Abstract
Transmembrane protein 45B (TMEM45B) is a member of the TMEM family of proteins and has been reported to be expressed abnormally in different kinds of human tumors. However, the biological function of TMEM45B in osteosarcoma remains unclear. The objective of this study was to investigate the role of TMEM45B in regulating the biological behavior of osteosarcoma cells. Our results demonstrated that the expression of TMEM45B at both the protein and mRNA levels was dramatically upregulated in human osteosarcoma cell lines. Knockdown of TMEM45B significantly suppressed the proliferation, migration, and invasion of U2OS cells in vitro. Mechanistically, knockdown of TMEM45B sharply downregulated the expression level of β-catenin, cyclin D1, and c-Myc in U2OS cells. Finally, knockdown of TMEM45B attenuated tumor growth in transplanted U2OS-derived tumors in nude mice. Taken together, our results demonstrated that TMEM45B plays an important role in regulating the proliferation, migration, and invasion of osteosarcoma cells and that its effects on proliferation and invasion were mediated partially through the Wnt/β-catenin signaling pathway. These observations support our belief that TMEM45B may serve as an oncogene in the development and progression of osteosarcoma.
Key words: Transmembrane protein 45B (TMEM45B), Osteosarcoma, Invasion, Wnt/β-catenin pathway
INTRODUCTION
Osteosarcoma is the most common primary bone tumor in children and adolescents1. Despite the development of multiple therapeutic strategies for osteosarcoma, including wide tumor excision, multiagent chemotherapy, and radiotherapy in the past few decades2–4, approximately 30% of children with osteosarcoma ultimately die from metastasis5. Therefore, dissecting the molecular mechanisms that regulate osteosarcoma invasion may facilitate the advancement of clinical treatment.
The transmembrane (TMEM) family of proteins plays a key role in a variety of physiological functions, including transepithelial ion transport, olfaction, phototransduction, smooth muscle contraction, nociception, cell proliferation, and control of neuronal excitability6–8. Transmembrane protein 45B (TMEM45B) is a member of TMEMs and has been reported to be expressed abnormally in different kinds of human tumors9. Recently, one study reported that TMEM45B showed a high expression in pancreatic cancer tissues and cell lines compared with the normal pancreatic tissues and cells. Also, downregulation of TMEM45B substantially repressed the proliferation, invasion, and migration of pancreatic cancer cells, accompanied by the induction of cell cycle arrest and apoptosis10. However, the biological function of TMEM45B in osteosarcoma remains unclear. The objective of this study was to investigate the role of TMEM45B in regulating the biological behavior of osteosarcoma cells. These results indicated that TMEM45B may serve as an oncogene in the development and progression of osteosarcoma, and knockdown of TMEM45B inhibited osteosarcoma cell proliferation and invasion, at least in part, through the inactivation of the Wnt/β-catenin signaling pathway.
MATERIALS AND METHODS
Cell Culture
Three human osteosarcoma cell lines (U2OS, SaOS2, and MG-63) and the normal osteoblast cell line (hFOB1.19) were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 U/ml penicillin, and 0.1 g/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified 5% CO2 environment.
Short Hairpin RNA-Mediated Knockdown of TMEM45B and Cell Transfection
A specific TMEM45B shRNA (shTMEM45B; 5′-GCCGCAATTAGGACTTTGT-3′) and the negative control shRNA (shNC) were synthesized by Sangon Biotech (Shanghai, P.R. China). U2OS cells were transfected with shTMEM45B or shNC using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Cell Proliferation Assay
Cell proliferation was detected by the cell counting kit-8 (CCK-8) assay. Briefly, infected cells (1 × 104 cells/well) were plated in 96-well plates and cultured at 24-h intervals for 4 days. Then 10 μl of CCK-8 solution was added into each well for 1 h of incubation. The absorbance at 450 nm was determined using a microplate reader (Bio-Rad, Hercules, CA, USA).
Cell Migration and Invasion Assays
For the cell migration assay, infected cells (1 × 104 cells/well) were suspended in serum-free medium and plated in the upper chamber (Corning Costar, Corning, NY, USA), and 600 μl of DMEM medium supplemented with 10% FBS was added into the lower chamber. After 24 h of incubation, the cells that had migrated to the lower surface were fixed in methanol, stained with 0.1% crystal violet for 15 min, and counted under a light microscope (Olympus, Tokyo, Japan). For the cell invasion assay, the inserts were coated with 100 μl of diluted (1:10) Matrigel (BD Biosciences, San Jose, CA, USA).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from osteosarcoma cell lines using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA (5 μg) was reverse transcribed using SuperScript Reverse Transcriptase III (Invitrogen). qRT-PCR was performed on the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Eugene, OR, USA) using the SYBR Green PCR Master Mix (Applied Biosystems). The sequences of the primers were as follows: TMEM45B, 5′-TCCTTCACCGCGCCTATAATC-3′ (forward) and 5′-TACCGGGTTCATGCCATTCTC-3′ (reverse); β-actin, 5′-GATCATTGCTCCTCCTGAGC-3′ (forward) and 5′-ACTCCTGCTTGCTGATCCAC-3′ (reverse). PCR products were separated on 1.2% agarose gels and visualized with ethidium bromide (EB). The relative expression level was calculated using the 2−ΔΔCt cycle threshold method.
Western Blot
Cells were lysed in RIPA buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM protease, and phosphatase inhibitor mixtures. Equivalent amounts of protein (30 μg) were separated using 10% SDS-PAGE and transferred to PVDF membrane (Millipore, Boston, MA, USA). After blocking with 5% skim milk in TBS/Tween 20 (0.05%, v/v) for 1 h, the membrane was incubated with specific primary antibodies (anti-TMEM45B, anti-β-catenin, anti-cyclin D1, anti-c-Myc, and anti-GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The target protein was visualized using enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Xenograft Tumor Formation Assay
For xenograft tumor formation, infected U2OS cells (1 × 106) were injected subcutaneously into 4-week-old female nude mice (n = 7 per group). Tumor growth was monitored with calipers once a week for a total of 4 weeks. Tumor volume was calculated from two perpendicular diameters using the formula: volume = (length/2) × (width2). Tumors were removed and weighed. All of the animal experiments were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University General Hospital (P.R. China).
Statistical Analysis
All data were presented as mean ± standard deviation (SD). The statistical significance of the difference was analyzed by ANOVA and post hoc Dunnett’s test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
TMEM45B Was Highly Expressed in Osteosarcoma Cell Lines
We examined the endogenous expression of TMEM45B in human osteosarcoma cell lines using qRT-PCR and Western blotting. The mRNA expression levels of TMEM45B were significantly higher in human osteosarcoma cell lines compared with that in the normal osteoblast cell line (hFOB1.19) (Fig. 1A). Consistent protein levels were observed by Western blotting (Fig. 1B).
Figure 1.
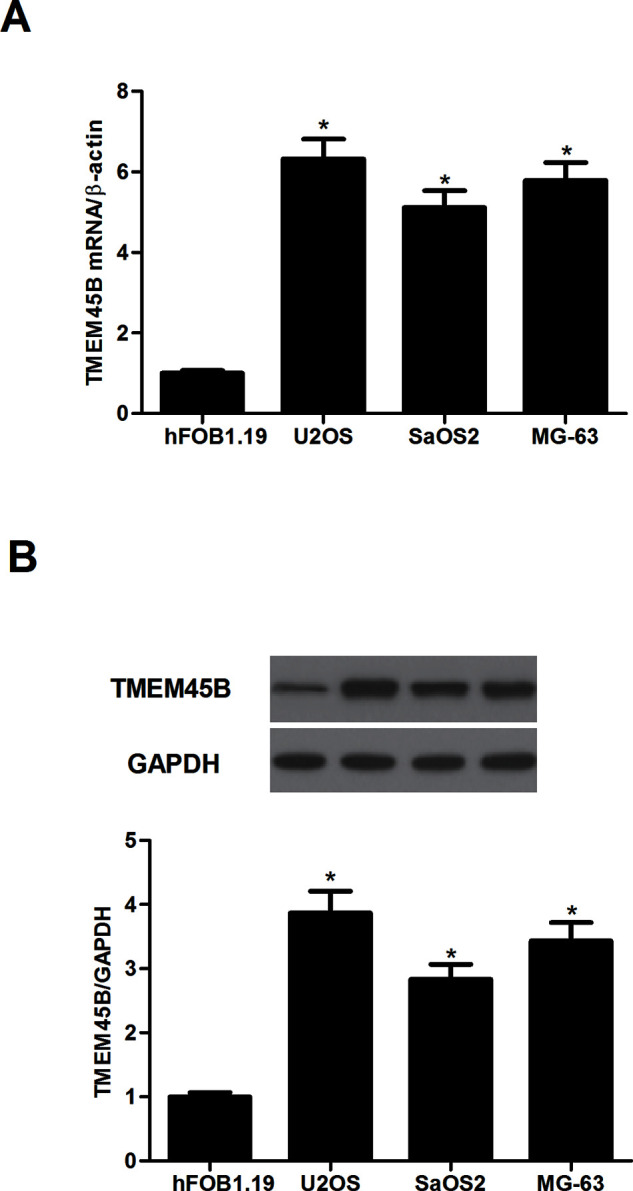
TMEM45B was highly expressed in osteosarcoma cell lines. (A) The mRNA expression level of TMEM45B was evaluated in osteosarcoma cell lines by qRT-PCR. (B) The protein expression level of TMEM45B was evaluated in osteosarcoma cell lines by Western blotting. GAPDH was used as a loading control. *p < 0.05, compared with the shNC group.
Knockdown of TMEM45B Inhibited Osteosarcoma Cell Proliferation In Vitro
To study the role of TMEM45B in the progression of osteosarcoma, we established a stably transfected U2OS cell line expressing shRNA against TMEM45B. The expression of TMEM45B at both the protein and mRNA levels was dramatically decreased in U2OS cells after transfection with shTMEM45B (Fig. 2A). These results indicate that the silencing of TMEM45B by shRNA targeting TMEM45B in U2OS cells was successful. The CCK-8 assay was then performed to determine the effect of shTMEM45B on cell proliferation, and the results indicated that knockdown of TMEM45B significantly suppressed U2OS cell proliferation, compared with the shNC group (Fig. 2B).
Figure 2.
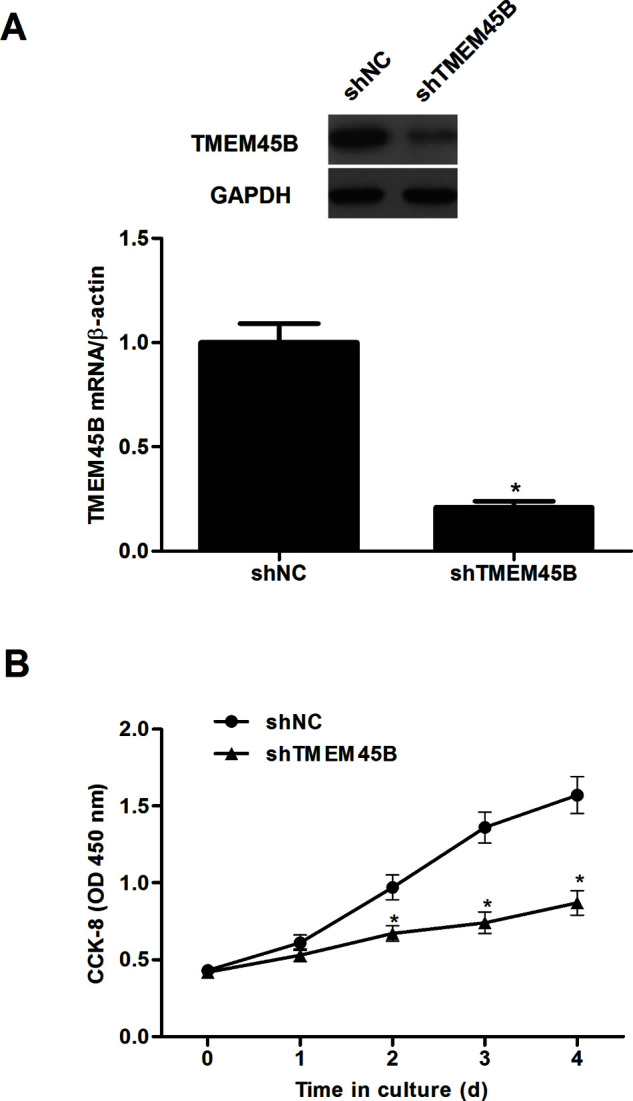
Knockdown of TMEM45B inhibited osteosarcoma cell proliferation in vitro. U2OS cells were transfected with shTMEM45B or shNC for 48 h, respectively. (A) The expression of TMEM45B at both the mRNA and protein levels was analyzed by qRT-PCR and Western blotting. (B) The CCK-8 assay was performed to determine the effect of shTMEM45B on cell proliferation. *p < 0.05, compared with the shNC group.
Knockdown of TMEM45B Inhibited Osteosarcoma Cell Migration and Invasion In Vitro
We next investigated the effects of shTMEM45B on cell migration and invasion in vitro using the Transwell migration chamber and Matrigel invasion assays. The cells in the lower chamber of the Transwell were obviously reduced in U2OS cells infected with shTMEM45B compared to shNC-treated cells (Fig. 3A). In addition, stable knockdown of TMEM45B significantly suppressed invasion in the U2OS cells (Fig. 3B).
Figure 3.
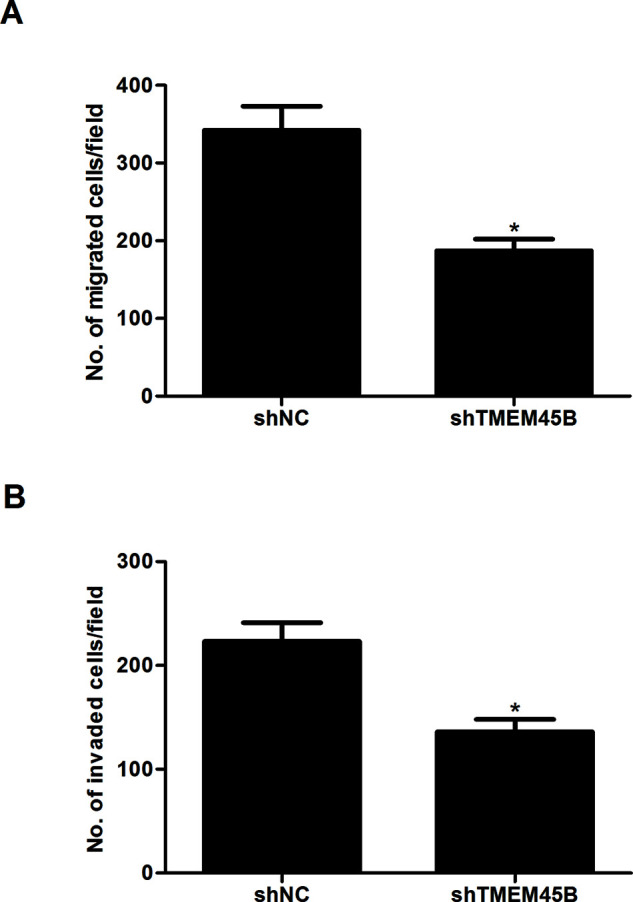
Knockdown of TMEM45B inhibited osteosarcoma cell migration and invasion in vitro. U2OS cells were transfected with shTMEM45B or shNC for 48 h, respectively. (A) Transwell migration assay was used to detect cell migration. (B) Matrigel invasion assay was used to detect cell invasion. *p < 0.05, compared with the shNC group.
Knockdown of TMEM45B Inhibited the Activation of the Wnt/β-Catenin Pathway in Osteosarcoma Cells
In order to explore the molecular mechanisms by which TMEM45B affects osteosarcoma growth and metastasis, we focused on the Wnt/β-catenin signaling pathway. Knockdown of TMEM45B sharply downregulated the expression level of β-catenin, cyclin D1, and c-Myc in U2OS cells, compared with the shNC group (Fig. 4).
Figure 4.
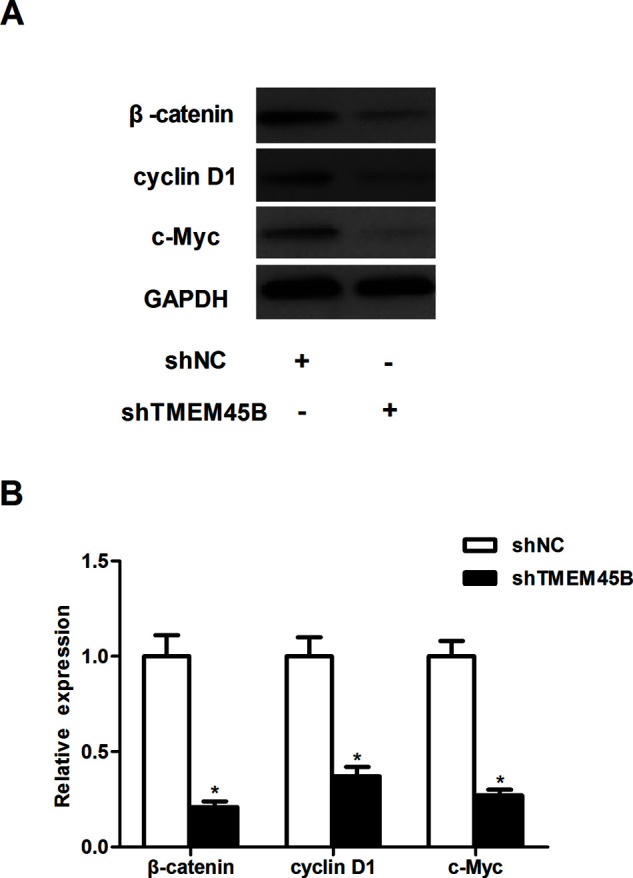
Knockdown of TMEM45B inhibited the activation of the Wnt/β-catenin pathway in osteosarcoma cells. (A) U2OS cells were transfected with shTMEM45B or shNC for 48 h, respectively. The expression of β-catenin, cyclin D1c, and c-Myc proteins was then analyzed by Western blotting. (B) Expression of β-catenin, cyclin D1, and c-Myc proteins was analyzed using Gel-Pro Analyzer version 4.0 software and normalized to GAPDH. *p < 0.05, compared with the shNC group.
Knockdown of TMEM45B Inhibited Tumor Growth In Vivo
Finally, we investigated the role of shTMEM45B in osteosarcoma in vivo using a xenograft model of osteosarcoma. We observed that the mean subcutaneous tumor size was lower in the shTMEM45B-treated group than in the shNC group over time (Fig. 5A). In addition, the mean tumor weight of the shTMEM45B-treated group was prominently reduced compared to the control groups (Fig. 5B).
Figure 5.
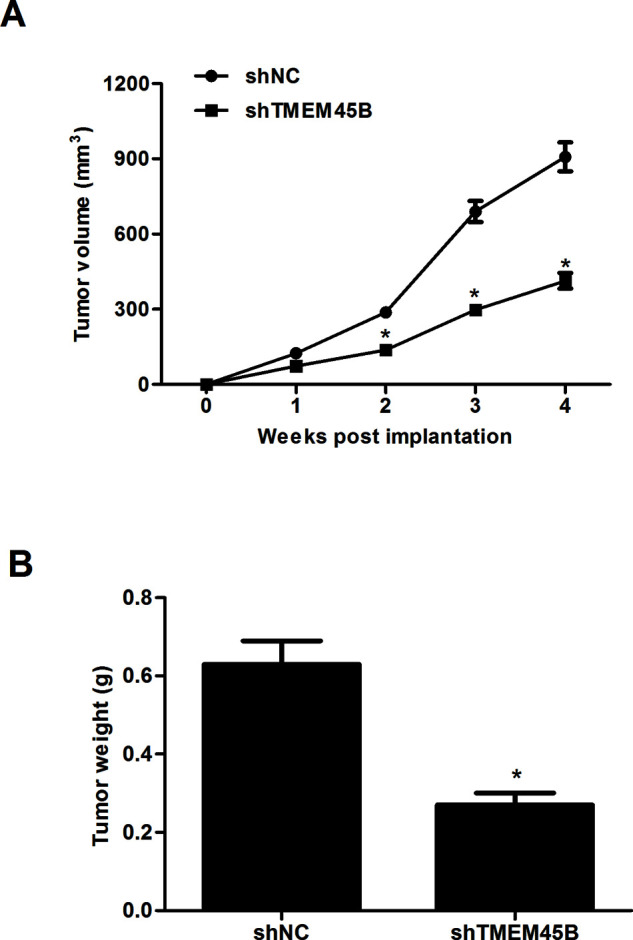
Knockdown of TMEM45B inhibited tumor growth in vivo. Infected U2OS cells (1 × 106) were injected subcutaneously into 4-week-old female nude mice. (A) Tumor growth was monitored with calipers once a week for a total of 4 weeks. (B) Tumors were removed and weighed after 20 days. *p < 0.05, compared with the shNC group.
DISCUSSION
Herein we present the first study on the expression and role of TMEM45B in osteosarcoma. Our results demonstrated that the expression of TMEM45B at both the protein and mRNA levels was dramatically upregulated in human osteosarcoma cell lines. Knockdown of TMEM45B significantly suppressed the proliferation, migration, and invasion of U2OS cells in vitro. Mechanistically, knockdown of TMEM45B sharply downregulated the expression level of β-catenin, cyclin D1, and c-Myc in U2OS cells. Finally, knockdown of TMEM45B attenuated tumor growth in transplanted U2OS-derived tumors in nude mice.
Previous studies have shown that TMEM45B plays an important role in cancer development and progression. TMEM45B was found to be overexpressed in lung cancer, and silencing of TMEM45B expression obviously inhibited lung cancer cell proliferation in vitro and in vivo11. In line with the results of previous studies, we observed that the expression of TMEM45B at both the protein and mRNA levels was dramatically upregulated in human osteosarcoma cell lines. Moreover, the in vitro and in vivo assays showed that knockdown of TMEM16A significantly suppressed the proliferation of U2OS cells in vitro, as well as attenuated tumor growth of transplanted U2OS-derived tumors in nude mice. These data imply that TMEM45B may be a novel oncogene in osteosarcoma.
Metastasis has been recognized as the main cause of fatal outcomes in osteosarcoma patients12. Once patients suffer metastasis, the 5-year survival rate drops to 17%13. In the current study, we showed that suppressed expression of TMEM45B significantly suppressed the migration and invasion of U2OS cells in vitro. These data imply that TMEM45B plays a critical role in the metastasis in osteosarcoma.
An accumulating body of evidence indicates that activation of the Wnt/β-catenin signaling pathway contributes to tumorigenesis and tumor progression14–16. Recently, it was reported that aberrant activation of the Wnt/β-catenin pathway is found in osteosarcoma and was associated with osteosarcoma progression17. β-Catenin is a crucial signaling molecule in the Wnt/β-catenin pathway18. In the nucleus, β-catenin interacts with T-cell factor/lymphocyte enhancer factor (TCF/LEF) and triggers downstream target genes, including c-Myc, cyclin D1, and matrix metalloproteinases (MMPs), which control cell proliferation, cell cycle, differentiation, metastasis, and apoptosis19,20. Thus, inhibition of the Wnt/β-catenin signaling may be a potential strategy for the treatment of osteosarcoma21–23. For example, Brun et al. confirmed that silencing four and half LIM protein 2 inhibited osteosarcoma cell proliferation, invasion, and migration in vitro by regulating the Wnt/β-catenin signaling pathway24. We observed that knockdown of TMEM45B sharply downregulated the expression level of β-catenin, cyclin D1, and c-Myc in U2OS cells. These results suggest that knockdown of TMEM45B suppressed the growth and metastasis of osteosarcoma, possibly by regulating the Wnt/β-catenin signaling pathway.
In summary, our results demonstrate that TMEM45B plays an important role in regulating the proliferation, migration, and invasion of osteosarcoma cells and that its effects on proliferation and invasion are mediated partially through the Wnt/β-catenin signaling pathway. These observations support our belief that TMEM45B may serve as an oncogene in the development and progression of osteosarcoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Chennupati SK, Norris R, Dunham B, Kazahaya K. Osteosarcoma of the skull base: Case report and review of literature. Int J Pediatr Otorhi. 2008;72:115–9. [DOI] [PubMed] [Google Scholar]
- 2. Hang TT, Dass CR, Choong PFM. Osteosarcoma treatment: State of the art. Cancer Metastasis Rev. 2009;28:247–63. [DOI] [PubMed] [Google Scholar]
- 3. Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–18. [PubMed] [Google Scholar]
- 4. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–8. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288:13305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Picollo A, Malvezzi M, Accardi A. TMEM16 proteins: Unknown structure and confusing functions. J Mol Biol. 2015;427:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scudieri P, Sondo E, Caci E, Ravazzolo R, Galietta LJ. TMEM16A-TMEM16B chimaeras to investigate the structure-function relationship of calcium-activated chloride channels. Biochem J. 2013;452:443–55. [DOI] [PubMed] [Google Scholar]
- 9. Hu R, Hu F, Xie X, Wang L, Li G, Qiao T, Wang M, Xiao H. TMEM45B, up-regulated in human lung cancer, enhances tumorigenicity of lung cancer cells. Tumour Biol. 2016;1–11. [DOI] [PubMed] [Google Scholar]
- 10. Zhao LC, Shen BY, Deng XX, Chen H, Zhu ZG, Peng CH. TMEM45B promotes proliferation, invasion and migration and inhibits apoptosis in pancreatic cancer cells. Mol Biosyst. 2016;12:1860–70. [DOI] [PubMed] [Google Scholar]
- 11. Rui H, Hu F, Xiao X, Lei W, Li G, Tong Q, Wang M, Xiao H. TMEM45B, up-regulated in human lung cancer, enhances tumorigenicity of lung cancer cells. Tumour Biol. 2016;1–11. [DOI] [PubMed] [Google Scholar]
- 12. Ren L, Khanna C. Role of ezrin in osteosarcoma metastasis. Adv Exp Med Biol. 2014;804:181–201. [DOI] [PubMed] [Google Scholar]
- 13. Kimura H, Tsuchiya H, Shirai T, Nishida H, Hayashi K, Takeuchi A, Ohnari I, Tomita K. Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J Orthop Sci. 2009;14:556–65. [DOI] [PubMed] [Google Scholar]
- 14. Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cletonjansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33. [DOI] [PubMed] [Google Scholar]
- 15. Zhang F, Chen A, Chen J, Yu T, Guo F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac J Cancer Prev. 2011;12:239–45. [PubMed] [Google Scholar]
- 16. Tian J, He H, Lei G. Wnt/β-catenin pathway in bone cancers. Tumor Biol. 2014;35:9439–45. [DOI] [PubMed] [Google Scholar]
- 17. Lin CH, Ji T, Chen CF, Bang HH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. 2014;804:33–45. [DOI] [PubMed] [Google Scholar]
- 18. Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. [DOI] [PubMed] [Google Scholar]
- 19. Moon RT. Wnt/β-catenin pathway. Sci STKE; 2005. [DOI] [PubMed] [Google Scholar]
- 20. SD, Doeberitz MVK. Wnt/β-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer 2005;113:515–24. [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, Gitelis S, O’Keefe RJ, Konttinen YT, Yin G. Inhibition of the Wnt-β-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun. 2013;431:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang RX, Li Y, Tian DD, Liu Y, Nian W, Zou X, Chen QZ, Zhou LY, Deng ZL, He BC. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int J Oncol. 2016;49:1973–82. [DOI] [PubMed] [Google Scholar]
- 23. Zhao H, Hou W, Tao J, Zhao Y, Wan G, Ma C, Xu H. Upregulation of lncRNA HNF1A-AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/β-catenin signaling pathway. Am J Transl Res. 2016;8:3503–12. [PMC free article] [PubMed] [Google Scholar]
- 24. Brun J, Dieudonné FX, Marty C, Müller J, Schüle R, Patiño-García A, Lecanda F, Fromigué O, Marie PJ. FHL2 silencing reduces Wnt signaling and osteosarcoma tumorigenesis in vitro and in vivo. PLoS One 2013;8:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


