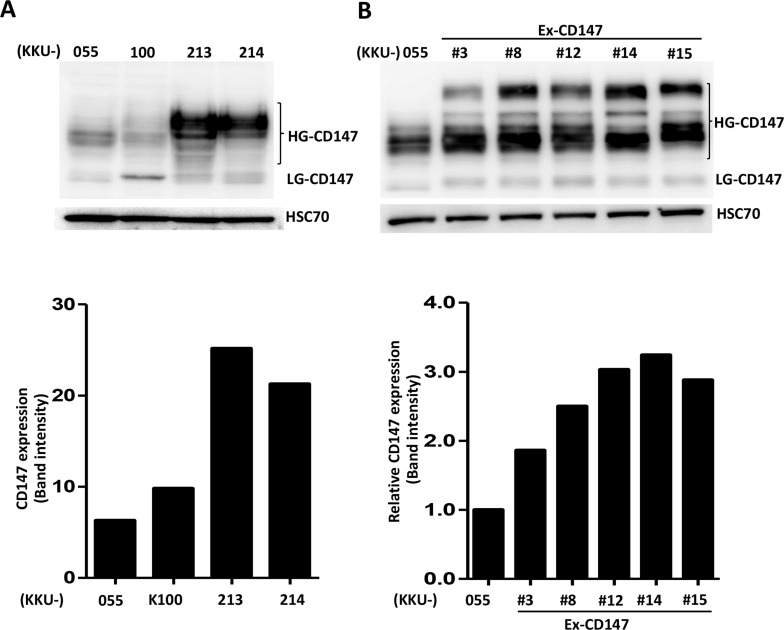
Endogenous CD147 expression in four CCA cell lines, KKU-055, KKU-100, KKU-213, and KKU-214, was first determined (Fig. 1A). CCA cell lines expressed different levels of CD147 in both high (HG) and low glycosylated (LG) forms. KKU-213 and KKU-214 had high CD147 expression, whereas KKU-055 and KKU-100 had low expression. Therefore, KKU-055 was selected to be established as the CD147-overexpressing cells.
Figure 1.
Expression of CD147 in cholangiocarcinoma (CCA) cell lines. The expression levels of CD147 were determined using Western blotting. (A) Endogenous expressions of CD147 in four CCA cell lines. (B) Expression of CD147 in the parental KKU-055 and the stable CD147-overexpressing clones. HSC70 was used as a loading control for Western blotting. Graphs represent band intensities of CD147 expression and are one representative from two independent experiments.
To create the CD147-overexpressing cell line, KKU-055 was transduced with lentivirus containing pReceiver-Lenti-CD147. Transduced cells were selected using 0.5 μg/ml puromycin. Single clones were selected using sterile clonal cylinders. Fifteen clones were randomly selected, and the expression of CD147 was determined using flow cytometry and Western blotting. Five CD147-overexpressing clones that possessed high levels of CD147 expression compared to their parental KKU-055 (Fig. 1B) were selected and designated as Ex-CD147 #3, #8, #12, #14, and #15. These five clones were used for further study.
CD147-Overexpressing Clones Exhibited High Metastatic Ability In Vivo
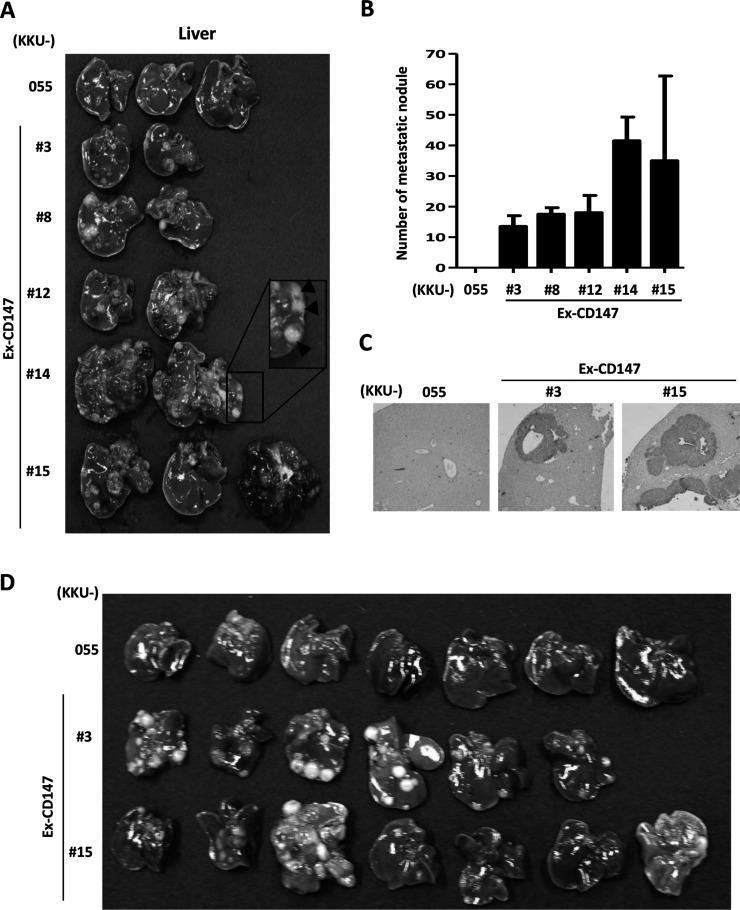
The effect of CD147 on CCA metastasis was investigated. Cells of five CD147-overexpressing cell clones and the parental cells, KKU-055, were intravenously injected via tail veins into Balb/c RJ mice (two to three mice/clone). Mice were sacrificed 24 days after injection. Lungs and livers were removed, and metastatic nodules were observed. No nodules were observed in the lungs from either parental or CD147-overexpressing cells in injected mice. However, multiple nodules were seen on the livers from mice injected with CD147-overexpressing cells but not from those injected with the parental cells (Fig. 2A). The visible nodules were counted, and the numbers are presented in Figure 2B. To confirm that no CCA cells metastasized to the liver from KKU-055-injected mice, liver sections from each group of animals were immunohistochemically stained with a specific antibody to human cells, anti-HLA class I A, B, C antibodies, and observed microscopically. There was no HLA+ staining observed in the livers from mice injected with parental cells. In contrast, HLA+ staining cells were frequently observed in the livers from the mice injected with CD147-overexpressing clones (Fig. 2C). To affirm the linkage between the expression of CD147 and the metastatic potential of CCA cells, two CD147-overexpressing cell line cells (Ex-CD147 #3 and #15) and their parental cells were injected via tail veins in a larger number of mice (six to seven mice/group). The results revealed that all livers obtained from mice injected with CD147-overexpressing cells had liver colonization, whereas none were found in the livers from mice injected with parental cells (Fig. 2D).
Figure 2.
Overexpression of CD147 promotes liver colonization of CCA cells in vivo. The stably expressed CD147 clones (Ex-CD147) or parental cells (KKU-055) were intravenously injected into mice. Lung and liver colonizations were inspected 24 days after injection. No lung colonization was observed. (A) Liver nodules were macroscopically observed in the livers from mice injected with CD147-overexpressing clones. The inset highlights the liver nodules. (B) Quantitative analysis of the number of liver nodules found in each group. (C) Histological analysis of anti-HLA staining indicated the presence of CCA cells in the liver tissues. Representatives of liver sections from Ex-CD147 #3- and #15-injected mice are presented. (D) KKU-055, Ex-CD147 #3 and #15 were injected via tail vein to six to seven Balb/c Rag-2/Jak3 double deficient (Balb/c RJ) mice. Livers were removed 24 days after injection and inspected for liver colonization.
CD147-Overexpressing Cells Exhibit High F-Actin Microfilament
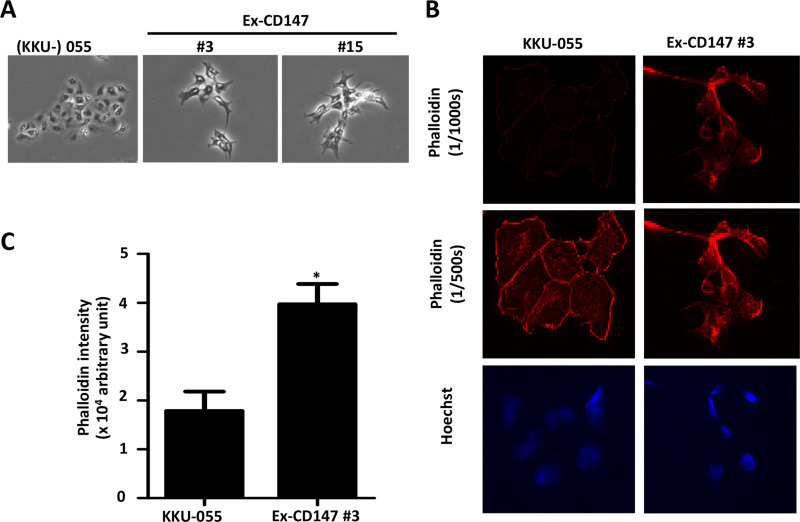
As observed under microscopy, the cell morphology of CD147-overexpressing cells exhibited more spindle-like shapes with multiple membrane protrusions compared with parental cells (Fig. 3A). The effect of CD147 on F-actin rearrangement was investigated using rhodamine phalloidin staining and observed under a fluorescence microscope. CD147-overexpressing cells presented the enrichment of cytoplasmic F-actin microfilaments on the protruding membranes, while it was generally dispersed in the cytoplasm and on the membranes of parental cells (Fig. 3B). In addition, the rhodamine fluorescence intensities of CD147-overexpressing cells were higher than those of parental cells (Fig. 3C).
Figure 3.
Overexpression of CD147 promotes cytoskeleton actin microfilament rearrangement. (A) Cell morphology of CD147-overexpressing cells compared with those of KKU-055. Phase contrast with 200× magnification. (B) F-actin arrangement analyzed by rhodamine phalloidin staining and detected by fluorescence microscopy with short (1/1,000 s) and long (1/500 s) exposure times. (C) Mean intensities of phalloidin/cell of the parental and CD147-overexpressing cells (Ex-CD147 #3) are compared. At least 100 cells were determined. The results are mean ± standard error of the mean (SEM) from one of two independent experiments. *p < 0.05.
CD147-Overexpressing Cells Possess Low Adhesion Ability
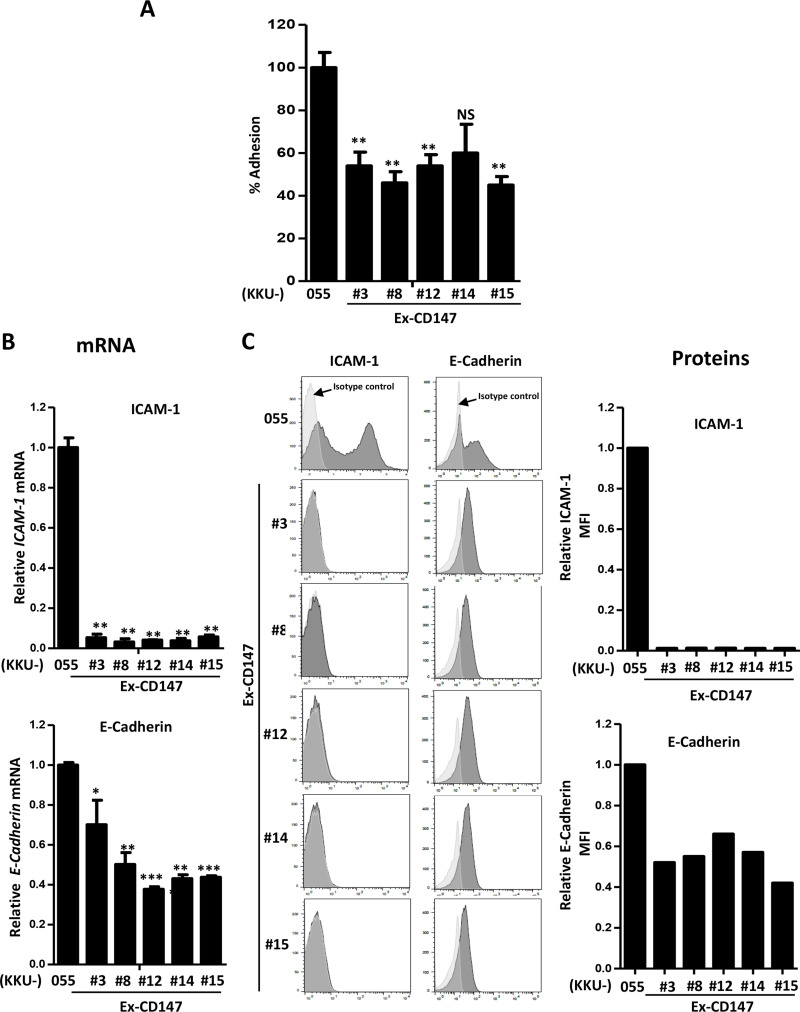
The adhesion ability and adhesion molecules may partly contribute to the morphological changes and F-actin rearrangements observed in CD147-overexpressing cells. Therefore, cell adhesion and adhesion molecules, namely, ICAM-1 and E-cadherin, were next investigated in CD147-overexpressing cells in comparison with parental cells. Almost all CD147-overexpressing cells had a significantly lower adhesion ability than their parental, KKU-055, cells (Fig. 4A). In addition, the expression of ICAM-1 and E-cadherin was also lower in CD147-overexpressing cells than in parental cells in both mRNAs and proteins (Fig. 4B and C).
Figure 4.
Overexpression of CD147 suppresses adhesion ability and expression of adhesion molecules in CCA cells. (A) Adhesion ability of CD147-overexpressing cells was compared with that of KKU-055. The data are mean ± SEM from one of two independent experiments. (B) Expression of ICAM-1 and E-cadherin was determined using real-time RT-PCR. The data are mean ± SEM from one of two independent experiments. (C) Expression of ICAM-1 and E-cadherin proteins was determined using flow cytometry. Histograms and mean fluorescent intensities of ICAM-1 and E-cadherin were compared. The data are one representative from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
CD147-Overexpressing Cells Had High Invasion Ability In Vitro
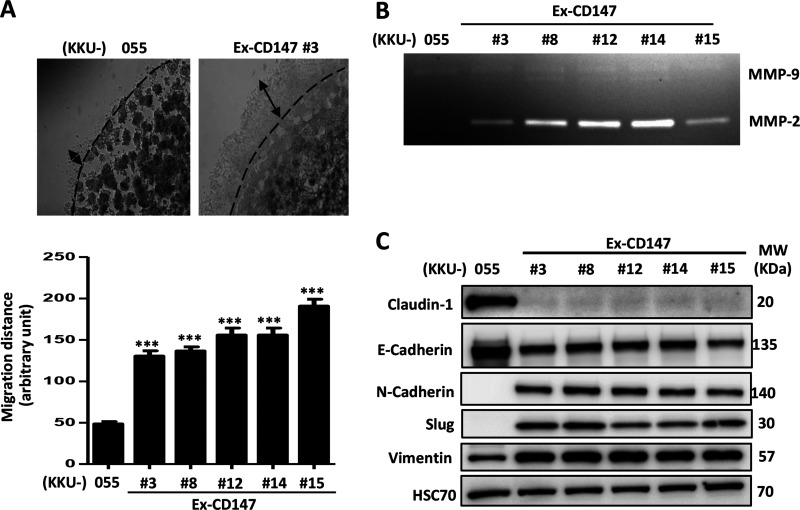
The effects of CD147 on cell migration and invasion were next investigated in vitro. Unfortunately, CD147-overexpressing cells had low adhesion properties, and hence data regarding their migratory ability using the wound-scratching and Boyden chamber assays could not be used. Therefore, 3D invasion or evasion assays were performed instead. The results showed that all CD147-overexpressing cells had a significantly higher invasion ability when compared with KKU-055 parental cells (Fig. 5A).
Figure 5.
Overexpression of CD147 promotes invasion by stimulating matrix metalloproteinase 2 (MMP-2) and the epithelial-to-mesenchymal transition process in CCA cells. (A) Invasion ability of CCA cells was investigated using an evasion assay. The migration distances were quantitatively analyzed and compared. The results (mean ± SEM) are the averages of three measurements from duplicate dots/sample. (B) Activities of MMPs were determined using a gelatin zymography assay. (C) Expression of epithelial markers (E-cadherin and claudin-1) and mesenchymal markers (N-cadherin, vimentin, and Slug) was determined using Western blotting. HSC70 protein was used as a loading control. The data are one representative from two independent experiments. ***p < 0.001.
Overexpression of CD147 Promotes MMP Activity and Expression of Mesenchymal Markers
To identify the mechanism underlying CD147-promoted cell invasion, the downstream targets of CD147, MMP-2, and MMP-9, which are involved in cancer cell invasion, were determined. Gelatin zymography revealed that the CD147-overexpressing cells had a dramatically higher MMP-2 activity than their parental cells (Fig. 5B). Western blot analysis of epithelial markers (E-cadherin and claudin-1) and mesenchymal markers (N-cadherin, vimentin, and Slug) indicated that CD147-overexpressing cells expressed lower levels of E-cadherin and claudin-1 and higher expressions of N-cadherin, vimentin, and Slug than their parental cells (Fig. 5C).
Suppression of CD147 Expression in High CD147-Expressing Cells Reduced Cell Migration and Invasion
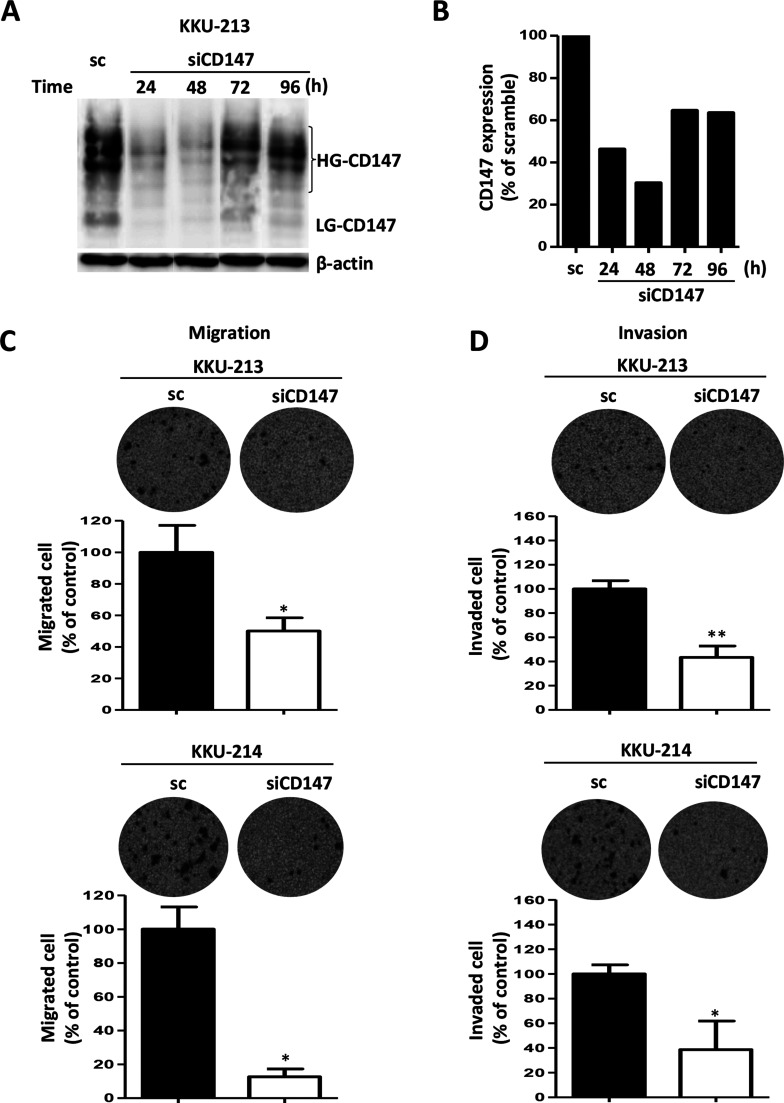
To explore whether CD147 could be a promising target for the effective treatment of CCA metastasis, KKU-213 and KKU-214, two CCA cell lines with a high CD147 expression, were transiently suppressed using siRNA against CD147. Scramble RNA-treated cells were used as the controls. The efficiency of siCD147 was checked according to time after transfection in KKU-213 cells using Western blot after 24 to 96 h. Compared with the scramble control, siCD147 could suppress CD147 expression up to 96 h (Fig. 6A and B). CD147 expression was suppressed to 30% of the control at 48 h and to 60% at 72–96 h after transfection.
Figure 6.
Suppression of CD147 expression in high CD147-expressing cell lines significantly inhibited cell migration and cell invasion. (A) siCD147 effectively suppressed CD147 expression of a high CD147-expressing cell, KKU-213, from 24 to 96 h. Expression levels of CD147 were determined using Western blot analysis. β-Actin was used as the loading control, and the nonspecific siRNA [scramble (sc)] was used as a negative control. (B) Quantitative analysis of CD147 expressions shown in (A). Cells were treated with siCD147 for 24 h and subjected to (C) migration and (D) invasion assay using the Boyden chamber. The data are mean ± SD of two independent experiments. *p < 0.05, **p < 0.01.
The proliferation rate of CD147 knockdown cells was not different than those of the scramble control cells, as observed using MTT assay from 24 to 96 h (data not shown). Cell migration and invasion were investigated using a Boyden chamber assay. The results revealed that silencing of CD147 could inhibit cell migration of KKU-213 to 40% and KKU-214 to 20% of the controls (Fig. 6C). Similar observations were made using the invasion assay. Inhibition of CD147 expression could reduce cell invasion of KKU-213 to 20% and KKU-214 to 40% of the controls (Fig. 6D).
DISCUSSION
CD147, a multifunctional transmembrane glycoprotein, has been documented to promote tumor cell invasion in several cancers26,27. In the present study, the crucial role of CD147 in the promotion of cell invasion and tumor metastasis in CCA cells was first demonstrated in vitro and in vivo. The pronounced effect of CD147 on metastasis was clearly shown in the tail vein metastasis mouse model. Forced overexpression of CD147 in KKU-055 cells that were Ex-CD147 clones exhibited higher invasion and lower adhesion abilities than their parent CCA cells. The molecular mechanism was shown to be by the induction of MMP-2 activity and mesenchymal markers N-cadherin, vimentin, and Slug expressions, and suppression of ICAM-1, E-cadherin, and claudin-1. Moreover, suppression of CD147 in high CD147-expressing cell lines could significantly reduce the cell migration and invasion ability of CCA cells. These findings suggest CD147 as an interesting target for the treatment of highly metastatic cancers, such as CCA.
Metastasis is a major problem in cancer patients, leading to treatment failure and mortality. Several clinical and preclinical studies indicate the relationship between CD147 and the metastasis of cancer13,28. In the present study, five clones of CD147-overexpressing cells were generated from the parental KKU-055 cells that possessed a low CD147 expression. The association of CD147 with metastatic potential in CCA cells was clearly demonstrated in the tail vein-injected metastatic mouse model. All mice injected with each clone of CD147-overexpressing cells had liver colonization, whereas mice injected with low CD147-expressing parental cells had none. The enhancing effect of CD147 on the metastasis of CCA cells seems to be generalized for cancer cells, as similar observations were reported in head and neck cancer and hepatocellular carcinoma. Overexpression of CD147 in head and neck cancer cells promoted lung colonization in the tail vein injection mouse model29. In addition, CD147 overexpression of hepatocellular carcinoma cells promoted liver metastasis in the intrasplenic injection mouse model30.
In 1889, Stephen Paget31 suggested the “seed and soil” theory that some tumor cells (the “seed”) metastasize to selected organs (the “soil”) based on the preferential microenvironment suitable for the seed to implant. On the other hand, some tumor cells follow the circulatory route and implant in the first organ encountered (the anatomical/mechanical hypothesis). Accumulated information suggests the influence of the tumor–stromal interaction in organ-specific metastasis32. In the present study, CD147-overexpressing cells metastasized preferentially to the liver without lung colonization, whereas KKU-213, a highly metastatic CCA cell line, colonized mainly in the lungs with no liver metastasis in the tail vein-injected mouse model18. The liver preferential metastasis of CD147-overexpressing cells observed in this study may fit the “seed and soil” theory. The association of CD147 and the liver microenvironment that promotes the hepatotropic properties of CD147-overexpressing CCA cells is of interest and should be further elucidated.
Epithelial-to-mesenchymal transition (EMT) is a crucial step in cancer metastasis. Several molecules are involved in this process, such as adhesion molecules, MMP, and EMT regulatory proteins, etc. Loss of epithelial cell markers, such as E-cadherin, and gain of mesenchymal cell markers, such as vimentin and N-cadherin, are common features of EMT33. In the current study, overexpression of CD147 augmented the metastatic phenotypes of CCA cells. The morphological changes accompanying the enrichment of cytoplasmic F-actin microfilaments were observed in the CD147-overexpressing cells. Rearrangement of F-actin microfilaments, the elevation in mesenchymal markers (N-cadherin, vimentin, and Slug), together with the decrease in ICAM-1 and E-cadherin on the cell surface may be responsible for the increase in cell motility, low adhesion, and high invasion ability of these CD147-overexpressing cells. It should be noted here that the influence of CD147 on the downstream target proteins is not proportional to the level of CD147 expression. In this study, the five clones of CD147-overexpressing cells exhibited similar invasive and adhesion abilities with comparable levels of EMT and MMP-2 activity, regardless of their CD147 expression levels. This phenomenon implies either that the lowest level of CD147 in the CD147-overexpressing cells (Ex-CD147 #3) is sufficient to activate the metastatic phenotypes of CCA cells or that the observed effects are not directly regulated by CD147. Extended experiments are needed to explore this further.
CD147 is identified as an inducer of MMP expression. Several MMPs, including MMP-1, -2, -3, -9, -11, and -14, are induced by CD14734. In the present study, overexpression of CD147 dramatically stimulated MMP-2 expression in CCA cells. Degradation of extracellular matrices by MMPs facilitates cancer invasion and metastasis35,36. Modulation of MMP expression by CD147 was well documented in a number of cancer cell types. Several preclinical studies have demonstrated the association of CD147 with tumor growth, invasion, and metastasis in many types of cancer cells in vitro and in vivo29,37–39. Silencing of CD147 expression reduced invasive ability and metastasis26,27,40, whereas overexpression of CD147 enhanced invasiveness and metastasis of cancer cells29.
Overexpression of CD147 together with a decrease in claudin-1 expression was first shown in this study. Claudin-1 is an integral membrane-tight junction protein in the claudin family. The role of claudin-1 in cancers is still controversial. Overexpression of claudin-1 was shown to enhance the invasion and metastasis in colon cancer, whereas depletion of claudin-1 was associated with the increase in cell invasion in lung and liver cancers41,42. In the present study, claudin-1 was prominent in parental cells with low CD147 expression but was almost suppressed in CD147-overexpressing cells. The underlying mechanism by which CD147 mediates the expression of claudin-1 and EMT should be further elucidated.
The collective data from CD147-overexpressing cells indicate the important role of CD147 in enhancing metastatic phenotypes of CCA cells and suggest CD147 as a target for mediating CCA metastasis. This possibility was revealed by the siCD147 experiments. Suppression of CD147 expression in the CCA cells with high CD147 expression effectively reduced the migration and invasion capabilities of these CCA cells. This observation was in agreement with reports in gastric and liver cancer cells28,43.
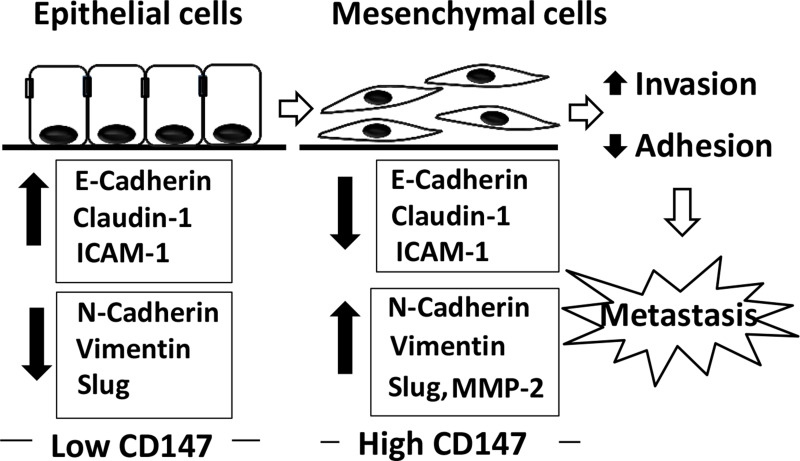
In conclusion, the present study demonstrated, for the first time, the promoting effect of CD147 on cell invasion and metastasis of CCA cells in vitro and in vivo. The molecular mechanisms by which CD147 modulated cell invasion and metastasis were shown to enhance mesenchymal characteristics together with the increase in MMP-2 activity and decrease in adhesion molecule expressions (Fig. 7). These findings suggested CD147 as a promising target for cancer treatment, particularly for the highly metastatic CCA.
Figure 7.
CD147 and the underlying mechanisms in promoting metastasis of CCA. Low CD147-expressing cells exhibit the epithelial phenotype with a high expression of adhesion molecules and epithelial markers (E-cadherin, claudin-1, and ICAM-1) and low expression of mesenchymal markers (N-cadherin, vimentin, and Slug). On the other hand, overexpression of CD147 mediates the development of mesenchymal phenotypes by suppression of adhesion molecules and epithelial markers and overexpression of mesenchymal markers. Moreover, overexpression of CD147 promotes MMP-2 activity. Altogether, overexpression of CD147 promotes metastasis of CCA cells.
ACKNOWLEDGMENTS
This work was cosupported by Grants-in-Aid for Science Research (No. 16K08742) from the Ministry of Education, Science, Sports, and Culture of Japan, the TRF Senior Research Scholar Grant to S. Wongkham (RTA5780012), the National Research University Project of Thailand through SHeP-GMS (NRU582010), Khon Kaen University (570601), Faculty of Medicine (I56326), and the Royal Golden Jubilee–PhD Program for P. Dana and S. Wongkham (PHD/0192/2552) cofunding with Khon Kaen University. We thank Mrs. I. Suzu for her technical assistance and Mrs. Yoshie Kanagawa and Ms. Y. Endo for their secretarial work; Professor James A. Will for editing the manuscript via the Faculty of Medicine Publication Clinic, KKU; and Professor Didier Trono for providing the lentiviral packaging vectors pMD2.G and pCMVR8.
REFERENCES
- 1. Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sonakul D, Koompirochana C, Chinda K, Stitnimakarn T. Hepatic carcinoma with opisthorchiasis. Southeast Asian J Trop Med Public Health 1978;9:215–9. [PubMed] [Google Scholar]
- 3. Obchoei S, Weakley SM, Wongkham S, Wongkham C, Sawanyawisuth K, Yao Q, Chen C. Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma. Mol Cancer 2011;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sawanyawisuth K, Wongkham C, Pairojkul C, Saeseow OT, Riggins GJ, Araki N, Wongkham S. Methionine aminopeptidase 2 over-expressed in cholangiocarcinoma: Potential for drug target. Acta Oncol. 2007;46:378–85. [DOI] [PubMed] [Google Scholar]
- 5. Obchoei S, Sawanyawisuth K, Wongkham C, Kasinrerk W, Yao Q, Chen C, Wongkham S. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumour Biol. 2014;36:849–59. [DOI] [PubMed] [Google Scholar]
- 6. Fossum S, Mallett S, Barclay AN. The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur J Immunol. 1991;21:671–9. [DOI] [PubMed] [Google Scholar]
- 7. Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149:847–54. [PubMed] [Google Scholar]
- 8. Koch C, Staffler G, Huttinger R, Hilgert I, Prager E, Cerny J, Steinlein P, Majdic O, Horejsi V, Stockinger H. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol. 1999;11:777–86. [DOI] [PubMed] [Google Scholar]
- 9. Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes 2002;51:3063–8. [DOI] [PubMed] [Google Scholar]
- 10. Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 11. Zhang C, Tu Z, Du S, Wang Y, Wang Q. Expression of matrix metalloproteinase 2 and extracellular matrix metalloproteinase inducer are unfavorable postoperative prognostic factors in intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2010;16:47–53. [DOI] [PubMed] [Google Scholar]
- 12. Han ZD, Bi XC, Qin WJ, He HC, Dai QS, Zou J, Ye YK, Liang YX, Zeng GH, Chen ZN, Zhong WD. CD147 expression indicates unfavourable prognosis in prostate cancer. Pathol Oncol Res. 2009;15:369–74. [DOI] [PubMed] [Google Scholar]
- 13. Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han X, Yao L, Lan M, Li Y, Zhang W. EMMPRIN/CD147 expression is associated with disease-free survival of patients with colorectal cancer. Med Oncol. 2013;30:369. [DOI] [PubMed] [Google Scholar]
- 14. Sawanyawisuth K, Wongkham C, Araki N, Zhao Q, Riggins GJ, Wongkham S. Serial analysis of gene expression reveals promising therapeutic targets for liver fluke-associated cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):89–93. [PubMed] [Google Scholar]
- 15. Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larbret F, Dubois N, Brau F, Guillemot E, Mahiddine K, Tartare-Deckert S, Verhasselt V, Deckert M. Technical advance: Actin CytoFRET, a novel FRET flow cytometry method for detection of actin dynamics in resting and activated T cell. J Leukoc Biol. 2013;94:531–9. [DOI] [PubMed] [Google Scholar]
- 17. Sribenja S, Sawanyawisuth K, Kraiklang R, Wongkham C, Vaeteewoottacharn K, Obchoei S, Yao Q, Wongkham S, Chen C. Suppression of thymosin beta10 increases cell migration and metastasis of cholangiocarcinoma. BMC Cancer 2013;13:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uthaisar K, Vaeteewoottacharn K, Seubwai W, Talabnin C, Sawanyawisuth K, Obchoei S, Kraiklang R, Okada S, Wongkham S. Establishment and characterization of a novel human cholangiocarcinoma cell line with high metastatic activity. Oncol Rep. 2016;36:1435–46. [DOI] [PubMed] [Google Scholar]
- 19. Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, Suzu S, Okada S. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;2011:539748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srikoon P, Kariya R, Kudo E, Goto H, Vaeteewoottacharn K, Taura M, Wongkham S, Okada S. Diethyldithiocarbamate suppresses an NF-kappaB dependent metastatic pathway in cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2013;14:4441–6. [DOI] [PubMed] [Google Scholar]
- 21. Uthaisar K, Seubwai W, Srikoon P, Vaeteewoottacharn K, Sawanyawisuth K, Okada S, Wongkham S. Cepharanthine suppresses metastatic potential of human cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 2012;13(Suppl):149–54. [PubMed] [Google Scholar]
- 22. Segatto I, Berton S, Sonego M, Massarut S, Fabris L, Armenia J, Mileto M, Colombatti A, Vecchione A, Baldassarre G, Belletti B. p70S6 kinase mediates breast cancer cell survival in response to surgical wound fluid stimulation. Mol Oncol. 2014;8:766–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995;55:2548–55. [PubMed] [Google Scholar]
- 24. Vaeteewoottacharn K, Kariya R, Matsuda K, Taura M, Wongkham C, Wongkham S, Okada S. Perturbation of proteasome function by bortezomib leading to ER stress-induced apoptotic cell death in cholangiocarcinoma. J Cancer Res Clin Oncol. 2013;139:1551–62. [DOI] [PubMed] [Google Scholar]
- 25. Phoomak C, Vaeteewoottacharn K, Sawanyawisuth K, Seubwai W, Wongkham C, Silsirivanit A, Wongkham S. Mechanistic insights of O-GlcNAcylation that promote progression of cholangiocarcinoma cells via nuclear translocation of NF-kappaB. Sci Rep. 2016;6:27853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y, He B, Song G, Bao Q, Tang Z, Tian F, Wang S. CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep. 2012;27:2003–9. [DOI] [PubMed] [Google Scholar]
- 27. Xu HY, Qian AR, Shang P, Xu J, Kong LM, Bian HJ, Chen ZN. siRNA targeted against HAb18G/CD147 inhibits MMP-2 secretion, actin and FAK expression in hepatocellular carcinoma cell line via ERK1/2 pathway. Cancer Lett. 2007;247:336–44. [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, Mi L, Wen N, Tian R, Wang L, Yao H, Feng Q, Zhang Y, Xing JL, Zhu P, Chen ZN. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res. 2007;5:605–14. [DOI] [PubMed] [Google Scholar]
- 29. Huang Z, Tan N, Guo W, Wang L, Li H, Zhang T, Liu X, Xu Q, Li J, Guo Z. Overexpression of EMMPRIN isoform 2 is associated with head and neck cancer metastasis. PLoS One 2014;9:e91596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene 2011;30:4410–27. [DOI] [PubMed] [Google Scholar]
- 31. Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889;133:571–3. [PubMed] [Google Scholar]
- 32. Langley RR, Fidler IJ. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011;128:2527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109:1026–34. [DOI] [PubMed] [Google Scholar]
- 35. Quemener C, Gabison EE, Naimi B, Lescaille G, Bougatef F, Podgorniak MP, Labarchede G, Lebbe C, Calvo F, Menashi S, Mourah S. Extracellular matrix metalloproteinase inducer upregulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007;67:9–15. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res. 2006;4:371–7. [DOI] [PubMed] [Google Scholar]
- 37. Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323–30. [DOI] [PubMed] [Google Scholar]
- 38. Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): Matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56:359–67. [DOI] [PubMed] [Google Scholar]
- 39. Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp Cell Res. 2012;318:1788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian AR, Zhang W, Cao JP, Yang PF, Gao X, Wang Z, Xu HY, Weng YY, Shang P. Downregulation of CD147 expression alters cytoskeleton architecture and inhibits gelatinase production and SAPK pathway in human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2008;27:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, Lee YC, Hong TM, Yang PC. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179:123–33. [DOI] [PubMed] [Google Scholar]
- 42. Higashi Y, Suzuki S, Sakaguchi T, Nakamura T, Baba S, Reinecker HC, Nakamura S, Konno H. Loss of claudin-1 expression correlates with malignancy of hepatocellular carcinoma. J Surg Res. 2007;139:68–76. [DOI] [PubMed] [Google Scholar]
- 43. Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, Qu LL, Wang SK. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61. [DOI] [PMC free article] [PubMed] [Google Scholar]