Abstract
Capecitabine has consistently demonstrated high efficacy and acceptable tolerability in salvage chemotherapy for advanced breast cancer. However, there remains no consensus on its role in adjuvant chemotherapy for early breast cancer (EBC). To estimate the value of capecitabine-based combination adjuvant treatment in EBC, eight randomized controlled trials with 14,072 participants were analyzed. The efficacy and safety outcomes included disease-free survival (DFS), overall survival (OS), relapse, breast cancer-specific survival (BCSS), and grades 3–5 adverse events. Capecitabine-based combination adjuvant chemotherapy demonstrated a 16% increase in BCSS (HR = 0.84, 95% CI = 0.71–0.98, p = 0.03) in the overall analysis and a 22% improvement in DFS (HR = 0.78, 95% CI = 0.64–0.96, p = 0.02) in the hormone receptor-negative (HR−) subgroup. However, there were no significant differences in DFS (HR = 0.96, 95% CI = 0.89–1.05, p = 0.38), OS (HR = 0.91, 95% CI = 0.82–1.00, p = 0.06), or relapse between capecitabine-based and capecitabine-free combination adjuvant chemotherapy. Analogous results were observed in the subgroup analyses of HR+, HER2−, HER2+, and triple-negative EBC. Regarding safety, reduced myelosuppression and hand–foot syndrome development were observed in capecitabine-treated patients. Capecitabine-based combination adjuvant chemotherapy might provide some BCSS benefit compared with capecitabine-free regimens in EBC, but the absolute survival gain is small, and the survival benefit appears to be restricted to patients with HR− EBC, which may indicate a target population for capecitabine-based combination adjuvant chemotherapy.
Key words: Early breast cancer (EBC), Capecitabine, Adjuvant chemotherapy, Meta-analysis
INTRODUCTION
Despite enormous improvements in therapeutics over the past few decades, breast cancer (BC) still accounts for approximately a half million deaths annually worldwide1. Survival outcomes of BC patients have substantially improved as a result of the development of systemic adjuvant chemotherapy. Recurrence and mortality rates have decreased 23% and 17%, respectively, because of the successful application of adjuvant chemotherapy2,3. In recent years, many prospective clinical trials have been undertaken to evaluate newer drugs or combination therapeutic regimens in the adjuvant setting to achieve further survival benefits and overcome therapeutic challenges, such as multidrug resistance and severe chemotherapy toxicity.
Capecitabine is an oral, tumor-selective, fluoropyrimidine prodrug that has consistently demonstrated high efficacy and good tolerability in salvage treatment for anthracycline-/taxane-pretreated advanced BC in both single and combined schedules4–9. The drug displays a favorable myelotoxicity profile and can be conveniently used as an oral agent, in contrast to conventional cytotoxic agents. Many prospective studies have demonstrated that the integration of capecitabine into traditional chemotherapy regimens results in a synergistic effect and a manageable overlapping toxicity profile10–14. Combining different chemotherapeutic agents for synergistic activity is an effective means of enhancing the efficacy of chemotherapy. The rationale for incorporating capecitabine into classical chemotherapeutic regimens is related to thymidine phosphorylase (TP). TP is a key rate-limiting enzyme that functions in converting capecitabine to 5-FU. Accordingly, the antitumor activity of capecitabine would be enhanced by an increase in TP expression10,15. TP is upregulated by numerous cytotoxic drugs including paclitaxel, cyclophosphamide, and docetaxel. Thus, a supra-additive activity is exhibited when these drugs are used in combination with capecitabine10,16. In addition to these synergistic effects, the overlapping toxicity profile is limited and largely manageable because of the favorable myelotoxicity profile of capecitabine. The minimal myelosuppression observed might be the greatest advantage of the combination of capecitabine with myelotoxic agents (e.g., anthracyclines and taxanes). Based on these advantages and encouraging results from previous trials, adjuvant chemotherapy with capecitabine has already entered clinical practice in treating early breast cancer (EBC).
An earlier meta-analysis17 of two trials comparing adjuvant anthracycline-/taxane-based schedules with or without capecitabine indicated that incorporating capecitabine into the adjuvant setting improved disease-free survival (DFS), overall survival (OS), metastasis, and breast cancer-specific survival (BCSS) for EBC. This drug was also effective in human epidermal growth factor receptor 2-negative (HER2−), hormone receptor-negative (HR−), and triple-negative breast cancer (TNBC). However, conflicting conclusions were reached in several recent randomized controlled trials (RCTs) that estimated the clinical value of integrating capecitabine into adjuvant chemotherapy for EBC patients. For example, OS was significantly improved [hazard ratio (HR) = 0.68, 95% confidence interval (CI) = 0.51–0.92, p = 0.01] with capecitabine in the USON 01062 trial18, whereas the CEICAM/2003-10 trial19 reported a decreased DFS (HR = 1.3, 95% CI = 1.03–1.64, p = 0.025). FinXX20 and CBCSG-1021, which were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in 2016, showed encouraging results based on the enhancement of both DFS and OS in HR− and TNBC subgroups in the former and relapse-free survival (RFS) in TNBC patients in the latter. By contrast, other RCTs, including TACT222, ICE II-GBG 5223, GAIN24, and Zhang et al.25, have reported no significant differences in survival outcomes between capecitabine-based and capecitabine-free combination adjuvant chemotherapy.
No consensus has been reached on the role of capecitabine-based combination adjuvant chemotherapy in EBC. This topic was intensely debated at the annual meeting of ASCO in 2016 and has attracted considerable attention. Accordingly, we systematically analyzed the existing evidence on the clinical value of capecitabine-based combination adjuvant chemotherapy in EBC.
MATERIALS AND METHODS
Publication Search and Trial Selection
To identify potential articles, the Web of Science, Cochrane Library, PubMed, and annual conference proceedings, including the San Antonio Breast Cancer Symposium (SABCS) and ASCO, were searched from the earliest record to December 2016. The MeSH term “Breast Neoplasm” and the keywords “capecitabine or Xeloda” were used with no restriction as to publication year or language.
The selection criteria included the following: (a) patients with operable, nonmetastatic BC; (b) RCTs that compared capecitabine-based regimens with capecitabine-free regimens in a combination adjuvant chemotherapy setting; and (c) sufficient efficacy and safety data for analysis. Two authors searched and selected literature independently (G.L.C. and Z.Z.G.).
We excluded studies that were reviews, non-RCTs, trials that focused on single-agent capecitabine, or trials for neoadjuvant or salvage chemotherapy. Studies with insufficient survival data, even after an attempt to contact the corresponding authors, were also excluded.
Quality Assessment and Data Extraction
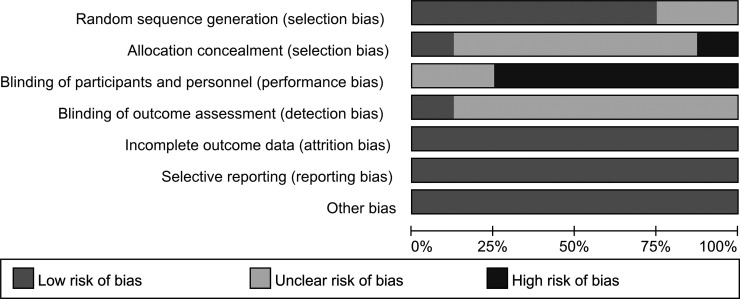
Two investigators (G.L.C. and Z.Z.G.) used the Cochrane’s risk of bias tool to evaluate the quality and potential bias of the eight studies separately. The risk of bias is summarized in a graph in Figure 1. These two authors extracted data independently from eligible trials. Discrepancies between the two investigators would be discussed by a third author (M.F.L.). Information including authors, year of publication, study period, study type and phase, randomization and allocation, baseline patient characteristics, adjuvant chemotherapy schedules, follow-up period, efficacy outcome results, and the occurrence of grades 3–5 adverse events (AEs) was extracted from the enrolled studies with internal consistency. The most recent and complete report was enrolled if duplicate publications were available for a trial. An attempt was made to contact the authors if important information could not be obtained from these articles.
Figure 1.
Risk of bias graph.
Statistical Analysis
The efficacy and safety outcomes included DFS, OS, relapse, BCSS, and the occurrence of grades 3–5 AEs. We performed subgroup analyses based on HR and HER2 status, study location, and median follow-up years to evaluate the potential causes of heterogeneity, assess the substantial contributions to survival outcomes, and determine the potential targeted patients who could benefit most from capecitabine-based adjuvant chemotherapy. We used intention-to-treat (ITT) analysis to evaluate data.
HRs and 95% CIs for DFS and OS were extracted from the enrolled studies, except for Zhang et al.’s trial25, for which these values were calculated based on Parmer et al.’s method26. The outcomes of relapse, BCSS, and AEs were evaluated based on relative risk (RR) and 95% CIs. All HRs and RRs were evaluated using time-to-events data and were pooled using the inverse-variance and Mantel–Haenszel method, respectively. A value of p < 0.05 or a 95% CI that did not include 1 was considered statistically significant.
The between-study heterogeneity was evaluated by both Cochrane’s Q statistic and the I 2 statistic: p < 0.10 and/or I 2 > 50% indicated high heterogeneity. A fixed- or random-effect model was selected according to the degree of heterogeneity. Cochrane Review Manager software 5.3 was used for all calculations.
RESULTS
Characteristics of Enrolled Trials
Based on predefined criteria, we identified 237 relevant articles through a search of databases and the Websites of two major annual conferences. Of the 237 articles, 173 records were excluded during the first screening of titles and abstracts, and 56 records were excluded because they were non-RCTs, trials that focused on single-agent capecitabine, trials for neoadjuvant or salvage chemotherapy, trials with insufficient efficacy and safety data for analysis, or reviews. After scrutiny, eight RCTs that included 14,072 patients who met the eligibility criteria were included. The search and selection process is summarized in the flow diagram (Fig. 2). Four trials with full text were published, whereas the other four trials were reported only as abstracts or posters at annual meetings; one trial25 enrolled only node-negative BC, another21 included TNBC only, and the six remaining trials enrolled patients with moderate- and/or high-risk nonmetastatic BC. One study23 enrolled only elderly patients (≥65). Of the 14,072 participants, 7,054 received capecitabine-based combination adjuvant chemotherapy regimens, and 7,018 received capecitabine-free combination adjuvant chemotherapy regimens. The range of median follow-up was 2 to 10.3 years. Table 1 presents the characteristics of the eligible studies.
Figure 2.
Flow diagram of the search and selection process for the trials.
Table 1.
Characteristics of the Included Studies
| Study | Year | Location | No. of Patients | Trial Phase | Capecitabine Based | Capecitabine Free | Capecitabine Schedule (mg/m2, Cycles) | Follow-Up (Median Years) |
|---|---|---|---|---|---|---|---|---|
| CBCSG-1021 | 2016 | China | 561 | III | TX-XEC | T-FEC | 1,000, 6 | 2.5 |
| FinXX20 | 2016 | Finland | 1,495 | III | TX-CEX | T-CEF | 900, 6 | 10.3 |
| GAIN24 | 2014 | Germany | 2,994 | III | EC-TX | ETC/idd-ETC | 1,000–1,250, 4 | 6.2 |
| GEICAM/2003-1019 | 2015 | Spain | 1,384 | III | ET-X | EC-T | 1,250, 4 | 6.6 |
| ICE II-GBC 5223 | 2015 | Germany | 391 | II | nPX/PX | EC/CMF | 1,000, 6 | 2 |
| TACT222 | 2014 | UK | 4,358 | III | E-X | E-CMF | 1,250, 4 | 5 |
| USON 0106218 | 2015 | USA | 2,611 | III | AC→TX | AC→T | 825, 4 | 5 |
| XH Zhang et al.25 | 2015 | China | 278 | II | AX | AC | 1,000, 4 | 4 |
T, docetaxel; X, Xeloda/capecitabine; E, epirubicin; F, fluorouracil; C, cyclophosphamide; ST, standard treatment; M, methotrexate; A, doxorubicin; idd, intense dose-dense; I, ibandronate; P, paclitaxel; nP, nab-paclitaxel.
Efficacy and Safety
Disease-Free Survival
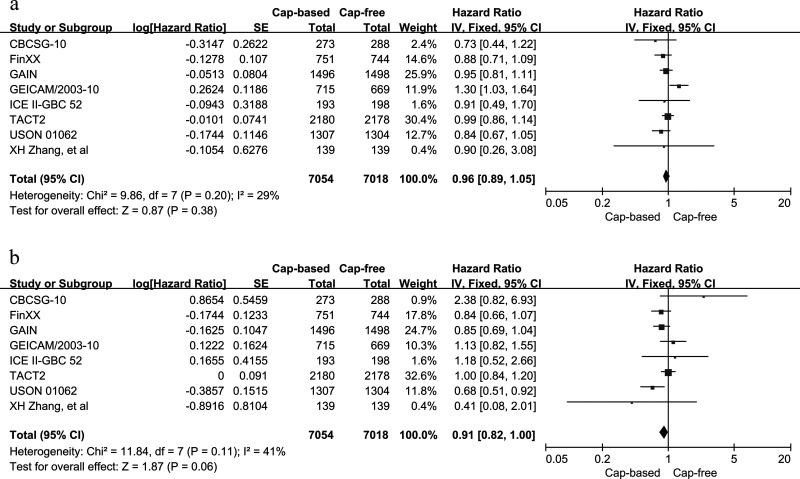
The HRs and 95% CIs for DFS were reported in eight RCTs that included 14,072 participants. The FinXX trial20 provided data on RFS instead of DFS. However, the definition of RFS in FinXX was the same as that of DFS in the other seven trials (i.e., the survival time without local recurrence and distant metastasis). Thus, we conducted a combined analysis of DFS and RFS from the FinXX trial for the overall analysis. A fixed-effects model was selected due to the lack of heterogeneity with respect to DFS for the capecitabine-based arm versus the capecitabine-free arm (p = 0.2, I 2 = 29%). The pooled HR was 0.96 with an associated 95% CI of 0.89–1.05 (p = 0.38), which corresponds to no improvement in DFS when comparing capecitabine-based and capecitabine-free combination adjuvant chemotherapy (Fig. 3). Publication bias was not assessed because, according to the guidelines in the Cochrane Handbook, the analysis contained fewer than 10 eligible trials.
Figure 3.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: (a) meta-analysis of disease-free survival (DFS) and (b) overall survival (OS).
Overall Survival
OS was assessed in eight RCTs with 14,072 participants using HRs and 95% CIs. No study-to-study heterogeneity was noted in OS (p = 0.11, I 2 = 41%) between the capecitabine-based and capecitabine-free arms. Thus, a fixed-effects model was selected. The overall analysis of capecitabine-based versus capecitabine-free group yielded a borderline significant result in OS (HR = 0.91, 95% CI = 0.82–1.00, p = 0.06) (Fig. 3).
Relapse
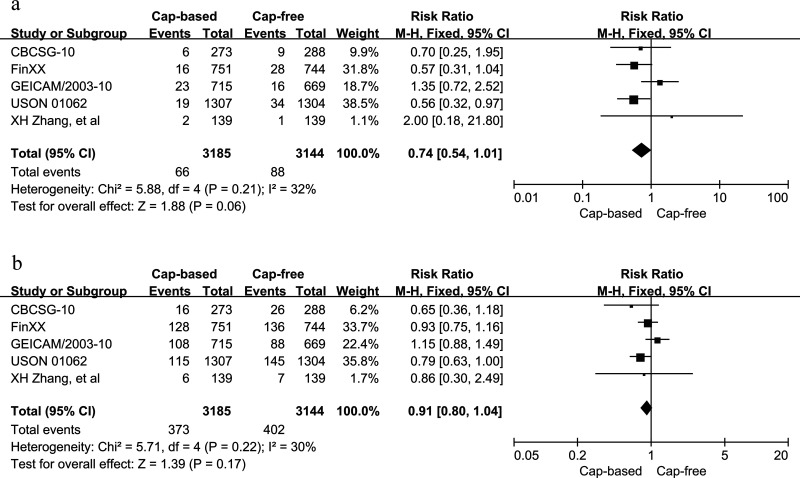
Data regarding tumor relapse were obtained from five studies that included 6,329 patients. No statistically significant heterogeneity was found among the trials in either local recurrence or distant metastasis between the capecitabine-based and capecitabine-free arms (p = 0.21, I 2 = 32%; p = 0.22, I 2 = 30%, respectively). Therefore, fixed-effects models were selected. The pooled HRs for local recurrence (HR = 0.74, 95% CI = 0.54–1.01, p = 0.06) and distant metastasis (HR = 0.91, 95% CI = 0.80–1.04, p = 0.17) did not show a significant advantage of the use of capecitabine (Fig. 4).
Figure 4.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: (a) meta-analysis of local recurrence and (b) distant metastasis.
Breast Cancer-Specific Survival
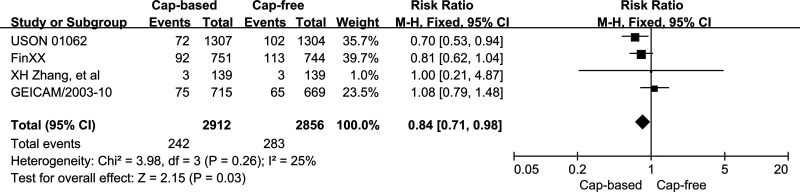
Four RCTs with 5,768 participants were analyzed for BCSS. A low level of heterogeneity was seen in BCSS between the capecitabine-based and capecitabine-free arms (p = 0.26, I 2 = 26%), and therefore, a fixed-effects model was selected. A statistically significant increase in BCSS of 16% with an associated 95% CI of 0.71–0.98 (p = 0.03) was found in the capecitabine-based versus the capecitabine-free groups (Fig. 5).
Figure 5.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: meta-analysis of breast cancer-specific survival.
Subgroups
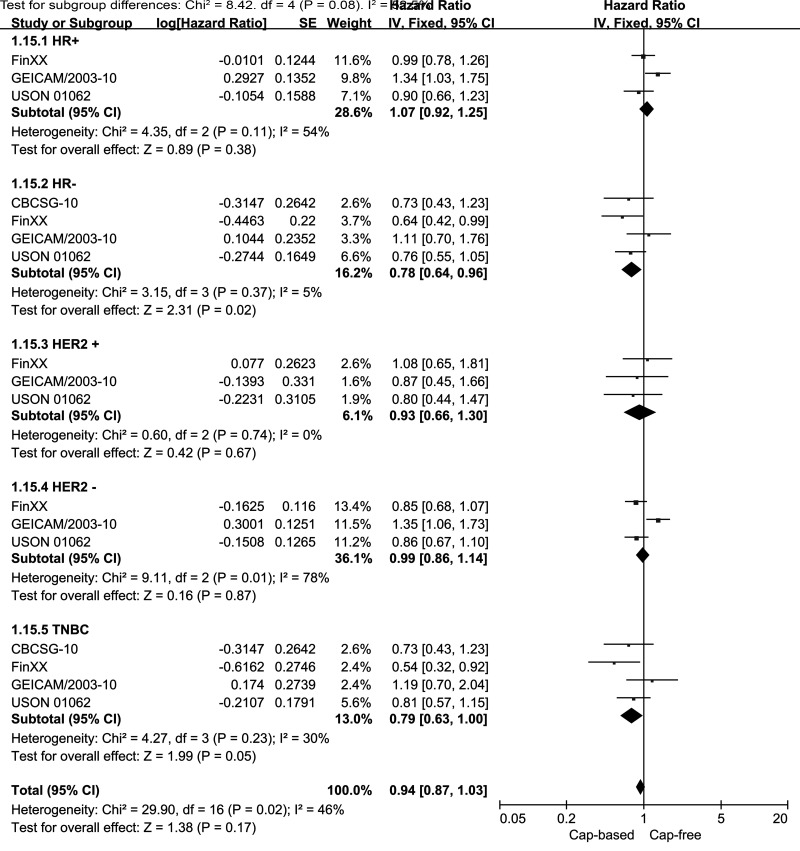
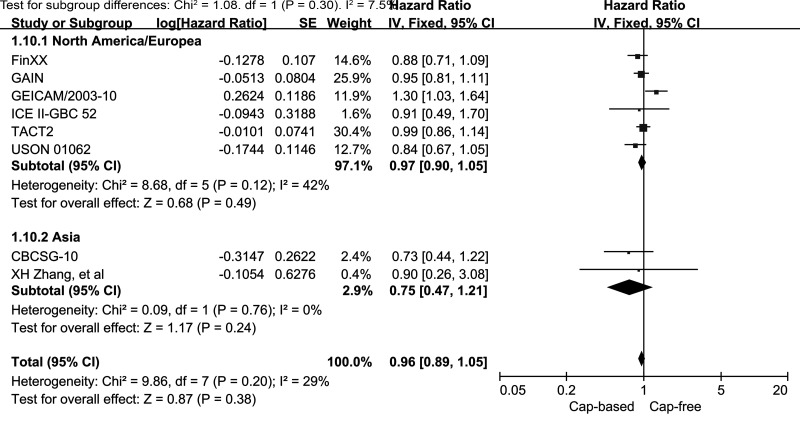
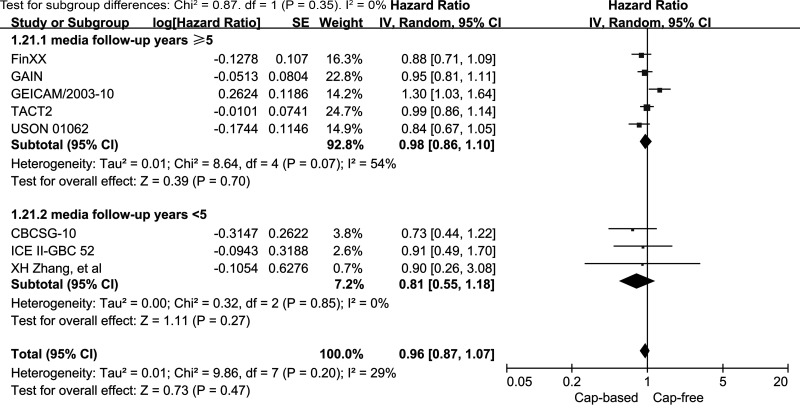
We performed subgroup analyses of DFS for capecitabine-based and capecitabine-free combination adjuvant chemotherapy according to HR and HER2 status (Fig. 6). The subgroup analysis of HR− EBC (including TNBC) indicated a significantly better DFS outcome in the capecitabine-based compared with the capecitabine-free groups (HR = 0.78, 95% CI = 0.64–0.96, p = 0.02; heterogeneity: p = 0.37, I 2 = 5%). By contrast, the analysis of the HR+ subgroup failed to reveal similar results (HR = 1.07, 95% CI = 0.92–1.25, p = 0.38; heterogeneity: p = 0.11, I 2 = 54%). No benefit was observed in the HER2+ or the HER2− subgroup for DFS in capecitabine-based compared with capecitabine-free combination adjuvant chemotherapy (HR = 0.93, 95% CI = 0.66–1.30, p = 0.67; HR = 0.99, 95% CI = 0.86–1.14, p = 0.87, respectively). In patients with TNBC, the pooled HR of DFS was 0.79 with an associated borderline 95% CI of 0.63–1.00 (p = 0.05; heterogeneity: p = 0.23, I 2 = 30%). This result indicated no significant improvement in DFS for TNBC patients who received capecitabine-based combination adjuvant chemotherapy. In addition, we also performed stratified analyses by study location and median years of follow-up. Neither the North America/Europe nor the Asia subgroup exhibited a significant improvement in DFS in the capecitabine-based versus capecitabine-free group (HR = 0.97, 95% CI = 0.9–1.09, p = 0.49, I 2 = 42%; HR = 0.75, 95% CI = 0.47–1.21, p = 0.24, I 2 = 0%, respectively) (Fig. 7). In addition, no benefit was seen in the subgroup with a median follow-up time of ≥5 years (HR = 0.98, 95% CI = 0.86–1.10, p = 0.7; heterogeneity: p = 0.07, I 2 = 54%) or in the subgroup with a median follow-up time of <5 years (HR = 0.81, 95% CI = 0.55–1.18, p = 0.27; heterogeneity: p = 0.85, I 2 = 0%) (Fig. 8).
Figure 6.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: meta-analysis of the subgroups based on hormone receptor (HR) and HER2 status.
Figure 7.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: meta-analysis of the subgroups based on study location.
Figure 8.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: meta-analysis of the subgroups based on median years of follow-up.
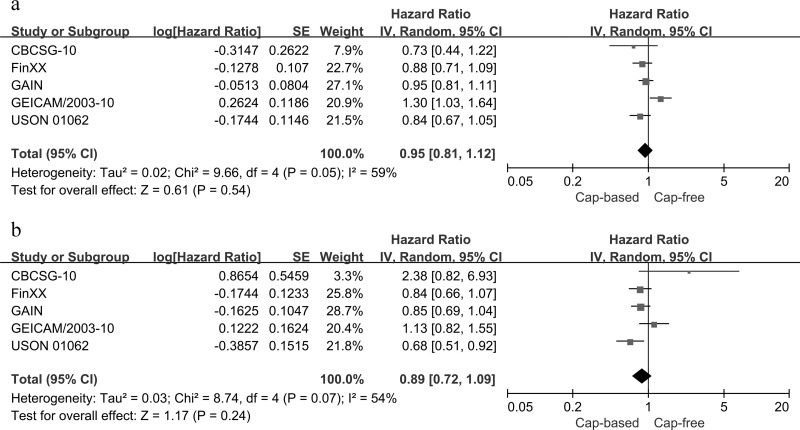
Anthracycline-/Taxane-Based Postoperative Chemotherapy in High-Risk EBC
The clinical value of adjuvant anthracycline-/taxane-based protocols with or without capecitabine was evaluated in a total of 9,045 participants with high-risk EBC in five RCTs. We analyzed DFS and OS in adjuvant anthracycline–/taxane–capecitabine regimens versus anthracycline/taxane regimens alone for high-risk EBC. No significant differences in either DFS (HR = 0.95, 95% CI = 0.81–1.12, p = 0.54; heterogeneity: p = 0.05, I 2 = 59%) or OS (HR = 0.89, 95% CI = 0.72–1.09, p = 0.24; heterogeneity: p = 0.07, I 2 = 54%) were observed between the two protocols using random-effect models for significant heterogeneity (Fig. 9).
Figure 9.
Capecitabine-based versus capecitabine-free combination adjuvant chemotherapy: (a) meta-analysis of DFS and (b) OS in an anthracycline-/taxane-based adjuvant setting.
Toxicity Analysis
Toxicity analysis was performed in seven RCTs with 9,675 patients. All AEs were grade ≥3 based on the NCI-CTC toxicity scale. The most commonly reported AEs included the following: hand–foot syndrome (HFS), anemia, neutropenia, thrombocytopenia, febrile neutropenia, diarrhea, vomiting, nausea, fatigue, mucositis, and myalgia. No significant heterogeneity was observed in the analyses of anemia, nausea, and vomiting (p > 0.1, I 2 < 50%) between the capecitabine-based and capecitabine-free arms. Therefore, fixed-effects models were used. However, significant heterogeneity (p < 0.1, I 2 > 50%) was found in the analyses of the other AEs, and thus random-effects models were selected. Neutropenia [odds ratio (OR) = 0.53, 95% CI = 0.31–0.91, p = 0.02], anemia (OR = 0.61, 95% CI = 0.43–0.85, p = 0.03), vomiting (OR = 0.74, 95% CI = 0.58–0.94, p = 0.01), and myalgia (OR = 0.42, 95% CI = 0.23–0.76, p = 0.004) were less frequent in the capecitabine-based versus the capecitabine-free group. HFS was reported in 672 of 4,839 (13.89%) patients in the capecitabine arm and in 80 of 4,836 (1.75%) patients in the control arm. More severe and frequent HFS occurred in patients who were treated with capecitabine (OR = 13.47, 95% CI = 6.96–26.07, p < 0.01; heterogeneity: p = 0.001, I 2 = 73%). In addition, more cases of diarrhea and mucositis arose in the capecitabine arm, and the ORs were 2.77 (95% CI = 1.64–4.67, p = 0.0001) and 2.24 (95% CI = 1.17–4.30, p = 0.02), respectively. The safety details of the treatments are presented in Table 2.
Table 2.
Outcomes of Grades 3–5 Drug-Related Adverse Events for Capecitabine-Based Versus Capecitabine-Free Combination Adjuvant Chemotherapy in Early Breast Cancer
| Grades 3–5 AEs | No. of Studies | Capecitabine Based n/N | Capecitabine Free n/N | OR [95% CI] | p |
|---|---|---|---|---|---|
| Hematologic | |||||
| Neutropenia | 7 | 1,977/4,839 | 2,460/4,836 | 0.53 (0.31–0.91) | 0.02 |
| Febrile neutropenia | 5 | 321/4,507 | 323/4,499 | 0.98 (0.54–1.79) | 0.94 |
| Anemia | 3 | 56/2,972 | 92/3,001 | 0.61 (0.43–0.85) | 0.03 |
| Thrombocytopenia | 4 | 44/2,706 | 11/2,725 | 3.39 (0.67–17.17) | 0.14 |
| Gastrointestinal | |||||
| Nausea | 5 | 111/2,632 | 117/2,671 | 0.96 (0.73–1.25) | 0.76 |
| Vomiting | 6 | 120/3,343 | 159/3,338 | 0.74 (0.58–0.94) | 0.01 |
| Diarrhea | 6 | 224/3,343 | 89/3,338 | 2.77 (1.64–4.67) | 0.0001 |
| Others | |||||
| HFS | 7 | 672/4,839 | 80/4,836 | 13.47 (6.96–26.07) | <0.001 |
| Fatigue | 5 | 311/3,204 | 309/3,199 | 1.05 (0.79–1.39) | 0.76 |
| Mucositis | 5 | 208/3,204 | 115/3,199 | 2.24 (1.17–4.30) | 0.02 |
| Myalgia | 5 | 72/3,204 | 181/3,199 | 0.42 (0.23–0.76) | 0.004 |
AEs, adverse events; HFS, hand–foot syndrome.
DISCUSSION
The role of capecitabine-based combination chemotherapy in an adjuvant setting has long been discussed. However, clinical trials focused on adjuvant capecitabine have reached conflicting conclusions. To systematically analyze the clinical value of capecitabine-based combination adjuvant chemotherapy for EBC, we conducted this study to compare the efficacy and toxicity of capecitabine-based versus capecitabine-free combination adjuvant chemotherapy; neither neoadjuvant chemotherapy nor salvage chemotherapy was included. Our meta-analysis indicates that capecitabine-based combination adjuvant chemotherapy might provide some BCSS benefit in EBC compared with capecitabine-free regimens, but the absolute survival gain is small, because no improvements in DFS, OS, or relapse were observed. Analogous results were obtained in subgroup analyses based on HR and HER2 status, study location, median follow-up years, and anthracycline-/taxane-based settings, with the exception of the HR− subgroup. The survival benefit of capecitabine-based combination adjuvant chemotherapy appeared to be restricted to patients with HR− EBC based on prolonged DFS. The toxicity profiles showed less-frequent grades 3–5 neutropenia, anemia, vomiting, and myalgia; however, grades 3–5 HFS, diarrhea, and mucositis occurred more frequently with the use of capecitabine.
HR− EBC accounts for more than 30% of the cases of disease27. Although endocrine therapy has produced noticeable survival benefits for patients with HR+ BC, it is not effective for HR− disease. Accordingly, more effective, targeted therapies are needed for HR− patients. Our subgroup analyses indicated a 22% increase in DFS in patients with HR− EBC upon the application of capecitabine-based combination adjuvant chemotherapy. This finding may indicate a target population for capecitabine-based combination adjuvant chemotherapy. Additionally, subgroup analysis of TNBC yielded a borderline statistically significant result. TNBC is accompanied by an inferior prognosis compared with other subtypes of BC due to unfavorable histopathological features. More importantly, no standard treatment strategy is currently available for TNBC; thus, novel and effective treatment strategies are also greatly needed28,29. The clinical value of capecitabine-based combination adjuvant chemotherapy in TNBC has not been adequately discussed, although previous studies and the borderline results of this study suggest that this therapy might be advantageous for TNBC12,30–32. Prolonged DFS was observed in the TNBC subgroup of the FinXX trial and the USON 01062 trial. In the CBCSG-10 trial, which recruited TNBC patients only, RFS was significantly enhanced in the capecitabine arm. More RCTs with larger sample sizes are required to corroborate the clinical value of capecitabine-based combination adjuvant chemotherapy in TNBC.
Although capecitabine-based regimens were superior to capecitabine-free regimens in terms of BCSS, only four RCTs included data regarding BCSS, with a total number of patients fewer than 3,000. Three other trials were reported as abstracts or posters, and one other trial with full text did not report BCSS data; thus, we failed to obtain additional data on BCSS. More trials are needed before a definitive conclusion can be reached regarding the impact of adjuvant capecitabine on BCSS. In addition to BCSS, all other endpoints in the overall analysis were negative, in marked contrast to the previous meta-analysis by Jiang et al.17. Their meta-analysis, which included two trials with 4,017 BC patients, was conducted to compare the efficacy of adjuvant anthracycline-/taxane-based schedules with or without capecitabine. A survival benefit of capecitabine-based adjuvant chemotherapy was found based on improvements in DFS, OS, metastasis, and BCSS in the overall analysis and subgroup analyses of TNBC, HR−, and HER2− EBC. In our meta-analysis, which included eight RCTs with a total of 14,072 patients, both anthracycline-/taxane-based and non-anthracycline-/taxane-based regimens were analyzed. Moreover, we did not restrict our analysis to high-risk EBC. The discordant results of these two meta-analyses might be attributable to differences in sample sizes, regimens combined with capecitabine, and types of patients. Thus, we performed a further analysis of five RCTs with 9,045 patients to compare DFS and OS for adjuvant anthracycline–/taxane–capecitabine with anthracycline/taxane schedules in patients with high-risk EBC to match the conditions of Jiang et al.’s meta-analysis. However, neither DFS nor OS was significantly improved under these conditions. The small sample size in Jiang et al.’s meta-analysis may be the most important reason for its limited conclusions. The present meta-analysis, which included more trials and patients with a longer follow-up duration, might therefore provide more conclusive findings.
Three ongoing trials are currently investigating this issue. EA113133 (NCT02445391), MINDACT34 (NCT00433589), and (NCT01354522)35 aim to assess the clinical value of capecitabine-based and capecitabine-free regimens as combination adjuvant chemotherapy regimens for EBC. These trials might provide a more definitive result in the future.
Compared with the capecitabine-free group, the toxicity profile in the capecitabine-based group, with minimal myelosuppression, was much more easily managed. We observed a significantly lower incidence of grades 3–5 anemia and neutropenia in patients who were treated with combined regimens that included capecitabine. Although HFS and diarrhea were more frequent in the capecitabine-based group, these events are reversible, non-life-threatening, and easier to manage than bone marrow toxicity.
Our meta-analysis has several limitations. First, we used data extracted from study publications rather than individual patient data, which might affect the reliability of the results. Second, four trials enrolled patients with moderate- or high-risk EBC, hindering a robust estimation of the overall population. Finally, four RCTs were reported as abstracts or posters only, complicating data extraction and making quality assessment difficult.
In conclusion, compared with capecitabine-free regimens, capecitabine-based combination adjuvant chemotherapy might provide some BCSS benefit in EBC. However, the absolute survival gain is small, as no improvement was observed in DFS, OS, or relapse. The survival benefit of capecitabine-based combination adjuvant chemotherapy appears to be restricted to patients with HR− EBC, which may indicate a target population for capecitabine-based adjuvant chemotherapy.
ACKNOWLEDGMENT
This work was supported by the National Foundation for Science and Technology Development (Grant No. 2013BAI05B05).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. [DOI] [PubMed] [Google Scholar]
- 2. Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365(9472):1687–717. [DOI] [PubMed] [Google Scholar]
- 4. Smorenburg CH, de Groot SM, van Leeuwen-Stok AE, Hamaker ME, Wymenga AN, de Graaf H, de Jongh FE, Braun JJ, Los M, Maartense E, van Tinteren H, Nortier JW, Seynaeve C. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: Results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann Oncol. 2014;25(3):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin W, Pei G, Liu G, Huang L, Gao S, Feng X. Efficacy and safety of capecitabine-based first-line chemotherapy in advanced or metastatic breast cancer: A meta-analysis of randomised controlled trials. Oncotarget 2015;6(36):39365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, Cortes J, Kiger C, Germa C, Wang K, Martin M, Baselga J, Kim SB. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–33. [DOI] [PubMed] [Google Scholar]
- 7. Miles D, Zielinski C, Martin M, Vrdoljak E, Robert N. Combining capecitabine and bevacizumab in metastatic breast cancer: A comprehensive review. Eur J Cancer 2012;48(4):482–91. [DOI] [PubMed] [Google Scholar]
- 8. Fornier MN. Approved agents for metastatic breast cancer. Semin Oncol. 2011;38(Suppl 2):S3–10. [DOI] [PubMed] [Google Scholar]
- 9. Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS, Nowak AK, Gainford MC, Fong A, Paksec L, Sourjina T, Zannino D, Gebski V, Simes RJ, Forbes JF, Coates AS. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498–504. [DOI] [PubMed] [Google Scholar]
- 10. Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4(4):1013–9. [PubMed] [Google Scholar]
- 11. Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, Chung KW, Kang HS, Kim EA, Kim SW, Shin KH, Kim SK. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109(3):481–9. [DOI] [PubMed] [Google Scholar]
- 12. Steger GG, Greil R, Lang A, Rudas M, Fitzal F, Mlineritsch B, Hartmann BL, Bartsch R, Melbinger E, Hubalek M, Stoeger H, Dubsky P, Ressler S, Petzer AL, Singer CF, Muss C, Jakesz R, Gampenrieder SP, Zielinski CC, Fesl C, Gnant M, ABCSG. Epirubicin and docetaxel with or without capecitabine as neoadjuvant treatment for early breast cancer: Final results of a randomized phase III study (ABCSG-24). Ann Oncol. 2014;25(2):366–71. [DOI] [PubMed] [Google Scholar]
- 13. Lam SW, de Groot SM, Honkoop AH, Jager A, Ten TA, Bos MM, Linn SC, van den Bosch J, Kroep JR, Braun JJ, van Tinteren H, Boven E, Dutch Breast Cancer Research Group. Paclitaxel and bevacizumab with or without capecitabine as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: A multicentre, open-label, randomised phase 2 trial. Eur J Cancer 2014;50(18):3077–88. [DOI] [PubMed] [Google Scholar]
- 14. von Minckwitz G, Rezai M, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Blohmer JU, Costa SD, Jackisch C, Paepke S, Schneeweiss A, Kümmel S, Denkert C, Mehta K, Loibl S, Untch M. Survival after adding capecitabine and trastuzumab to neoadjuvant anthracycline-taxane-based chemotherapy for primary breast cancer (GBG 40--GeparQuattro). Ann Oncol. 2014;25(1):81–9. [DOI] [PubMed] [Google Scholar]
- 15. Terranova-Barberio M, Roca MS, Zotti AI, Leone A, Bruzzese F, Vitagliano C, Scogliamiglio G, Russo D, D’Angelo G, Franco R, Budillon A, Di Gennaro E. Valproic acid potentiates the anticancer activity of capecitabine in vitro and in vivo in breast cancer models via induction of thymidine phosphorylase expression. Oncotarget 2016;7(7):7715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toi M, Atiqur RM, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005;6(3):158–66. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Yin W, Zhou L, Yan T, Zhou Q, Du Y, Shen Z, Shao Z, Lu J. First efficacy results of capecitabine with anthracycline- and taxane-based adjuvant therapy in high-risk early breast cancer: A meta-analysis. PLoS One 2012;7(3):e32474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Shaughnessy J, Koeppen H, Xiao Y, Lackner MR, Paul D, Stokoe C, Pippen J, Krekow L, Holmes FA, Vukelja S, Lindquist D, Sedlacek S, Rivera R, Brooks R, McIntyre K, Brownstein C, Hoersch S, Blum JL, Jones S. Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clin Cancer Res. 2015;21(19):4305–11. [DOI] [PubMed] [Google Scholar]
- 19. Martin M, Ruiz SA, Ruiz BM, Ribelles N, Rodriguez-Lescure A, Munoz-Mateu M, Gonzalez S, Margeli VM, Barnadas A, Ramos M, Del Barco Berron S, Jara C, Calvo L, Martínez-Jáñez N, Mendiola Fernández C, Rodríguez CA, Martínez de Dueñas E, Andrés R, Plazaola A, de la Haba-Rodríguez J, López-Vega JM, Adrover E, Ballesteros AI, Santaballa A, Sánchez-Rovira P, Baena-Cañada JM, Casas M, del Carmen Cámara M, Carrasco EM, Lluch A. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: Results from the GEICAM/2003-10 Study. J Clin Oncol. 2015;33(32):3788–95. [DOI] [PubMed] [Google Scholar]
- 20. Joensuu H, Huovinen PKR. Adjuvant capesitabine in combination with docetaxel (T) epirubicin (E), and cyclophosphamide (C) in the treatment of early breast cancer (BC): 10-Year survival results from the randomized FinXX trial (2005-2016). 2016. ASCO Annual Meeting|Abstracts|Meeting Library. Finnish Breast Cancer Group; [cited 2016 Sep 10] Available from http://meetinglibrary.asco.org/content/168306-176.html [Google Scholar]
- 21. Zhimin Shao JLDP. Cbcsg-10: Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for triple negative breast cancer (2012-2016). 2016. ASCO Annual Meeting|Virtual Meeting|Meeting Library. China Breast Cancer Clinical Study Group; [cited 2016 Aug 9] Available from http://meetinglibrary.asco.org/content/123895?media=vm.html [Google Scholar]
- 22. Canney P, Barrett-Lee P, Bartlett J, Bertelli G, Coleman R, Earl H, Ellis P, Morden J, Murray N, Stein R, Cameron D, Bliss J. The UK TACT2 Trial: Non-inferiority of capecitabine compared with CMF after epirubicin in patients requiring chemotherapy for early breast cancer (EBC) (CRUK/05/019). Eur J Cancer 2014;50:S99–S100. [Google Scholar]
- 23. von Minckwitz G, Conrad B, Reimer T, Decker T, Eidtmann H, Eiermann W, Hackmann J, Mobus V, Marme F, Potenberg J, Stickeler E, Simon E, Thomssen C, Huober J, Denkert C, Alfer J, Jackisch C, Nekljudova V, Burchardi N, Loibl S. A randomized phase 2 study comparing EC or CMF versus nab-paclitaxel plus capecitabine as adjuvant chemotherapy for nonfrail elderly patients with moderate to high-risk early breast cancer (ICE II-GBG 52). Cancer 2015;121(20):3639–48. [DOI] [PubMed] [Google Scholar]
- 24. Moebus VJ, Von Minckwitz G, Jackisch C, Lueck HJ, Schneeweiss A, Tesch H, Elling D, Harbeck N, Conrad B, Fehm T, Huober JB, Muller V, Bauerreind I, Schmidt M, Loibl S, Nekljudova V, Untch M, Thomssen C. German adjuvant intergroup node positive (GAIN) study: A phase III trial to compare IDD-ETC versus EC-TX in patients with node-positive primary breast cancer—Final efficacy analysis. J Clin Oncol. 2014;32S(15):1009. [Google Scholar]
- 25. Zhang X, Zhou Y, Mao F, Lin Y, Guan J, Sun Q. Efficacy and safety of pirarubicin plus capecitabine versus pirarubicin plus cyclophosphamide in Chinese node-negative breast cancer patients: A 4-year open-label, randomized, controlled study. Med Oncol. 2015;32(10):240. [DOI] [PubMed] [Google Scholar]
- 26. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 27. Cazzaniga M, Bonanni B. Prevention of ER-negative breast cancer: Where do we stand? Eur J Cancer Prev. 2012;21(2):171–81. [DOI] [PubMed] [Google Scholar]
- 28. Engebraaten O, Vollan HK, Borresen-Dale AL. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol. 2013;183(4):1064–74. [DOI] [PubMed] [Google Scholar]
- 29. Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, Sinha N. Therapeutic targets of triple-negative breast cancer: A review. Br J Pharmacol. 2015;172(17):4228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrero JM, Hardy-Bessard AC, Capitain O, Lortholary A, Salles B, Follana P, Herve R, Deblock M, Dauba J, Atlassi M, Largillier R. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: Results from the GINECO A-TaXel phase 2 study. Cancer 2016. (1097-0142 (Electronic); - 0008-543X (Linking)). [DOI] [PubMed] [Google Scholar]
- 31. Masuda N, Higaki K, Takano T, Matsunami N, Morimoto T, Ohtani S, Mizutani M, Miyamoto T, Kuroi K, Ohno S, Mizutani M, Miyamoto T, Kuroi K, Ohno S, Morita S, Toi M. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer. Cancer Chemother Pharmacol. 2014;74(2):229–38. [DOI] [PubMed] [Google Scholar]
- 32. Kotsori AA, Dolly S, Sheri A, Parton M, Shaunak N, Ashley S, Walsh G, Johnston S, Smith IE. Is capecitabine efficacious in triple negative metastatic breast cancer? Oncology 2010;79(5-6):331–6. [DOI] [PubMed] [Google Scholar]
- 33. Ingrid Mayer E-ACRG. Platinum based chemotherapy or capecitabine in treating patients with residual triple-negative basal-like breast cancer following neoadjuvant chemotherapy (2015-2016). ECOG-ACRIN Cancer Research Group; [cited 2016 Mar 12] Available from https://www.clinicaltrials.gov/show/NCT02445391.html [Google Scholar]
- 34. Emiel Rutgers MP-G, Fatima Cardoso, EORTC. Genetic testing or clinical assessment in determining the need for chemotherapy in women with breast cancer that involves no more than 3 lymph nodes (MINDACT) (2007-2016). European Organisation for Research and Treatment of Cancer-EORTC; [cited 2016 Dec 12] Available from https://www.clinicaltrials.gov/show/NCT00433589.html [Google Scholar]
- 35. Sun Q. TAC versus TCX as adjuvant treatment for node-positive Her2-negative breast cancer (2011-2016). Peking Union Medical College Hospital; [cited 2016 Dec 12] Available from https://www.clinicaltrials.gov/show/NCT01354522.html [Google Scholar]