Abstract
miRNAs play a key role in the carcinogenesis of many cancers, including bladder cancer. In the current study, the role of miR-5195-3p, a quite recently discovered and poorly studied miRNA, in the proliferation and invasion of human bladder cancer cells was investigated. Our data displayed that, compared with healthy volunteers (control) and SU-HUC-1 normal human bladder epithelial cells, miR-5195-3p was sharply downregulated in bladder cancer patients and five human bladder cancer cell lines. The oligo miR-5195-3p mimic or miR-5195-3p antagomir was subsequently transfected into both T24 and BIU-87 bladder cancer cell lines. The miR-5195-3p mimic robustly increased the miR-5195-3p expression level and distinctly reduced the proliferation and invasion of T24 and BIU-87 cells. In contrast, the miR-5195-3p antagomir had an opposite effect on miR-5195-3p expression, cell proliferation, and invasion. Our data from bioinformatic and luciferase reporter gene assays identified that miR-5195-3p targeted the mRNA 3′-UTR of Krüppel-like factor 5 (KLF5), which is a proven proto-oncogene in bladder cancer. miR-5195-3p sharply reduced KLF5 expression and suppressed the expression or activation of its several downstream genes that are kinases improving cell survival or promoting cell cycle regulators, including ERK1/2, VEGFA, and cyclin D1. In conclusion, miR-5195-3p suppressed proliferation and invasion of human bladder cancer cells via suppression of KLF5.
Key words: Bladder cancer, miR-5195-3p, Cell proliferation, Cell invasion, Krüppel-like factor 5 (KLF5)
INTRODUCTION
Bladder cancer is one of the most common cancers among malignant tumors of the urinary tract, of which the incidence in males is three to four times higher than that in females1. The causes of bladder cancer are varied: smoking history, genetic variables, occupational hazards, and many other unexplained factors. Early clinical manifestation may not be obvious, making early diagnosis difficult1,2. Nearly three fourths of patients experienced a recurrence after transurethral electrotomy2,3. Therefore, it is urgent to discover novel targets for bladder cancer diagnosis and therapy.
miRNAs are very short, nonprotein coding RNAs (ncRNAs) with a length of 19–23 nucleotides (nt) that have been shown to regulate gene expression in many biological processes under physiological and pathological conditions, including cell survival and death, cell movement, and stress response and development. During the past two decades, miRNAs have been tightly connected with cancer for its significant regulatory role in cell proliferation, apoptosis, invasion, and cycle control4–6. Numerous miRNAs are regarded as diagnostic, monitoring, and therapeutic biomarkers in multiple cancers7,8.
Recently, it was revealed that miRNAs played a key role in bladder cancer carcinogenesis9,10. Some miRNAs played a negative role in the progression of bladder cancer. For instance, miR-143 and -145 synergistically inhibited the growth of human bladder cancer cells by suppressing the PI3K/Akt and MAPK signaling pathways11. Other miRNAs had a promoting effect on the progression of bladder cancer, such as miR-200c, which increased bladder cancer cell migration and invasion via suppression of the metastasis-suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK)12. In this study, we investigated the role of miR-5195-3p, a quite newly discovered miRNA, in the proliferation and invasion of bladder cancer cells. We then explored its potential mechanism.
MATERIALS AND METHODS
Tissue Sampling
This study protocol was approved by the ethics committee of the First Affiliated Hospital of Nanchang University (P.R. China). The subjects were patients in the Department of Urology, The First Affiliated Hospital of Nanchang University (P.R. China). They were informed of the experimental details and gave written consent. Bladder cancer tissues were obtained from a total of 90 patients (63 ± 10.6 years), including 30 patients with primary bladder cancer, 30 patients with bladder cancer at T1 or T2 stages, and 30 patients with bladder cancer at T3 or T4 stages. As a control, bladder epithelial tissues were isolated from 15 healthy volunteers (50 ± 7.6 years).
Cell Culture and Transfection
Normal human bladder epithelial cell SV-HUC-1, human bladder cancer cell lines (including vs. BIU-87, T24, EJ-28, Pumc-91, and J82), and HEK293 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) that had been added to 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin (Gibco), and 100 U/ml streptomycin (Gibco). The cells were incubated at a humidified atmosphere at 37°C in a 5% CO2 incubator.
For transfection, T24 and BIU-87 bladder cancer cells were subcultured into 12-well plates (Corning, Corning, NY, USA). On reaching 75% confluence, 60 nM of the miR-5195-3p mimic (mimic-5195-3p), 80 nM of the miR-5195-3p antagomir (anta-5195-3p), or 80 nM of relative negative controls (mimic-NC or anta-NC) was transfected into the cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. For knockdown of Krüppel-like factor 5 (KLF5), KLF5 siRNA and scrambled control siRNA (ctrl-siRNA) were transfected into T24 and BIU-87 cells at a density of 100 nM. The mimic-5195-3p, anta-miR-5195-3p, and their NCs, as well as KLF5 siRNA and its negative control, were designed and synthesized by the Ribobio Co., Ltd. (Guangzhou, P.R. China).
Cell Proliferation
The cells were seeded into 12-well culture plates at 3 × 104/well. After transfection for 48 h, cell proliferation was evaluated using the cell counting kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer’s instructions.
In Vitro Invasion Assay
Cells (5 × 104) with serum-free medium were plated into the top chamber with a noncoated membrane (24-well insert; 8 μm; Corning). DMEM plus 10% FBS was used to chemoattract cells in the lower chamber. After incubation for 24 h, the nonmigrated cells in the upper chamber were removed, and the filters were stained with 2.5% crystal violet. The invading cells were captured under a light microscope (100×; Olympus Corporation, Osaka, Japan).
Real-Time Quantitative PCR
In a 25-μl system, SYBR Premix Ex Taq (TaKaRa, Dalian, P.R. China), 0.4 mM of primers, and 200 ng of cDNA template were applied in quantitative PCRs (qPCRs). The primers of miR-5195-3p, KLF5, and U6 RNA (internal reference) were designed and synthesized by Ribobio Co., Ltd. The reactions were initially denatured at 95°C for 3 min and followed by 35 cycles of 95°C for 15 s and 55°C for 1 min. Transcript abundance was calculated by using the 2−ΔΔCt method.
Western Blotting
Total protein (50 μg) from each sample was separated by 12% SDS-PAGE. The protein was transferred onto a PVDF membrane (Millipore, Billerica, MA, USA) for immunoblotting analysis. The primary antibodies anti-KLF5 (1:400; Abcam, Cambridge, MA, USA), anti-VEGFA (1:400; Abcam), anti-cyclin D1 (1:300; Abcam), anti-ERK1/2 (1:400; Abcam), anti-pERK1/2 (1:200; Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (1:500; Abcam) were used to incubate in the membrane at 4°C overnight. After incubation with the HRP-conjugated secondary antibodies for 1 h at room temperature, proteins were then detected with the ECL (Millipore, Boston, MA, USA) method and analyzed with Quantity One software (Bio-Rad, Hercules, CA, USA).
3′-UTR Luciferase Gene Reporter Assay
The 3′-UTR sequence of human KLF5 mRNA was amplified by PCR. The mutant was created by a Site-Directed Mutagenesis Kit (TaKaRa). The wild-type (WT) and mutant (MUT) 3′-UTRs were purified and inserted into a psiCHECK™-2 Vector (Promega, Madison, WI, USA). The mimic-NC and mimic-5195-3p were cotransfected with psiCHECK-KLF5 3′-UTR WT or -KLF5 3′-UTR MUT into the HEK293 cells using Lipofectamine 3000. After incubation for 72 h, the fluorescence intensity was measured using a microplate reader (PT-3502; Potenov, Beijing, P.R. China).
Statistical Analysis
All data were obtained from at least three independent experiments. The data were expressed as means ± SEM. Statistics were calculated with SPSS v22.0. Multiple comparisons were assessed by one-way ANOVA followed by Dunnett’s tests. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-5195-3p Was Dramatically Downregulated in Human Bladder Cancer Tissues and Cell Lines
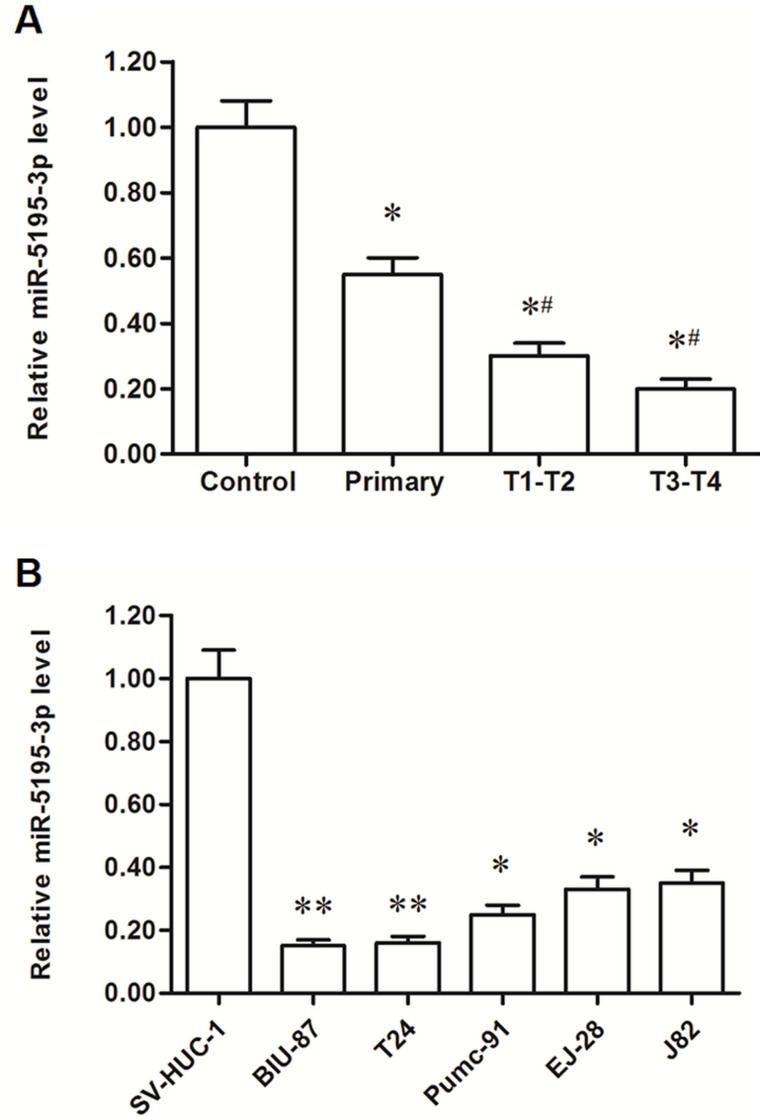
Expression levels were detected in tissues from 90 bladder cancer patients and 15 healthy volunteers (control group). It was shown that, compared with the control, miR-5195-3p expression was significantly reduced by about a half in bladder tissues from primary bladder cancer patients and further decreased (about 70%) in patients with metastatic bladder cancer (Fig. 1A). miR-5195-3p levels were then detected in human normal bladder epithelial cell line SV-HUC-1 and five bladder cancer cell lines. The results showed that miR-5195-3p was reduced by >60% in all five cancer cell lines and by >80% in T24 and BIU-87 cells (Fig. 1B). These results suggest that miR-5195-3p may be associated with the progression of bladder cancer.
Figure 1.
miR-5195-3p was significantly downregulated in human bladder cancer tissues and cell lines. The expression level of miR-5195-3p was detected in tissues with real-time quantitative PCR (qPCR). (A) miR-5195-3p was significantly downregulated in bladder cancer tissues. *p < 0.05 versus control, #p < 0.05 versus primary bladder cancer patients. The expression of miR-5195-3p was detected in human normal bladder epithelial cell SV-HUC-1 and five human bladder cancer cell lines with real-time qPCR, including BIU-87, EJ-28, T24, Pumc-91, and J82. (B) miR-5195-3p was significantly downregulated in bladder cancer cell lines. *p < 0.05, **p < 0.01 versus SV-HUC-1.
miR-5195-3p Played a Negative Role in the Proliferation and Invasion of Human Bladder Cancer Cells
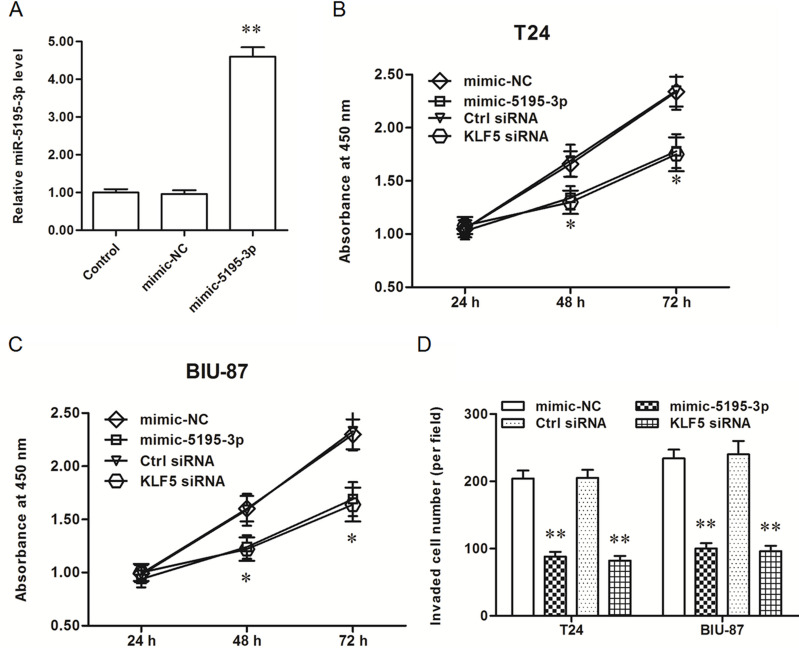
In order to explore the role of miR-5195-3p, miR-5195-3p mimic or miR-5195-3p antagomir was transfected into T24 and BIU-87 bladder cancer cells. After incubation for 48 h, miR-5195-3p expression, cell proliferation, and cell invasion were detected. The results showed that miR-5195-3p expression was increased about 4.8-fold (Fig. 2A), and the proliferation and invasion of T24 and BIU-87 bladder cancer cells were both significantly inhibited (Fig. 2B–D). Consistent with results from the miR-5195-3p transfection, knockdown of KLF5, the potential target of miR-5195-3p, significantly inhibited the proliferation and invasion of T24 and BIU-87 cells (Fig. 2B–D).
Figure 2.
miR-5195-3p mimic decreased the proliferation and invasion of T24 and BIU-87 bladder cancer cell lines. (A) miR-5195-3p expression was distinctly increased by the miR-5195-3p mimic transfection. (B, C). The miR-5195-3p mimic and KLF5 siRNA both reduced the proliferation of T24 and BIU-87 cells. (D) The miR-5195-3p mimic and KLF5 siRNA both suppressed the invasion of T24 and BIU-87. *p < 0.05, **p < 0.01 versus mimic-NC or ctrl-siRNA.
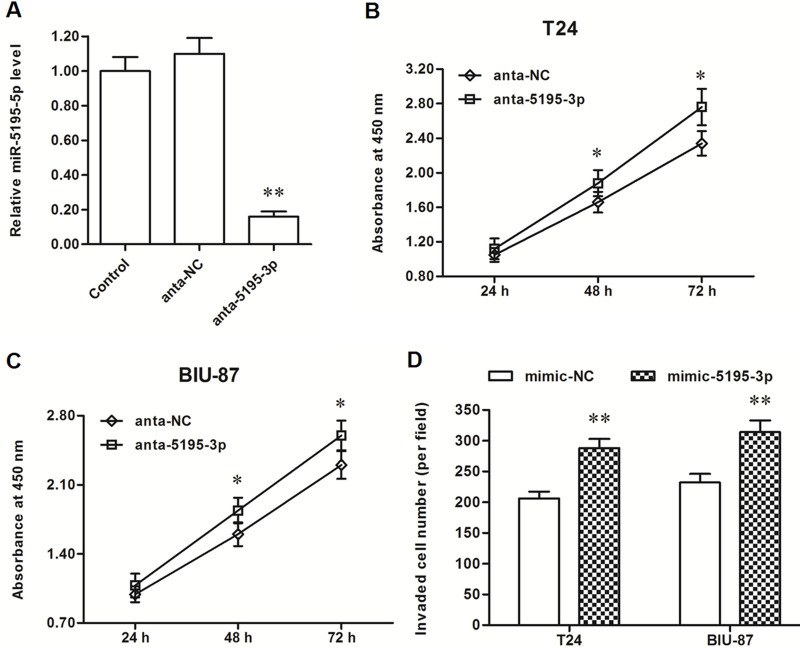
In contrast with results from the miR-5195-3p mimic transfection, miR-5195-3p expression was sharply decreased by the miR-5195-3p antagomir transfection (Fig. 3A), and the proliferation and invasion of T24 and BIU-87 cells were markedly increased (Fig. 3B–D). These data on gain-and-loss experiments indicated that miR-5195-3p had a negative effect on the proliferation and invasion of bladder cancer cells.
Figure 3.
miR-5195-3p antagomir increased the proliferation and invasion of T24 and BIU-87 cells. (A) The miR-5195-3p level was sharply decreased by the anta-miR-5195-3p transfection. (B, C) The anta-5195-3p significantly promoted the proliferation of T24 and BIU-87 cells. (D) The anta-miR-5195-3p significantly promoted the invasion of T24 and BIU-87 cells. *p < 0.05, **p < 0.01 versus anta-NC.
miR-5195-3p Directly Targeted KLF5 and Suppressed its Expression
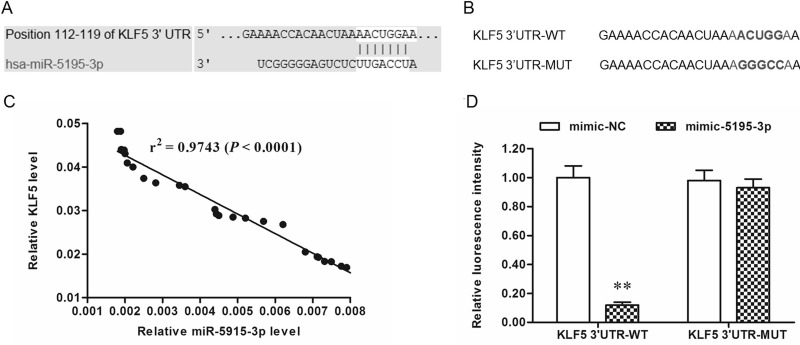
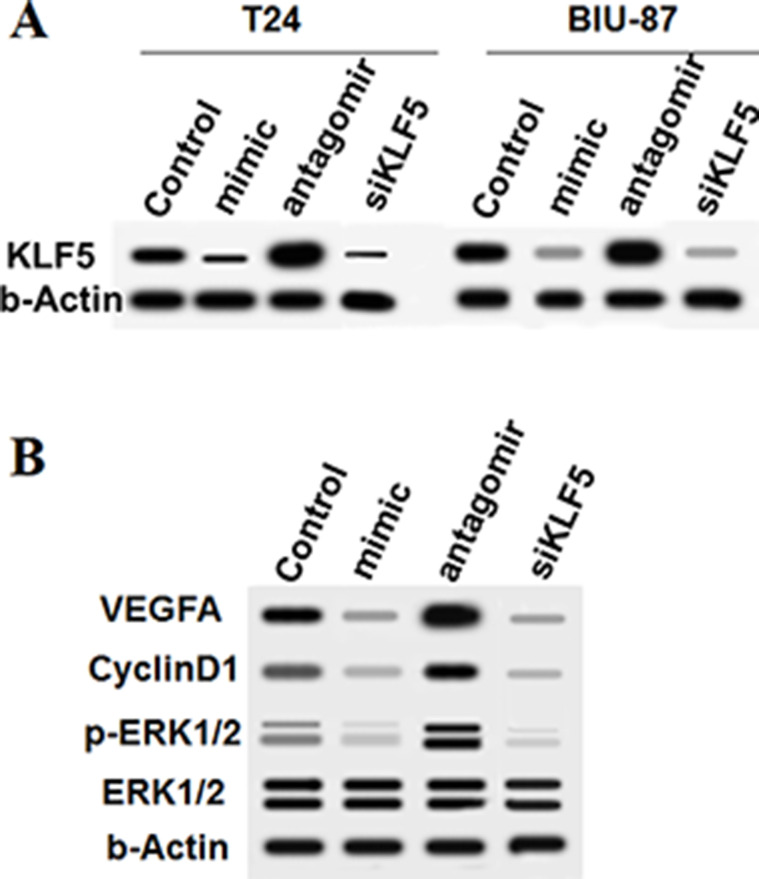
Bioinformatics analysis was used to assess the interaction between miR-5195-3p and KLF5. Seven consecutive bases in the 3′-UTR of KLF5 mRNA completely matched the seed of miR-5195-3p (Fig. 4A), suggesting that miR-5195-3p potentially targeted KLF5 mRNA at this region. A correlation analysis in 30 randomly selected patients showed that there was quite a significant correlation between miR-5195-3p and KLF5 mRNA levels (p < 0.0001) (Fig. 4C). WT and MUT 3′-UTR luciferase reporter gene check reporters were constructed (Fig. 4B) and then transfected into HEK293 cells with mimic-5195-3p or mimic-NC. The data revealed that miR-5195-3p markedly weakened the luciferase activity in the WT group but not in the MUT group (Fig. 4D). To further validate that KLF5 was a target of miR-5195-3p, the control or miR-5195-3p mimic was transfected into T24 and BIU-87 cells. Western blotting indicated that the KLF5 protein level was sharply reduced by the miR-5195-3p mimic transfection and increased by the miR-5195-3p antagomir (Fig. 5A). Moreover, results from downstream gene detection in T24 cells depicted that VEGFA, cyclin D1, and ERK1/2 were negatively regulated by miR-5195-3p as a result of a decrease in KLF5 (Fig. 5B). Similarly, siRNA-mediated knockdown of KLF5 suppressed the expression and activation of VEGFA, cyclin D1, and ERK1/2 (Fig. 5A and B).
Figure 4.
miR-5195-3p potentially targeted KLF5 mRNA at the 3′-UTR. Alignment of human miR-5195-3p and KLF5 mRNA sequences among mammals. (A) The output of the TargetScan online server on the binding of miR-5195-3p and the 3′-UTR of KLF5 mRNA. (B) WT and mutant KLF5 mRNA 3′-UTR sequences. (C) Correlation analysis between miR-5195-3p and KLF5 mRNA levels. (D) The miR-5195-3p mimic suppressed the luciferase activity of the WT vector but not the mutant vector. **p < 0.01.
Figure 5.
miR-5195-3p negatively regulated the expression of KLF5 and its downstream genes. (A) Protein levels of KLF5 and its downstream genes were detected with Western blotting. (B) The miR-5195-3p and KLF5 knockdown both negatively regulated the expression of VEGFA and cyclin D1 and the activation of ERK1/2.
DISCUSSION
miR-5195-3p was discovered by small RNAome deep sequencing in human acute lymphoblastic leukemia in 201113. However, the function of miR-5195-3p has hardly been reported, either in carcinogenesis or normal biological processes, in the past 5 years. Quite recently, a study revealed that miR-5195-3p plays a key role in the migration and invasion of A172 and T98G malignant glioma cells14. In this study, we found that miR-5195-3p was decreased in bladder cancer tissues and cell lines compared with normal bladder epithelial tissue and cell lines. We then observed that miR-5195-3p played a suppressive role in bladder cancer cell proliferation and invasion.
The Krüppel-like family of transcription factors (KLFs) is a 17-member family of zinc finger DNA-binding proteins that regulates a wide range of biological processes15–17. Some KLF members have been shown to regulate the progression of multiple cancers, including KLF4, 5, 6, and 1518,19. KLF5, also known as intestinal enriched Krüppel-like factor (IKLF), can bind p300 to acetylate the first zinc finger that gave it a transactivating function20. KLF5 can function as either a tumor suppressor or a promoter in the carcinogenesis of different types of cancers. For example, in esophageal squamous cell cancer, KLF5 activated the JNK pathway and increased apoptosis and reduced cell survival21, whereas in breast and lung cancers, and in intestinal tumors, KLF5 functioned as a tumor promoter22–24. In urothelial tissue, KLF5 is required for the formation of the normal bladder urothelium and played a promoting role in the proliferation, invasion, migration, and angiogenesis25,26. In bladder cancer, KlF5 was identified as a tumor promoter, regulating upregulation and activation of many downstream genes that are kinases improving cell survival or promoting cell cycle regulators, such as extracellular signal-regulated protein kinases (ERK1/2), vascular endothelial growth factor (VEGFA), p53, and cyclin D127,28. As we know, the function of an miRNA is basically dependent on which genes it targets. In this study, results from the bioinformatic prediction and 3′-UTR luciferase reporter gene assay confirmed that KlF5 is a target gene of miR-5195-3p, and KlF5 suppression by miR-5195-3p increased the proliferation and invasion of T24 and BIU-87 human bladder cancer cell lines.
In conclusion, miR-5195-3p inhibits the proliferation and invasion of human bladder cancer cells by directly targeting oncogene KLF5. Thus, it may be regarded as a potential therapeutic target for bladder cancer.
REFERENCES
- 1. Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005;66:4–34. [DOI] [PubMed] [Google Scholar]
- 2. Ploeg M, Aben KKH, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alfred WJ, Lebret T, Compérat EM, Cowan NC, De SM, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2016;30290–1. [DOI] [PubMed] [Google Scholar]
- 4. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122:6–7. [DOI] [PubMed] [Google Scholar]
- 5. Calin GA, Croce CM. MicroRNA-cancer connection: The beginning of a new tale. Cancer Res. 2006;66:7390–4. [DOI] [PubMed] [Google Scholar]
- 6. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Croce CM. 27 Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2012;18:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iorio MV, Croce CM. MicroRNA dysregulation in cancer: Diagnostics, monitoring, and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baumgart S, Jeppesen D, Heinzelmann J, Stöckle M, Ostenfeld MS, Junker K. 228 Characterization of miRNA expression pattern from in-vitro obtained exosomes of different urinary bladder cancer cell lines. Eur Urol Suppl. 2015;14:e228. [Google Scholar]
- 10. Itesako T, Enokida H, Yoshino H, Chiyomaru T, Seki N, Nakagawa M. The microRNA expression signature of bladder cancer by deep sequencing: The functional significance of the miR-195/497 cluster. PLoS One 2014;9:e84311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S, Akao Y. Replacement treatment with microRNA-143 and -145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett. 2013;328:353–61. [DOI] [PubMed] [Google Scholar]
- 12. Cheng Y, Zhang X, Li P, Yang C, Tang J, Deng X, Yang X, Tao J, Lu Q, Li P. MiR-200c promotes bladder cancer cell migration and invasion by directly targeting RECK. OncoTargets Ther. 2016;9:5091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schotte D, Akbari MF, Lange-Turenhout EA, Chen C, van Ijcken WF, Pieters R, den Boer ML. Discovery of new microRNAs by small RNAome deep sequencing in childhood acute lymphoblastic leukemia. Leukemia 2011;25:1389–99. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, He T, Ye M, Xu F, Yang L, Wang H, Song X. Upregulation of p72 enhances malignant migration and invasion of glioma cells by repressing Beclin1 expression. Biochemistry 2016;81:574–82. [DOI] [PubMed] [Google Scholar]
- 15. Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: Bring in the family. Genomics 2005;85:551–6. [DOI] [PubMed] [Google Scholar]
- 16. Knoedler JR, Denver RJ. Krüppel-like factors are effectors of nuclear receptor signaling. Gen Comp Endocr. 2014;203:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Krüppel-like transcription factors: A functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. [DOI] [PubMed] [Google Scholar]
- 18. Tetreault MP, Yang Y, Katz JP. Krüppel-like factors in cancer. Nat Rev Cancer 2013;13:701–13. [DOI] [PubMed] [Google Scholar]
- 19. Mcconnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chanchevalap S. Krüppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006;34:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarapore RS, Yang Y, Katz JP. Restoring KLF5 in esophageal squamous cell cancer cells activates the JNK pathway leading to apoptosis and reduced cell survival. Neoplasia 2013;15:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia L, Liu R, Chen C. Abstract 4042: Kruppel-like factor 5 promotes breast cancer proliferation, migration, and invasion partially through upregulating the transcription of TNFα-induced protein 2. Cancer Res. 2015;75(15 Suppl). [Google Scholar]
- 23. Li X, Liu X, Xu Y, Liu J, Xie M, Ni W, Chen S. KLF5 promotes hypoxia-induced survival and inhibits apoptosis in non-small cell lung cancer cells via HIF-1α. Int J Oncol. 2014;45:1507–14. [DOI] [PubMed] [Google Scholar]
- 24. Nakaya T, Kuroda M, Nagai R. KLF5 is essential for oncogenesis of intestinal tumors and control of intestinal stem cells. Cancer Res 2013;73(8 Suppl):2293. [Google Scholar]
- 25. Bell SM, Zhang L, Mendell A, Xu Y, Haitchi HM, Lessard JL, Whitsett JA. Kruppel-like factor 5 is required for formation and differentiation of the bladder urothelium. Dev Biol. 2011;358:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, Bao Y, Zhou Z, Cheng X, Simons JW. KLF5 promotes cell proliferation and tumorigenesis through gene regulation in the TSU-Pr1 human bladder cancer cell line. Int J Cancer 2006;118:1346–55. [DOI] [PubMed] [Google Scholar]
- 27. Seo KL, Hyun Y, Lee CS. Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer. Cancer Res. 2011;71:6475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang G, Wu K, Chen Y, Zhou J, Chong D, Qi S, Shan X, Jing J, Tang X, Feng L. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget 2015;6:43791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]