Abstract
Choriocarcinoma is one of the gestational trophoblastic neoplasias (GTNs) that originate in the chorionic villi and the extravillous trophoblast. Long noncoding RNAs (lncRNAs) are a type of non-protein-coding RNAs that have recently been implicated in human tumorigenesis. The present study investigated the role of the lncRNA LINC00261 in cell proliferation, metastasis, and apoptosis in choriocarcinoma cell lines. The transcription level of LINC00261 was significantly lower in choriocarcinoma tissues and in choriocarcinoma cell lines. Overexpression of LINC00261 caused a decrease in cell proliferation and arrested the cell cycle at the G0/G1 phase. Furthermore, overexpression of LINC00261 inhibited cell migration and invasion. Meanwhile, it promoted cell apoptosis and the relative activities of caspase 3 and caspase 9 in choriocarcinoma JEG-3 and JAR cells. These data suggested that LINC00261 promotes cell proliferation and metastasis in choriocarcinoma. Our data might provide novel insight into the early diagnosis and treatment of choriocarcinoma in clinics.
Key words: Choriocarcinoma, LINC00261, Proliferation, Metastasis, Apoptosis
INTRODUCTION
Choriocarcinoma is one of the gestational trophoblastic neoplasias (GTNs) that originate in the chorionic villi and the extravillous trophoblast1,2. As a malignant lesion, choriocarcinoma often arises after molar pregnancies. However, it can also occur after any form of gestation, such as miscarriages and full-term pregnancies3. Choriocarcinoma is generally responsive to chemotherapy at a localized stage. The therapeutic approach against this malignancy has been highly effective in patients with choriocarcinoma at an early stage. However, at an advanced stage of the disease, the prognosis remains dim, and a combined chemotherapy is needed. It should also be noted that despite the excellent effectiveness of a combined regime, in some cases an increased risk for secondary malignancies might be associated with it4. Therefore, early diagnosis and treatment are urgently needed to control the health threat caused by choriocarcinoma.
Long noncoding RNAs (lncRNAs) are a class of RNAs that are more than 200 nucleotides in length but are unable to translate proteins5. lncRNAs have been implicated in various biological processes including chromatin modification, transcription, translation, posttranscriptional processing, and posttranslational processing6,7. Because of their active involvement in many functional processes such as cell proliferation8 and metastasis7, lncRNAs have recently received greater attention in tumor biology. Emerging evidence has demonstrated the critical role of lncRNAs in a variety of cancers, with limited focus on the link between lncRNAs and choriocarcinoma. To the best of our knowledge, only the untranslated transcript of the H19 gene has been indicated to exert a tumor suppressor role in choriocarcinoma9.
The long intergenic noncoding RNA 00261 (LINC00261) is an lncRNA that was initially found to be differentially expressed in gastric and pancreatic cancers10,11. Aside from the expression profile, the functional role of LINC00261 in human cancers has remained largely unknown. On the basis of the current literature, it is only recently that LINC00261 has been found to have a clinical indication and exert a functional role in cell proliferation and metastasis in gastric cancer12, suggesting that LINC00261 may have a pivotal functional role in tumorigenesis. However, its involvement in human cancers aside from gastroenteric tumors remains to be uncovered.
The present study aimed to investigate the functional role of LINC00261 in choriocarcinoma. To this end, its expression level was initially examined in clinical choriocarcinoma tissues and in choriocarcinoma cell lines. An expression plasmid of LINC00261 was next employed to upregulate the expression of LINC00261 in choriocarcinoma cell lines JEG-3 and JAR. The involvement of LINC00261 in cell proliferation, metastasis, and apoptosis was systemically examined in this study. Our data suggest that, aside from gastroenteric tumors, LINC00261 also exerted a tumor suppressor role in choriocarcinoma. LINC00261 might be a promising approach to the early diagnosis and treatment of choriocarcinoma.
MATERIALS AND METHODS
Human Sample
This study was approved by the ethical committee of The Second Hospital of Jilin University. A total of 60 patients diagnosed with choriocarcinoma who undertook clinical surgeries in our hospital were admitted to this study. Tumor tissue and its adjacent noncancerous tissues were collected and stored in liquid nitrogen after dissection. All patients showed their full intention to participate in this study, and written consent from each patient was collected.
Cell Culture and Transfection
A total of three choriocarcinoma cell lines, namely, BeWo CCL-98, JEG-3, and JAR, were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), and a normal cell line HTR8/SVneo was commercially obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, P.R. China). All of these cells were cultured in the recommended medium supplied with 10% fetal bovine serum (FBS; Gibco, USA) at a 5% CO2 atmosphere in a 37°C incubator. Transfection was performed in JEG-3 and JAR cells with Lipofectamine 2000 according to the manufacturer’s instruction.
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from human tissues and cultured cell lines by TRIzol reagent (TaKaRa Biotechnology, Dalian, P.R. China) with a concentration of 1 ml for each well in six-well plates. The quality and quantity of the RNA samples were measured by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was reversely transcribed by a Transcriptor First Strand cDNA Synthesis Kit (TaKaRa Biotechnology). RT-PCR was then performed using an ABI 7900 Fast Real-Time PCR system. GAPDH was included as an internal control.
Cell Proliferation
Cell proliferation was explored with 3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) as per the protocols. Briefly, a total of 1 × 104 JEG-3 or JAR cells were seeded into a 96-well plate and transfected with plasmids in the presence or absence of LINC00261 overexpression in triplicate. Forty-eight hours after treatment, 10 μl of MTT (5 μg/ml) was added into the medium in each well and incubated for another 3 h at 37°C in the dark. Formazan crystals that formed were then dissolved in 100 μl of DMSO solution, and the absorbance was collected at a wavelength of 570 nM with a TECAN reader (NY, USA).
Colony Formation Assay
Both JEG-3 and JAR cells were seeded into six-well plates and pretreated with LINC00261-expressing plasmid. After 48 h, these were spread into 12-well plates (100 cells/well) in triplicate. The plates were incubated at 37°C for a continuous 14 days, and the colonies were fixed with precold methanol and stained with crystal violet (1%) for 5 min. Colonies that contain more than 50 cells were counted as survivors under a Nikon microscope with a magnification of 200×.
Cell Cycle Assay
Both JEG-3 and JAR cells were seeded into six-well plates (3 × 105 cells per well) and pretreated with LINC00261-expressing plasmids for 48 h until a confluence of 85% was reached. Cells were then collected by low-speed centrifugation (1,000 rpm, 5 min), and cell pellets were resuspended in 1 ml of PBS solution, fixed in 75% ice-cold ethanol, and incubated at −20°C for 48 h. Prior to flow cytometry (FCM) analysis, JEG-3 and JAR cells were lysed, centrifuged, and resuspended in propidium iodide (PI) staining buffer with 50 μl/ml of PI and 250 μl/ml of RNase A. The cell mixtures were then incubated at 4°C for an additional 30 min in a dark environment and examined by the fluorescence-activated cell sorting (FACS) technique (Beckman, Germany).
Transwell Assay
JEG-3 and JAR cells were seeded into six-well plates and transfected with LINC00261-expressing plasmid for 48 h. Cells were then collected by low-speed centrifugation and resuspended with the recommended medium (FBS free), and 1 × 104 cells were seeded into the upper chamber of a 24-well plate (Corning, Corning, NY, USA) with a volume of 100 μl, before which 600 μl of medium with 10% FBS was added into the lower chamber. After incubation for 12 h, cells were washed with PBS three times, fixed with ice-cold methanol for 10 min, and stained with crystal violet for 5 min. For cell invasion assays, the membrane of the chamber was precoated with Matrigel (BD Biosciences, San Jose, CA, USA) for 6 h at 37°C. The ability of cells to migrate and invade was quantified by counting the cells on the lower surface of the membrane. Five fields were randomly chosen and calculated under a Nikon microscope. Each experiment was repeated at least three times in triplicate.
Wound Healing Assay
Wound healing assays were conducted by scratching identical wounds for anchorage-dependent cells JEG-3 and JAR with 10-μl sterile pipette tips, before which cells were seeded into six-well plates and transfected with LINC00261-expressing plasmid for 48 h. Both cell lines were then washed with warmed PBS twice and scraped in a cross in the center of each well. Cells were washed again with PBS and immediately replaced with fresh medium in the absence of FBS. After growth for 24 h, cells were observed and photographed with a Nikon microscope at a magnification of 200×.
Cell Apoptosis Detection
Cell apoptosis was examined by Hoechst-33258 (Beyotime, Nanjing, P.R. China) according to the manufacturer’s protocol. JEG-3 and JAR cells were seeded into 12-well plates in triplicate and transfected with plasmids in the presence or absence of LINC00261 overexpression for 48 h. Hoechst-33258 staining buffer was then mixed into the medium and incubated for 15 min. Fluorescence images were then captured randomly under an inverted fluorescence microscope (Nikon, Japan). The percentages of apoptotic neurons were obtained with the following formula: apoptotic rate = (apoptotic cells/total cells) × 100%.
Caspase Activities Detection
The relative activity of caspase 3, caspase 8, and caspase 9 was detected with relative caspase activity kits (Beyotime), as per the manufacturer’s protocols. Briefly, both JEG-3 and JAR cells were administrated with LINC00261-expressing plasmid for 48 h, and cell lysates were collected by lysis buffer (Beyotime). Activity was examined in a 96-well plate by incubating proteins and reaction buffers with substrates for caspase 3, caspase 8, and caspase 9, respectively. Each sample was then detected with a TECAN reader at an absorbance of 405 nm.
Western Blot Analysis
Both JEG-3 and JAR cells were seeded into a six-well plate and transfected with LINC00261-expressing plasmid for 48 h, after which total proteins were extracted by NP40 lysis buffer and quantified with the standard BCA method (Thermo Scientific, Waltham, MA, USA). Equal amounts of protein from each sample (50 μg) were loaded onto an SDS-PAGE gel and electroblotted onto nitrocellulose (NC) membranes. The membrane was then blocked with TBST supplemented with 5% milk for 1 h at room temperature and incubated with primary antibodies at 4°C overnight. Primary antibodies against Bax (cyto. and mito.) were purchased from Abcam (Cambridge, UK). Primary antibodies against caspase 9, caspase 3, cytochrome C, and GAPDH, as well as secondary antibodies, were commercially obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical Analysis
All data were presented as mean ± standard deviation (SD). Each experiment was repeated at least three times in triplicate, except when stated otherwise. The Student’s t-test was included to compare the difference between groups. Any value of p < 0.05 was considered statistically significant.
RESULTS
The Expression of Long Noncoding RNA LINC00261 Was Decreased in Human Choriocarcinoma
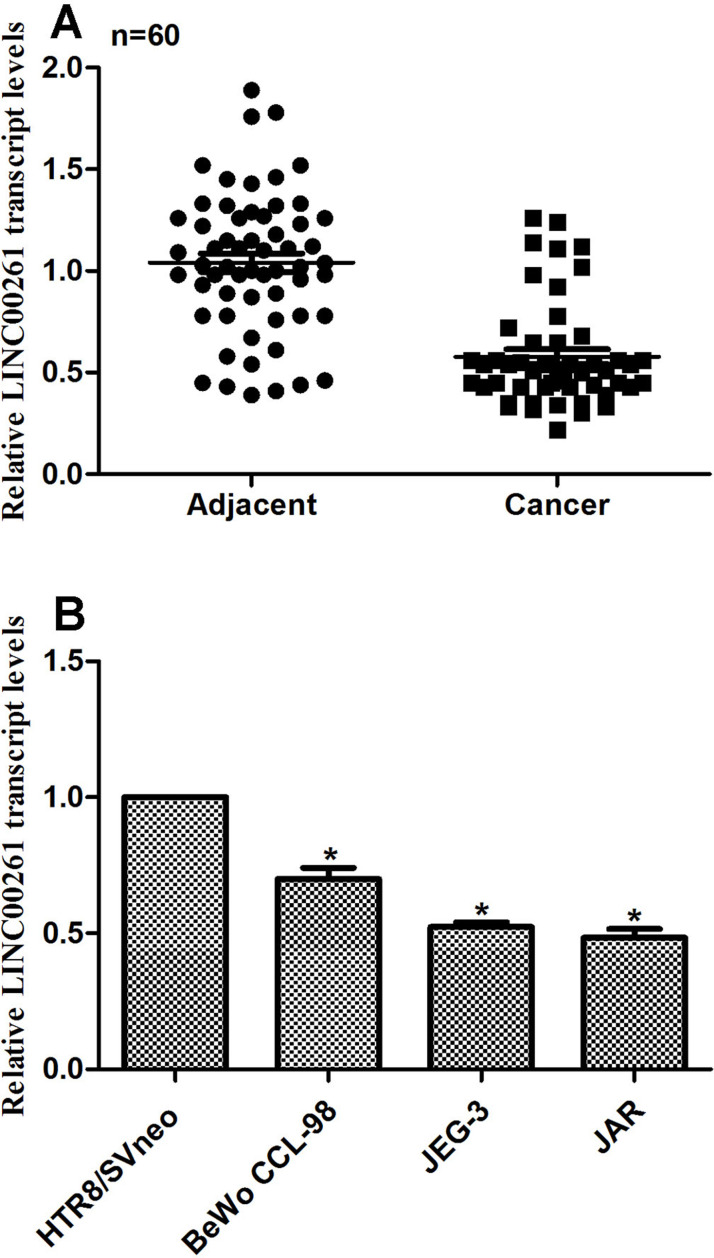
First we examined the relative expression of lncRNA LINC00261 in human choriocarcinoma in vivo and in vitro. To this end, 60 choriocarcinoma patients were included in this study, and their tumor tissue, as well as adjacent noncancerous tissue, was collected for RT-PCR analysis. The average expression of lncRNA LINC00261 was significantly lower than their counterparts (Fig. 1A). BeWo CCL-98, JEG-3, and JAR cells were derived from human choriocarcinoma patients, while HTR/SVneo cells were from normal placentas. The relative expression of LINC00261 was also remarkably decreased in three choriocarcinoma cell lines compared with normal cells (Fig. 1B). Among the choriocarcinoma cell lines, JEG-3 and JAR cells showed the lowest transcription level of LINC00261; thus, these two cell lines were selected for subsequent analysis. These results suggested that the relative expression of LINC00261 was notably decreased in human choriocarcinoma.
Figure 1.
The expression of long noncoding RNA LINC00261 was decreased in human choriocarcinoma. (A) A total of 60 choriocarcinoma patients were included into this study, and RT-PCR analysis showed that their expression of LINC00261 was significantly lower than their adjacent noncancerous tissues. (B) The relative transcript level of LINC00261 was remarkably decreased in human choriocarcinoma cell lines BeWo CCL-98, JEG-3, and JAR. *p < 0.05 versus HTR8/SVneo.
Overexpression of LINC00261 Inhibited Cell Proliferation in Human Choriocarcinoma Cells
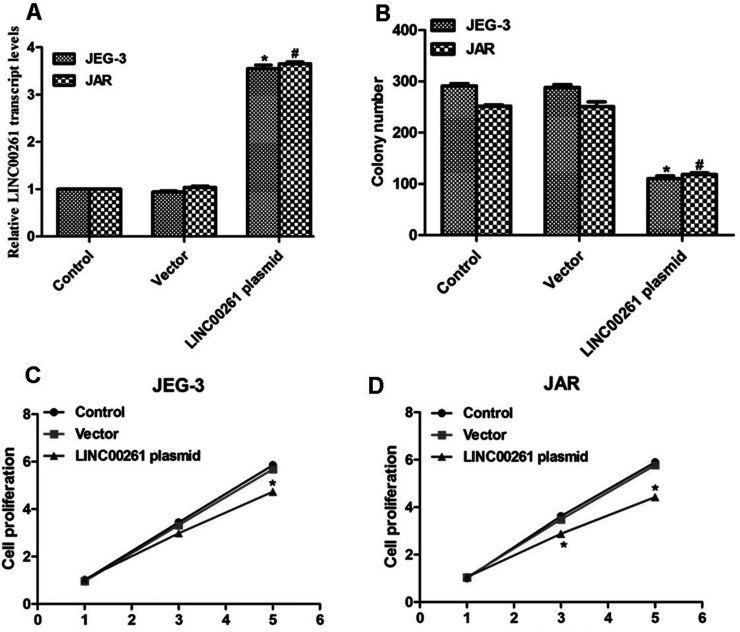
Next we examined the detailed role of LINC00261 in human choriocarcinoma. The long length of the LINC00261 gene was cloned from the human genome, and LINC00261-expressing plasmid was constructed. Both cell lines showed increased expression of LINC00261 when cells were transfected with this expressing plasmid (Fig. 2A). A colony formation assay was performed in JEG-3 and JAR cells. Approximately 300 colonies were formed in control JEG-3 cells, while only 100 colonies were observed in LINC00261 plasmid-transfected cells (Fig. 2B). A similar phenomenon was shown in JAR cells (Fig. 2B). Transfection of LINC00261-expressing plasmid inhibited cell proliferation on the fifth day upon treatment of JEG-3 cells (Fig. 2C). Likewise, the cell proliferative rate of JAR cells was retarded by 30% on the third day and 40% on the fifth day when cells were transfected with LINC00261-expressing plasmid (Fig. 2D).
Figure 2.
Overexpression of LINC00261 inhibited colony formation and cell proliferation. (A) Transfection of LINC00261-expressing plasmid increased the transcript level of LINC00261 in JEG-3 and JAR cells. (B) Transfection of LINC00261-expressing plasmid inhibited colony formation for both JEG-3 and JAR cells. *p < 0.05, versus Control in JEG-3 cells. #p < 0.05, versus Control in JAR cells. (C) Overexpression of LINC00261 in JEG-3 cells inhibited cell proliferation on the fifth day. (D) Overexpression of LINC00261 in JAR cells suppressed cell proliferation on the third and fifth days upon transfection. *p < 0.05 versus Control.
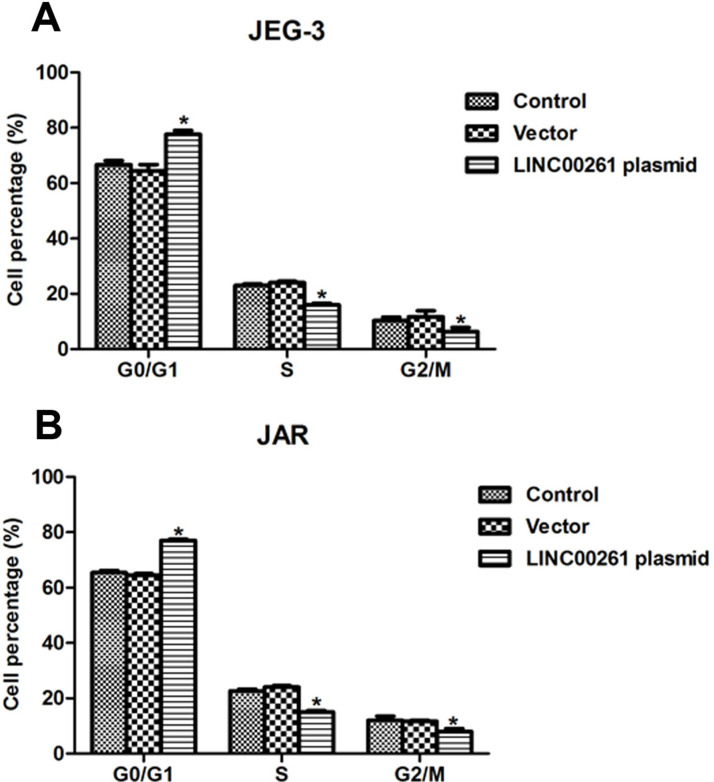
Cell cycle analysis was performed to further explore the effects of LINC00261 on cell proliferation. Approximately 12% of JEG-3 cells in the S phase and G2/M phase were shifted to the G0/G1 phase upon transfection of LINC00261-expressing plasmid, while treatment with vector caused little effect on the cell cycle (Fig. 3A). Analogously, the JAR cell percentage in the G0/G1 phase was increased by 14%, while the cell percentage in the S phase and in the G2/M phase was decreased by 8% and 6%, respectively (Fig. 3B). All of these data revealed that overexpression of LINC00261 suppressed cell proliferation in human choriocarcinoma cells.
Figure 3.
Overexpression of LINC00261 arrested cell cycle in the G0/G1 phase in JEG-3 and JAR cells. (A) Transfection of LINC00261-expressing plasmid caused cell cycle arrest in the G0/G1 phase, while the cell percentage was decreased in the S phase and the G2/M phase. (B) Overexpression of LINC00261 in JAR cells increased the cell percentage in the G0/G1 phase and decreased that in the S phase and the G2/M phase. *p < 0.05 versus Control
Overexpression of LINC00261 Inhibited Cell Metastasis in Human Choriocarcinoma Cells
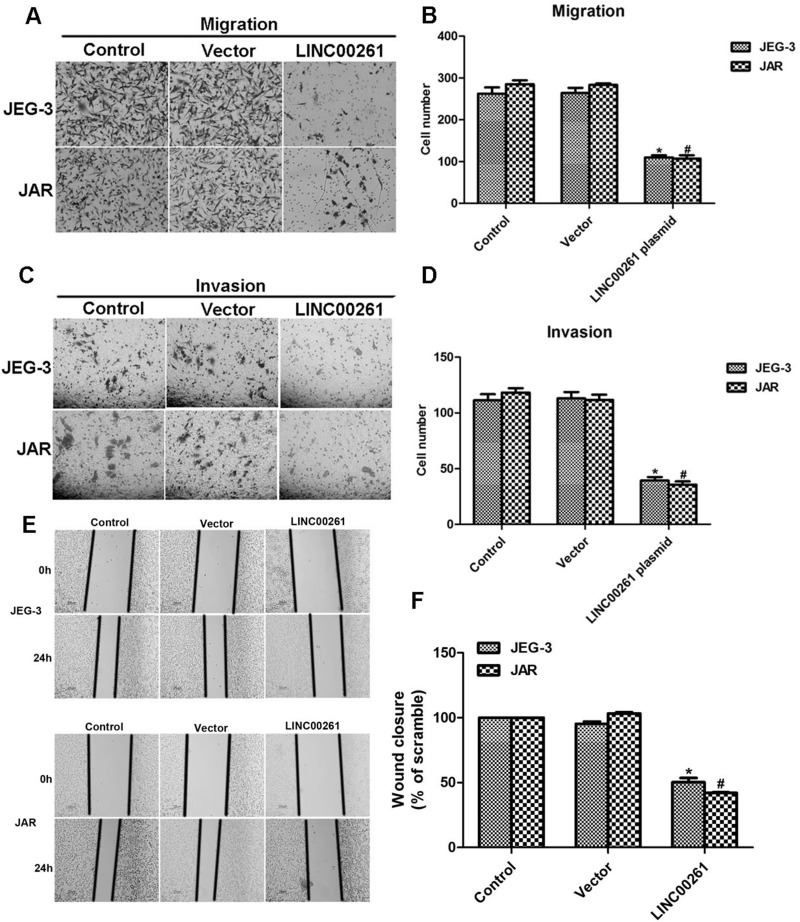
The effect of LINC00261 on cell metastasis was also explored by Transwell and wound healing assays. Prior to the experiments, both JEG-3 and JAR cells were transfected with LINC00261-expressing plasmid. A total of 260 JEG-3 control cells were observed on the lower surface of the membrane, while only 100 cells migrated through the membrane upon transfection with LINC00261-expressing plasmid (Fig. 4A and B). The migration potential of JAR cells was suppressed by approximately 63.5% when cells were treated with LINC00261 plasmid. Similarly, the invasive capacity of JEG-3 cells was inhibited by 63.6%, and about 72.0% of JAR cells were suppressed from invading through the membrane upon transfection of LINC00261-expressing plasmid (Fig. 4C and D).
Figure 4.
Overexpression of LINC00261 inhibited cell metastasis in JEG-3 and JAR cells. (A) Representative images of cell migration assays in JEG-3 and JAR cells when cells were transfected with LINC00261-expressing plasmid. (B) Transfection of LINC00261 inhibited cell migration potential for JEG-3 and JAR cells. (C) Representative images of cell invasion assays in JEG-3 and JAR cells when cells were transfected with LINC00261-expressing plasmid. (D) Transfection of LINC00261 suppressed cell invasive capacity for JEG-3 and JAR cells. *p < 0.05 versus Control in JEG-3 cells. #p < 0.05 versus Control in JAR cells. (E) Representative images of wound healing assays in JEG-3 cells and JAR cells when cells were transfected with LINC00261-expressing plasmid. (F) Quantification of wound healing assays for both JEG-3 and JAR cells upon transfection of LINC00261-expressing plasmid. *p < 0.05 versus Control in JEG-3 cells. #p < 0.05 versus Control in JAR cells.
A wound healing assay was performed on JEG-3 and JAR cells. Cells were transfected with LINC00261-expressing plasmid 48 h before the experiments. The ability of JEG-3 cells to migrate was inhibited by approximately 50% upon LINC00261 treatment, while more than 53% of JAR cells were suppressed from migration toward the scraped wounds (Fig. 4E and F). These results show that overexpression of LINC00261 in human choriocarcinoma cells inhibits cell migration and invasion, providing further evidence of the expression of LINC00261 in three choriocarcinoma cell lines (Fig. 1B).
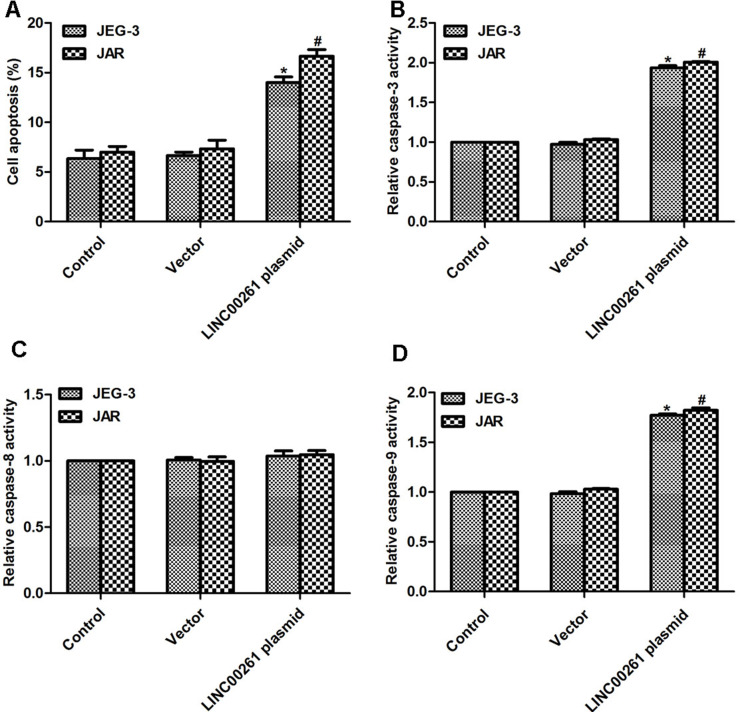
Upregulation of LINC00261 in Human Choriocarcinoma Increased Cell Apoptotic Rate and the Relative Activities of Caspase 3 and Caspase 9
Cell apoptosis was examined in these two cell lines. Cell apoptotic rate was increased by 9% in JEG-3 cells and by 11% in JAR cells when cells were transfected with LINC00261-expressing plasmid, while transfection of the vector caused no effect on cell apoptosis (Fig. 5A). We tried to figure out the detailed mechanism underlying the regulatory role of LINC00261 in cell apoptosis in human choriocarcinoma cells. Thus, we examined the activity of caspase 3, caspase 8, and caspase 9 by relative caspase activity kit and Western blot. The relative activity of caspase 3 was increased by twofold upon transfection with LINC00261 plasmid compared to JEG-3 control cells and JAR cells, respectively (Fig. 5B). The activity of caspase 8 remained stable in the presence or absence of LINC00261-expressing plasmid (Fig. 5C). However, treatment with LINC00261 caused increased activity in caspase 9 in both cell lines (Fig. 5D), suggesting the potential effect of LINC00261 on cell apoptosis.
Figure 5.
Overexpression of LINC00261 increased cell apoptosis and the activities of caspase 3 as well as caspase 9. (A) Transfection of LINC00261 plasmid increased cell apoptotic rate in both JEG-3 and JAR cells. (B) Overexpression of LINC00261 in JEG-3 and JAR cells increased the relative activities of caspase 3. (C) Overexpression of LINC00261 in JEG-3 and JAR cells caused little effects on the activity of caspase 8. (D) Overexpression of LINC00261 in JEG-3 and JAR cells increased the relative activities of caspase 9. *p < 0.05 versus Control in JEG-3 cells. #p < 0.05 versus Control in JAR cells.
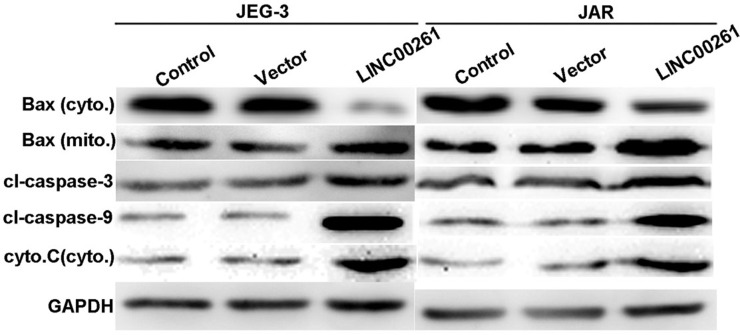
Upregulation of LINC00261 Modulated Apoptosis-Related Proteins in Human Choriocarcinoma Cells
When cells were overexpressed with LINC00261, it was observed that the protein level of Bax in the cytoplasm was significantly decreased, whereas its level in the mitochondria was increased. Concomitantly, the protein level of cytochrome C in the cytoplasm was upregulated. The cleaved caspases, including cleaved caspase 3 and cleaved caspase 9, were consistently increased in LINC00261-overexpressed JEG-3 cells and JAR cells. These proteins were related to mitochondria-mediated cell apoptosis (Fig. 6), and therefore this observation suggested that LINC00261 might promote cell apoptosis through a mitochondria pathway.
Figure 6.
Upregulation of LINC00261 modulated apoptosis-related proteins in human choriocarcinoma cells. JEG-3 and JAR cells were overexpressed with LINC00261 and subject to protein extraction for apoptosis-related analysis. It was observed that Bax in the cytoplasm was significantly decreased, whereas its level in the mitochondria was increased. The protein levels of cytochrome C in the mitochondria, cleaved caspase 3, and cleaved caspase 9 were consistently increased in LINC00261-overexpressed JEG-3 cells and JAR cells. Bax (cyto.), Bax in the cytoplasm; Bax (mito.), Bax in the mitochondria; cyto. C (cyto.), cytochrome C in the cytoplasm.
DISCUSSION
Choriocarcinoma represents a large proportion of GTNs. Traditionally, prognosis of this malignancy is desirable because of the development of chemotherapy. However, updated statistics have shown that approximately 10%–30% of patients have an incomplete response to first-line multiagent chemotherapy13,14. Moreover, choriocarcinoma is featured by a high potential for malignancy with a tendency for widespread dissemination metastases15. In the metastatic stage of this disease, the prognosis remains dim. Despite the excellent effectiveness of a combined regime, in some cases an increased risk for secondary malignancy might be associated with it4. It is therefore urgent to find novel markers that could serve as an early diagnostic and therapeutic target for choriocarcinoma.
The present study, for the first time, identified an lncRNA termed LINC00261 as a critical suppressor of cell proliferation and metastasis in choriocarcinoma. The transcription level of LINC00261 was significantly lower in choriocarcinoma tissues and in choriocarcinoma cell lines, which is consistent with its expression profile in gastroenteric tumors10–12. After overexpression of LINC00261 in choriocarcinoma JAR and JEG-3 cells, it was observed that clonogenic potential and cell proliferation were significantly inhibited. Instead, cell cycle progression was arrested at the G0/G1 phase. Since cell cycle dysregulation is a well-recognized hallmark of tumor growth16, these observations suggest that LINC00261 may function as an inhibitor to tumor growth in choriocarcinoma. Interestingly, cell apoptosis was also induced by LINC00261 overexpression. Upregulation of LINC00261 caused a significant increase in caspase 3 and caspase 9 activity. The activity of caspase 8 remained unchanged. Two major pathways are known to mediate cell apoptosis: the mitochondria-mediated intrinsic pathway and the death receptor-induced extrinsic pathway17. The mitochondria-dependent pathway is regulated by the cytochrome C and Bax families of proteins. Release of cytochrome C from the mitochondria signals the initiation of apoptosis18, while Bax in the mitochondria indicates the proapoptotic process and that in the cytoplasm suggests an antiapoptotic outcome. Upon release, cytochrome C activates caspase 3 by cleaving it, leading to a cascade of activating a series of downstream caspases such as caspase 9. Distinct from the intrinsic mitochondria-dependent pathway, the extrinsic pathway is triggered by the binding of extracellular ligands to specific transmembrane receptors, primarily members of the tumor necrosis factor receptor (TNFR) family19. Receptor binding by the TNF family ligands activates caspase-dependent pathways. Caspases 8 and 10 are two such prodeath caspases that initiate the extrinsic apoptosis pathway20. The current study has found that in addition to the increased activity of caspase 3 and caspase 9, the protein levels of Bax in the mitochondria, cleaved caspase 3, cleaved caspase 9, and cytochrome C in the cytoplasm were concordantly upregulated by LINC00261, suggesting that the intrinsic mitochondria-dependent apoptosis pathway was initiated. In contrast, the activity of caspase 8 remained unchanged upon LINC00261 overexpression, indicating that the extrinsic apoptosis pathway might not be critically involved in LINC00261-mediated cell apoptosis. Given that cell apoptosis is a good basis for tumor growth inhibition, our data suggest that LINC00261 inhibits cell proliferation by inducing a mitochondria-dependent apoptosis pathway.
In addition to growth inhibition, our data also provided evidence that LINC00261 inhibited cell metastasis in choriocarcinoma cells. After overexpressing LINC00261, cell migration was inhibited by 61.5% in JEG-3 cells and 63.5% in JAR cells. Cell invasive capacity was inhibited by 63.6% in JEG-3 cells and 72.0% in JAR cells. Wound recovery rate was concordantly suppressed by up to 50.1% in JEG-3 cells and 53.2% in JAR cells after LINC00261 overexpression. The suppression of cancer metastasis was also observed in gastric cancer where a molecular mechanism of epithelial–mesenchymal transit (EMT) was proposed12. Though the detailed molecular mechanism of how LINC00261 suppresses cell metastasis is unclear, there should be mechanisms other than EMT processes since N-cadherin and vimentin had only a twofold change upon transfection with the LINC00261 plasmid in a previous report12. It would be very interesting to explore the underlying mechanism hereafter.
In all, our data show that the lncRNA LINC00261 was significantly downregulated in choriocarcinoma tissues and cell lines. Overexpression of LINC00261 suppresses the cell proliferation, migration, and invasion abilities in choriocarcinoma. LINC00261 might serve as a drug target for the sake of clinical application. Further insight into the detailed mechanism underlying LINC00261-mediated functional roles and its targets may aid in the early diagnosis and treatment of choriocarcinoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Lurain JR. Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11–8. [DOI] [PubMed] [Google Scholar]
- 2. Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C. Gestational trophoblastic disease: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):i39–i50. [DOI] [PubMed] [Google Scholar]
- 3. Biscaro A, Braga A, Berkowitz RS. Diagnosis, classification and treatment of gestational trophoblastic neoplasia. Rev Bras Ginecol Obstet. 2015;37:42–51. [DOI] [PubMed] [Google Scholar]
- 4. May T, Goldstein DP, Berkowitz RS. Current chemotherapeutic management of patients with gestational trophoblastic neoplasia. Chemother Res Pract. 2011;2011:806256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010;142:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23:205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin Z, Ding H, He E, Chen J, Li M. Overexpression of long non-coding RNA MFI2 promotes cell proliferation and suppresses apoptosis in human osteosarcoma. Oncol Rep. 2016;36:2033–40. [DOI] [PubMed] [Google Scholar]
- 9. Wake N, Arima T, Matsuda T. Involvement of IGF2 and H19 imprinting in choriocarcinoma development. Int J Gynaecol Obstet. 1998;60(Suppl 1):S1–S8. [PubMed] [Google Scholar]
- 10. Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19:3658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muller S, Raulefs S, Bruns P, Afonso-Grunz F, Plotner A, Thermann R, Jager C, Schlitter AM, Kong B, Regel I, Roth WK, Rotter B, Hoffmeier K, Kahl G, Koch I, Theis FJ, Kleeff J, Winter P, Michalski CW. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer 2015;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan Y, Wang YF, Su HF, Fang N, Zou C, Li WF, Fei ZH. Decreased expression of the long noncoding RNA LINC00261 indicate poor prognosis in gastric cancer and suppress gastric cancer metastasis by affecting the epithelial-mesenchymal transition. J Hematol Oncol. 2016;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Abrao RA, de Andrade JM, Tiezzi DG, Marana HR, Candido DRF, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: Comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecol Oncol. 2008;108:149–53. [DOI] [PubMed] [Google Scholar]
- 14. Lurain JR, Singh DK, Schink JC. Primary treatment of metastatic high-risk gestational trophoblastic neoplasia with EMA-CO chemotherapy. J Reprod Med. 2006;51:767–72. [PubMed] [Google Scholar]
- 15. Yousefi Z, Mottaghi M, Rezaei A, Ghasemian S. Abnormal presentation of choriocarcinoma and literature review. Iran J Cancer Prev. 2016;9:e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sherr CJ. Cancer cell cycles. Science 1996;274:1672–7. [DOI] [PubMed] [Google Scholar]
- 17. Spencer SL, Sorger PK. Measuring and modeling apoptosis in single cells. Cell 2011;144:926–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vladimirov YA, Proskurnina EV, Alekseev AV. Molecular mechanisms of apoptosis: Structure of cytochrome c-cardiolipin complex. Biochemistry (Mosc) 2013;78:1086–97. [DOI] [PubMed] [Google Scholar]
- 19. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–9. [DOI] [PubMed] [Google Scholar]
- 20. Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–32. [DOI] [PMC free article] [PubMed] [Google Scholar]