Abstract
E2F3a, as a member of the E2F family, is essential for cell division associated with the progression of many cancers. However, the biological effect of E2F3a on glioma is not understood as well. To investigate the functional mechanism of E2F3a in glioma, we examined the expression of E2F3a in glioma tissue and cell lines. We found that E2F3a was upregulated in glioma tissue compared with adjacent tissue, and this was associated with a poor survival rate. E2F3a was highly expressed in glioma cell lines compared with normal HEB cell lines. Knockdown of E2F3a significantly inhibited cell proliferation, promoted G0/G1 phase arrest, elevated apoptosis rates, and suppressed cell migration and invasion. However, overexpression of E2F3a markedly promoted cell proliferation, migration, and invasion and inhibited apoptosis. Moreover, in vivo studies showed that knockdown of E2F3a expression dramatically inhibited U373 tumor growth in a nude mouse model. Results of real-time PCR and Western blot showed that the depletion of E2F3a upregulated the expression levels of cell apoptosis-related proteins and downregulated migration-related proteins. Conversely, E2F3a overexpression downregulated the expression levels of cell apoptosis-related proteins and upregulated migration-related proteins. In conclusion, our results highlight the importance of E2F3a in glioma and provide new insights into the diagnostics and therapeutics of gliomas.
Key words: Glioma, E2F3a, Apoptosis, Migration
INTRODUCTION
Malignant gliomas are the most common primary brain tumors, and they arise from the glial cells within the central nervous system, which are characterized by morphological and genetic complexities that contribute to uncontrolled proliferation, high resistance to apoptosis, and diffuse infiltration into normal brain parenchyma1,2. Glioblastoma multiforme (GBM) is the most aggressive glioma with a survival time of only 12 months from the time of diagnosis, even with multimodal therapy such as irradiation and chemotherapy3,4. Furthermore, conventional strategies are limited due to the glioma’s ability to efficiently infiltrate surrounding normal brain tissue, making it difficult to fully resect the tumors during surgery. Recently, a wide range of other therapeutic strategies, including immunotherapy and gene therapy, have been tried or are under evaluation. However, treatments for GBM remain essentially ineffective, and the recurrence of tumors is still poorly understood. Moreover, glioblastoma tumors are heavily infiltrated by microglia and macrophages, which contribute at least one third of the total tumor mass implicated in several roles in GBM progression including proliferation, survival, motility, and immunosuppression5–7, and secrete a soluble motogenic factor that acts on glioblastoma cells. Therefore, investigating the mechanism of glioblastoma cell invasion and discovering new therapeutic targets for glioma have received a great deal of interest.
The E2F family of transcription factors is essential for cell proliferation, differentiation, cell cycle, DNA replication, and apoptosis8,9. To date, eight different E2F family members (E2F1–8) have been identified. Among them, E2F3 encodes two protein products, E2F3a and E2F3b, through the use of two distinct promoters10. E2F3a has its peak expression at the G1/S transition, whereas E2F3b is constitutively expressed in quiescent cells that are functional in the G0 phase and remain constant throughout the cell cycle11. E2F3a transcriptionally activates E2F target genes such as thymidine kinase and cyclin E, unlike E2F3b, which represses the transcription of E2F target genes. Members of the E2F family have dual roles in tumorigenesis, as oncogenes and as tumor suppressor genes. E2F3a has been shown to be overexpressed in several types of human cancers, including liver, bladder, prostate, and ovarian cancers and acute lymphoblastic leukemia, and is correlated with cancer cell proliferation, apoptosis, invasion, and prognosis10,12–14. E2F3a overexpression also induced DNA damage in primary human fibroblasts that were inhibited by blocking DNA replication15. Although E2F3a has been reported at a higher level in glioma tissue than in normal brain tissue, and proliferation rate and the percentage of S phase cells were reduced after E2F3a depletion16, no apoptosis or invasion functional studies of E2F3a, or the related molecular mechanism of glioma cells, have been performed.
In this study, we demonstrate the effects of silencing and overexpressing of E2F3a on cell proliferation, cell cycle, apoptosis, invasion, and migration, and the changes in the gene expression pattern in glioma cell lines. Our results suggest that E2F3a could regulate glioma carcinogenesis and may serve as a potential target for glioma therapies.
MATERIALS AND METHODS
Bioinformatics Analysis
TCGA RNA-Seq and the corresponding clinical data were downloaded from TCGA following the approval of this project by the consortium. RNA-Seq analysis used data from 68 GBM and 5 nontumor brain samples. Survival rate data were downloaded from the NCBI’s Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series Accession No. GSE82009 following the approval of this project by the consortium.
Cell Lines and Culture Conditions
Human glioma cell lines T98G, U251, and U373 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured according to the guidelines recommended by the ATCC. SHG44 was from the Cell Bank of Shanghai Biological Institute, Chinese Academy of Science (Shanghai, P.R. China) and was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone). All cells were maintained at 37°C under an atmosphere of 5% CO2/95% air.
Protein Extraction and Western Blot
Total protein was extracted from cells using ice-cold radioimmunoprecipitation assay buffer (RIPA; Beyotime Institute of Biotechnology) containing 0.01% protease and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO, USA). After being incubated on ice for 30 min, cell lysis was centrifuged 12,000 × g at 4°C for 20 min, and proteins in the supernatant were collected for experiments. Lysate was separated by 10%–15% SDS-gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Boston, MA, USA). The protein was probed with anti-E2F3a (Cat. No. sc-879; 1:4,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Bim (Cat. No. ab7888; 1:1,000; Abcam, Cambridge, MA, USA), anti-Bax (Cat. No. ab69643; 1:1,000), anti-caspase 3 (Cat. No. ab2302; 1:500), anti-caspase 9 (Cat. No. ab2014; 1:1,000), MMP-2 (Cat. No. ab37150; 1:500), anti-MMP-9 (Cat. No. ab73734; 1:1,000), anti-MMP-14 (Cat. No. ab3644; 1:1,000), anti-MMP-19 (Cat. No. ab53146; 1:1,000), anti-PCNA (Cat. No. ab18197; 1:1,000), anti-Ki67 (Cat. No. ab15580; 1:1,000), and anti-GAPDH (Cat. No. 5174; 1:1,500; Cell Signaling Technology, Danvers, MA, USA).
Construction and Infection
Oligonucleotide encoding shRNA-targeted human E2F3a mRNA and a scramble shRNA were both obtained from Sangon Biotech Inc. (Beijing, P.R. China). These oligonucleotides were annealed and digested using AgeI and EcoRI and were then ligated into pLKO.1-EGFP, giving rise to a pLKO.1-EGFP-E2F3a-shRNA construct. For E2F3a expression constructs, mRNA was isolated from adult human normal brain tissue and reversed transcribed, and the full-length cDNA was first amplified using the following primers: 5′-CGAGAGATGAGAAAGGGAATCCAG-3′ (forward) and 5′-GAGAGTTCACACGAAGCATAATCAAC-3′ (reverse), and then cloned into the pLVX-Puro vectors using XhoI and KpnI (Shanghai Genomics Inc., Shanghai, P.R. China). The recombinant pLKO.1-EGFP-E2F3a-shRNA and pLVX-Puro-E2F3a were stably infected into U373 and U251 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The recombinant pLKO.1-EGFP-scramble shRNA (shNC) and black pLVX-Puro (NC) were used as the negative control.
CCK-8 Assay
Cell viability was determined using the cell counting kit-8 (CCK-8) in accordance with the manufacturer’s protocol (Sigma-Aldrich). Cells infected with pLKO.1-EGFP-E2F3a-shRNA or pLVX-Puro-E2F3a were seeded into 96-well plates at 3 × 103 per well for 48 h, and the CCK-8 assay was performed after 24, 48, and 72 h. The optical density (OD) was measured using a microplate reader (Bio-Tek Company, Winooski, VT, USA) at 450 nm wavelength.
Cell Cycle and Cell Apoptosis Assay
Cells infected with pLKO.1-EGFP-E2F3a-shRNA or pLVX-Puro-E2F3a were seeded into 96-well plates at 3 × 103 per well for 48 h. For cell cycle analysis, cells were digested by trypsin, fixed with ice-cold 70% ethanol for 4 h, and stained with 50 μg/ml propidium iodide (PI) in the presence of RNase A at 37°C for 30 min. For cell apoptosis analysis, cells were harvested and washed twice in phosphate-buffered saline (PBS), stained with 10 μl of annexin V–FITC and 5 μl of PI, and analyzed using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell Migration and Cell Invasion Assay
Cells (1 × 105) infected with pLKO.1-EGFP-E2F3a-shRNA or pLVX-Puro-E2F3a in 100 μl of MEM or DMEM without FBS were seeded into the Transwell upper chamber. In the lower chamber, 500 μl of MEM or DMEM with 10% FBS was added as a chemoattractant. After a 48-h incubation period, cells that did not migrate were scraped from the upper chamber, and the adherent cells were stained with crystal violet and photographed (200×). The procedure for the cell invasion assay was similar to the cell migration assay, except that the Transwell membranes were precoated with Matrigel (BD Biosciences, San Jose, CA, USA).
Real-Time PCR
Total RNA was isolated from tissue specimens or cells with the TRIzol reagent (Invitrogen) and reverse transcribed to cDNA according to the manufacturer’s protocol (Fermentas, Hanover, MD, USA). Quantitative real-time PCR (qRT-PCR) was carried out using an ABI 7300 real-time PCR machine (Applied Biosystems, Foster City, CA, USA) and SYBR Green PCR Kit (Thermo Fisher Scientific, Rockford, IL, USA). The relative expression of genes was calculated with the 2−(ΔCt) method. The primers used are listed in Table 1.
Table 1.
Primer Sequences Used in This Study
| Gene | Sequences |
|---|---|
| Bim forward | 5′-CCACCAGCACCATAGAAG-3′ |
| Bim reverse | 5′-GAGCAGGCACAGAGAAAG-3′ |
| Bax forward | 5′-AGCTGAGCGAGTGTCTCAAG-3′ |
| Bax reverse | 5′-TGTCCAGCCCATGATGGTTC-3′ |
| Caspase 9 forward | 5′-CATGCTGGCTTCGTTTCTG-3′ |
| Caspase 9 reverse | 5′-AGGGGCAAACAACAGATGG-3′ |
| Caspase 3 forward | 5′-AACTGGACTGTGGCATTGAG-3′ |
| Caspase 3 reverse | 5′-ACAAAGCGACTGGATGAACC-3′ |
| MMP-2 forward | 5′-TTGACGGTAAGGACGGACTC-3′ |
| MMP-2 reverse | 5′-GGCGTTCCCATACTTCACAC-3′ |
| MMP-9 forward | 5′-AAGGGCGTCGTGGTTCCAACTC-3′ |
| MMP-9 reverse | 5′-AGCATTGCCGTCCTGGGTGTAG-3′ |
| MMP-14 forward | 5′-GAGCTCAGGGCAGTGGATAG-3′ |
| MMP-14 reverse | 5′-GGTAGCCCGGTTCTACCTTC-3′ |
| MMP-19 forward | 5′-CTTTCAAGGGGGACTATGTG-3′ |
| MMP-19 reverse | 5′-TATTCAGCTTCTTGGGGAAG-3′ |
| GAPDH forward | 5′-AATCCCATCACCATCTTC-3′ |
| GAPDH reverse | 5′-AGGCTGTTGTCATACTTC-3′ |
In Vivo Tumor Growth
The in vivo tumor growth experiments were authorized by The Affiliated Hospital of Xuzhou Medical University. pLKO.1-EGFP-E2F3a-shRNA stable expressed U373 cells (1 × 106 cells/0.1 ml) were injected subcutaneously into the right armpit of 4-week-old male athymic nude mice (n = 6; Shanghai Laboratory Animal Co., Ltd., Shanghai, P.R. China) every 3 days for 45 days. Afterward, the mice were sacrificed, and tumor tissues were excised and weighed. The shortest and longest diameters of the tumors were measured with calipers every 4 days, and tumor volume (mm3) was calculated using the following standard formula: (the shortest diameter)2 × (the longest diameter) × 0.5.
Statistical Analyses
All data were obtained from at least three independent tests with triplicate samples and shown as mean ± SD. GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis (one-way ANOVA for multiple groups and unpaired, two-tailed t-test for two groups). Overall survival in relation to WWP2 expression was evaluated by the Kaplan–Meyer survival curve and log-rank nonparametric test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
E2F3a Expression in Glioma Tissues and Cell Lines
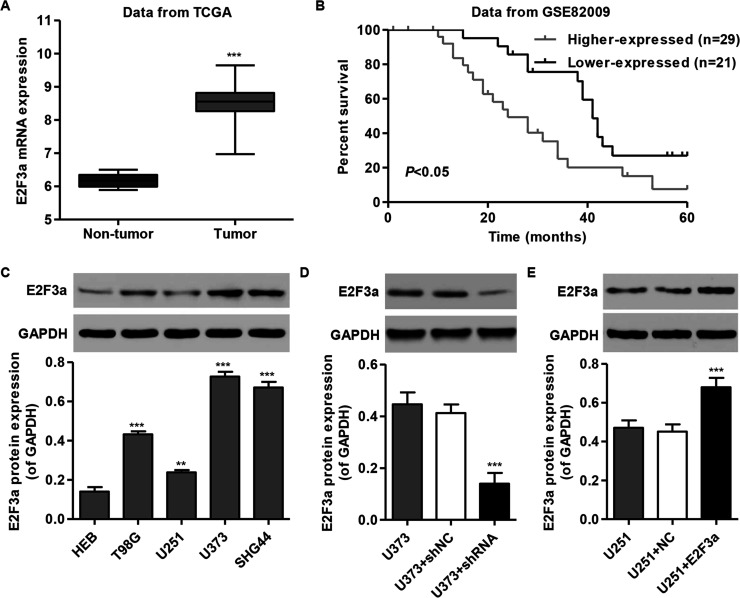
E2F3a was significantly increased in glioma tissue when compared with the nontumor tissue of patients in the TCGA database (Fig. 1A). We next compared the survival time of patients from the GSE82009 database. The glioma patients were divided into two groups according to the median level of E2F3a. The cumulative survival rate was significantly higher in patients with lower E2F3a-expressing tumors than in those with higher E2F3a-expressing tumors (Fig. 1B). These results indicate that E2F3a expression could represent a new prognostic factor in glioma patients, and therefore we chose to focus our experimental research on E2F3a.
Figure 1.
E2F3a expression in glioma tissues and cell lines. (A) Comparison of the expression level of E2F3a between glioma and nontumor tissues in TCGA. (B) Kaplan–Meyer survival curves for two groups of patients in GSE82009. Black, data for patients with low prognostic scores (group 1, higher survival time); gray, patients with high prognostic scores (group 2, poorer survival). (C) E2F3a expression level in four glioma cell lines and normal human embryonic brain (HEB) cells was analyzed by Western blot. (D, E). Expression of E2F3a in U373 and U251 cells was analyzed by Western blot. GAPDH was also detected as the internal control. Representative Western blots (top) and quantitative results are shown (bottom). **p < 0.01 compared with HEB cells; ***p < 0.001 compared with HEB cells or corresponding control cells.
To investigate the expression pattern of E2F3a in glioma cell lines, we assessed the E2F3a protein levels in human embryonic brain (HEB) and four glioma cell lines. Four glioma cell lines showed a higher expression of E2F3a when compared with the normal HEB cell line, with the highest expression in U373 cells and the lowest expression in U251 cells (Fig. 1C). These two cell lines were therefore used in the following experiments. To study the function of E2F3a on glioma cell lines, construct and knockdown of E2F3a were generated and stably infected into U373 and U251 cells. E2F3a expression was significantly decreased in E2F3a-shRNA-infected U373 cells (Fig. 1D), whereas E2F3a expression was significantly increased in E2F3a construct-infected U251 cells (Fig. 1E).
Downregulation of E2F3a Inhibits Cell Proliferation of Glioma
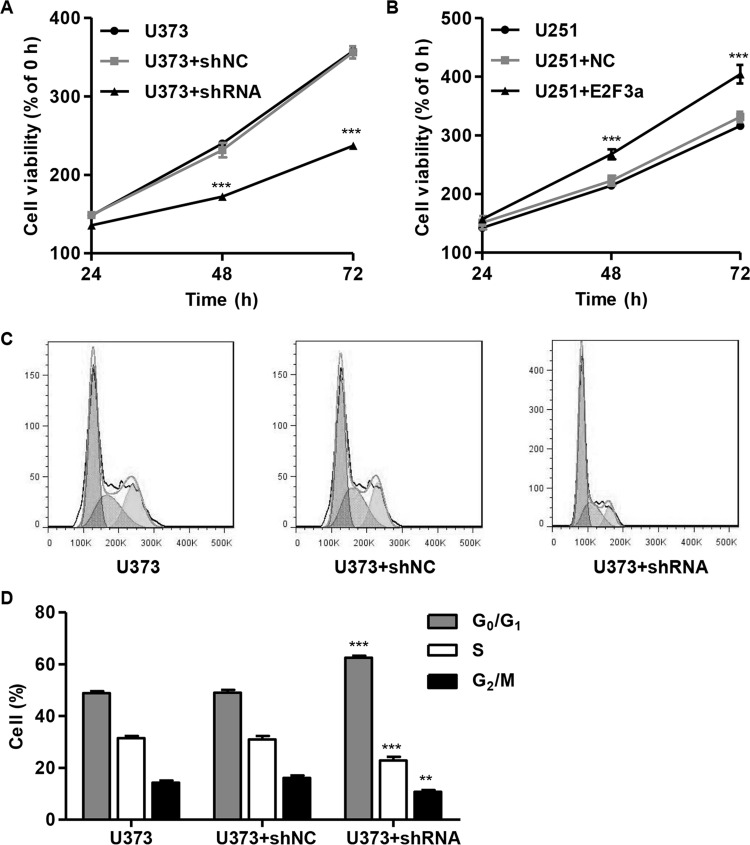
To examine the effects of E2F3a knockdown and overexpression on the proliferation of glioma cell lines, CCK-8 assays were performed on both U373 and U251 cells following lentivirus infection for 48 h. E2F3a knockdown can inhibit U373 cell proliferation, and E2F3a overexpression can promote U251 cell proliferation at both 48 and 72 h (Fig. 2A and B).
Figure 2.
Suppressing E2F3a expression inhibited cell proliferation and induced the G0/G1 phase arrest. U373 cells were infected with E2F3a-shRNA and collected 48 h later, and U251 cells were infected with E2F3a constructs and collected 48 h later. (A, B) Cell proliferation was detected in U373 and U251 cells by cell counting kit-8 (CCK-8) assay, respectively. (C, D) Cell cycle profile of U373 cells was analyzed using flow cytometry. **p < 0.01, ***p < 0.001 compared with corresponding control cells.
Considering that downregulation of E2F3a significantly slowed cell proliferation, we further performed cell cycle analysis to reveal the mechanism of the inhibitory effect of E2F3a shRNA lentivirus on cell proliferation. E2F3a shRNA lentivirus infection significantly increased the percentage of cells in the G0/G1 phase and decreased the percentage of cells in both the S and G2/M phases, compared with the U373 control cells (Fig. 2C and D).
Downregulation of E2F3a Promotes Cell Apoptosis of Glioma
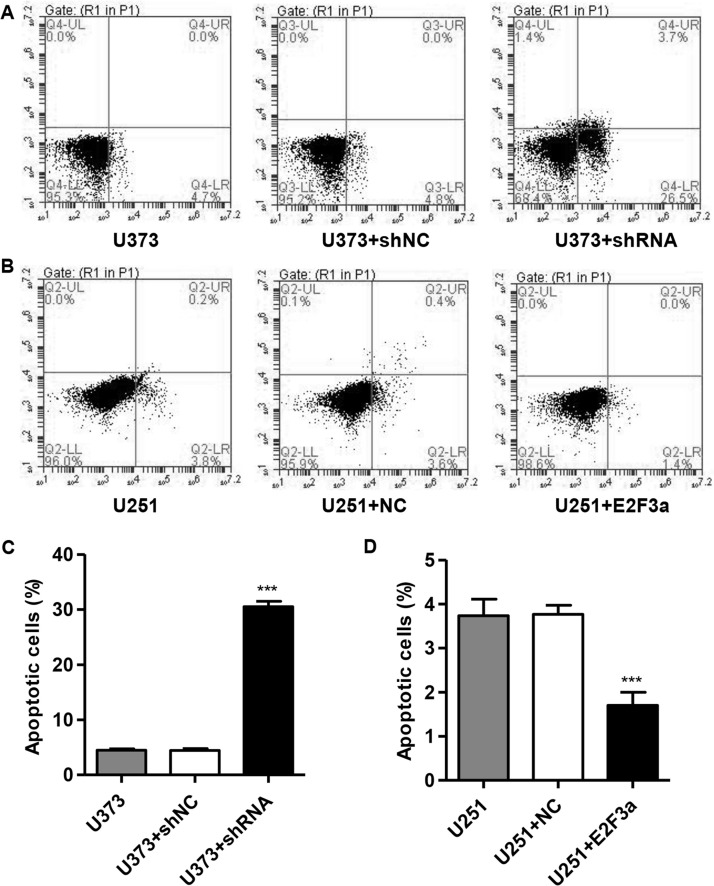
We then investigated the effect of E2F3a knockdown and overexpression on the apoptosis of glioma cell lines. E2F3a knockdown resulted in an approximately sixfold increase in the apoptotic ratio, compared with the U373 control cells (Fig. 3A and C). E2F3a overexpression resulted in an approximately 54% decrease in the apoptotic ratio, compared with the U251 control cells (Fig. 3B and D).
Figure 3.
Suppressing E2F3a expression induced apoptosis in glioma cells. U373 cells were infected with E2F3a-shRNA and collected 48 h later, and U251 cells were infected with E2F3a constructs and collected 48 h later. Cell apoptosis of U373 (A, C) and U251 cells (B, D) was analyzed by annexin V/propidium iodide (PI) staining. ***p < 0.001 compared with corresponding control cells.
Downregulation of E2F3a Reduces Cell Migration and Invasion of Glioma
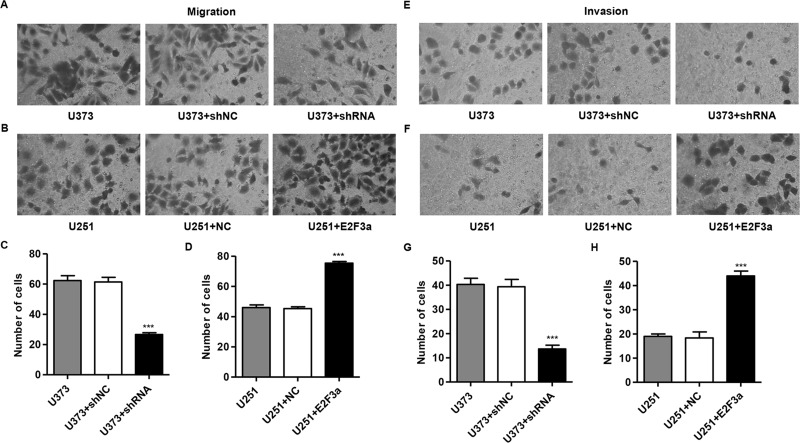
To determine the function of E2F3a on glioma migration, Transwell assays were carried out to quantitatively determine the effect of E2F3a on cell migration and invasion. Similar numbers of control and shNC or vector (NC)-infected cells migrated to the lower face of the Transwell membrane, whereas the E2F3a knockdown cells exhibited a strongly inhibited motility, with only 43% of cells migrating. E2F3a overexpression cells exhibited a strongly increased motility with approximately 1.6-fold cells migrating (Fig. 4A–D). Additionally, E2F3a knockdown cells exhibited a strongly inhibited invasion, with only 34% of cells invading, and the E2F3a overexpression cells exhibited a strongly increased invasion, with approximately 2.3-fold cells invading (Fig. 4E–H).
Figure 4.
Silencing of E2F3a inhibited cell migration and invasion in glioma cells. U373 cells were infected with E2F3a-shRNA and collected 48 h later, and U251 cells were infected with E2F3a constructs and collected 48 h later. Migration (A–D) and invasion (E–H) of U373 and U251 cells were measured by Transwell assay. ***p < 0.001 compared with corresponding control cells.
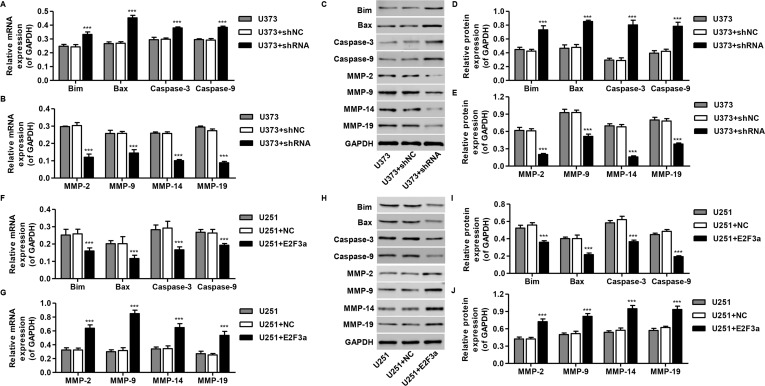
E2F3a Affected the Expression of Cell Apoptosis and Migration-Related Protein
The expression levels of apoptosis (Bim, Bax, cleaved caspase 3, and caspase 9) and migration-regulating proteins (MMP-2, MMP-3, MMP-9, and MMP-13) were then detected by RT-PCR and Western blotting, respectively. Interestingly, Bim, Bax, cleaved caspase 3, and caspase 9 mRNA and protein levels were increased by E2F3a knockdown in U373 cells (Fig. 5A, C, and D) but repressed by E2F3a overexpression in U251 cells (Fig. 5F, H, and I). While MMP-2, MMP-3, MMP-9, and MMP-13 mRNA and protein levels were repressed by E2F3a knockdown in U373 cells (Fig. 5B, C, and E), they were increased by E2F3a overexpression in U251 cells (Fig. 5G, H, and J). These data suggest antiapoptosis and migration-promoting roles for E2F3a in glioma cells.
Figure 5.
Mechanisms of E2F3a exert their functions in glioma cells. U373 cells were infected with E2F3a-shRNA and collected 48 h later, and U251 cells were infected with E2F3a constructs and collected 48 h later. The expression of apoptosis and migration-related protein in U373 (A–E) and U251 cells (F–J) was measured by real-time PCR and Western blot analysis. ***p < 0.001 compared with corresponding control cells.
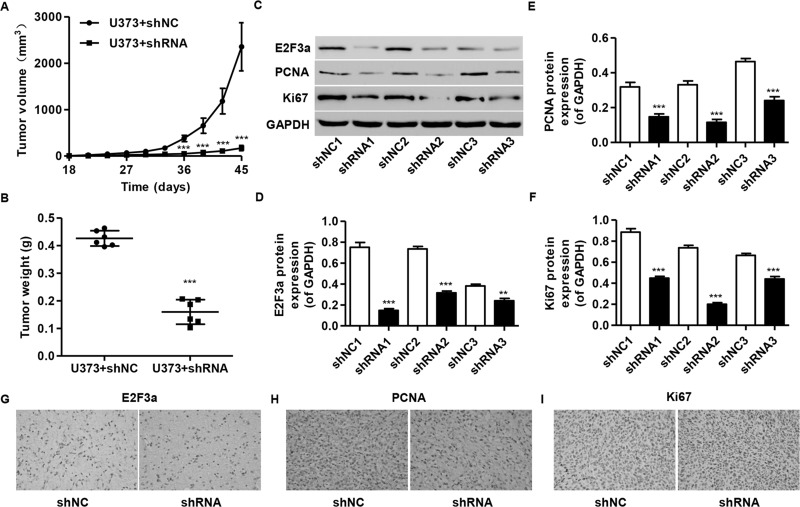
Silencing of E2F3a Suppresses Tumorigenesis of Glioma Cells In Vivo
To confirm the growth inhibitory effect of E2F3a-shRNA in vivo, a xenograft tumor-bearing model was established by inoculating E2F3a-shRNA-infected U373 cells into nude mice. E2F3a-shRNA-treated tumors grew much slower than the control shNC-treated tumors in mice (Fig. 6A), with average tumor weights of 0.42 ± 0.03 g and 0.16 ± 0.04 g in the shNC and E2F3a-shRNA mice, respectively (Fig. 6B). Furthermore, compared with shNC ones, a significant decrease in E2F3a, PCNA, and Ki-67 expression was observed in tumors formed from knockdown E2F3a cells by Western blot analysis (Fig. 6C–F) and immunohistochemical analysis (Fig. 6G–I).
Figure 6.
Knockdown of E2F3a in glioma cells reduced tumor growth in vivo. U373 cells infected with shRNA control (shNC) or E2F3a-shRNA were subcutaneously injected into athymic nude mice. Tumor growth was shown 45 days after injection. (A) Time course analysis of tumor growth after injection. (B) Tumor growth was significantly reduced in E2F3a knockdown tumors. (C–F) The expression of E2F3a, PCNA, and Ki-67 in xenografts from the nude mice was determined by Western blot and immunohistochemical analysis (G–I). **p < 0.01, ***p < 0.001 compared with corresponding control cells.
DISCUSSION
It is known that members of the E2F family act individually or synergistically to regulate cell proliferation and apoptosis, as well as other cellular processes. Among these, depletion of E2F3 has emerged as profoundly suppressing ectopic proliferation and inappropriate apoptosis in the central nervous system (CNS), peripheral nervous system (PNS), placenta, and a number of other tissues including embryos17,18. E2F3 can also function as tumor suppressors and oncogenes. For instance, lacking one or two copies of E2F3 (E2F3a and E2F3b) decreased the development of pituitary tumors, but the incidence and invasion abilities of medullary thyroid carcinomas are increased19. Low expression of E2F3a was associated with inferior prognosis in childhood acute lymphoblastic leukemia14 but was associated with improved progression-free survival and overall survival of patients with ovarian cancer13. Although E2F3a has been widely reported to be involved in tumorigenesis, there are few reports on its role in gliomagenesis. E2F3a protein expression is more upregulated in glioma tissues than in normal brain tissues and is greater in grade III and grade IV than in grade II, suggesting an important role of E2F3a in gliomagenesis16. In this study, the phenotypic and molecular effects of E2F3a knockdown and overexpression on glioma cells were characterized.
E2F3a plays an important role in regulating cell proliferation and cell cycle progression. E2F3a functions mainly at the G1/S phase transition and is recognized as one of the key factors in cell cycle control. Transgenic mice expressing E2F3a have a hyperplastic and hyperproliferative epidermis and are predisposed to developing spontaneous tumors in the skin15. More recently, several miRNAs such as miR-34a, miR-20a, miR-125b, and miR-200c have been reported to inhibit cell proliferation by downregulating E2F3 in tumor cells20,21. On the other hand, E2F3a overexpression in hepatocellular carcinoma did not lead to alterations in proliferation10. Thus, not all tissues respond to deregulated E2F3a expression in the same way. In this study, by taking advantage of the level of endogenous E2F3a expression in glioma cells, E2F3a was silenced in U373 cells and was overexpressed in U251 cells. We found that silencing E2F3a results in significantly decreased proliferation and percentage of S and G2/M phase, and an increase in the percentage of the G0/G1 phase in U373 cells. In contrast, E2F3a overexpression results in increased proliferation in U251 cells. Moreover, our in vivo study showed that knockdown of E2F3a in U373 cell-injected mice showed decreased tumor growth in vivo, evidenced by decreased expression of PCNA and Ki-67. This suggests that E2F3a has a major effect on cell proliferation and cell cycle of the glioma cells both in vitro and in vivo.
Given that E2F3a plays an important role in the G1/S transition and cell proliferation, low expression of this gene in glioma cells would result in an increase in apoptosis. This finding coincided with the results of many studies. For instance, E2F3a increases the apoptosis of the epidermis through the activation of caspase 3 in a transgenic mouse model independent of p539. Very recently, E2F3a has been shown to mediate DNA damage-induced apoptosis22, which is significantly reduced by the absence of ataxia telangiectasia mutated (ATM) kinase in transgenic mouse tissue and in primary human fibroblasts15. Consistent with these results, silencing E2F3a significantly increases the apoptotic events in U373 cells and decreases apoptotic events in U251 cells, suggesting an antiapoptotic role for the E2F3a proteins in glioma cells. However, overexpression of E2F3a proteins results in an increase in apoptotic events in HepG2 cells10, suggesting a contrary role for E2F3a in cell apoptosis between these two cancers.
To determine the oncogenic role of E2F3a in glioma cells, we detected the cell invasion and migration abilities of U373 and U251 cells with the knockdown or overexpression of E2F3a. We found that the invasive and migration activities were inhibited by the downregulation of E2F3a expression, but were promoted by the upregulation of E2F3a expression. In line with our findings, several miRNAs, such as miR-144, miR-497, miR-874, and miR-203, have been reported to inhibit cell migration and invasion of tumor cells by targeting E2F323–26, and E2F3 mediates neuronal migration in a manner beyond cell cycle regulation27.
Many of the genes that are targets of E2F3a are involved in apoptosis, transcriptional control, and cell cycle regulation10,27. XAF1 and TNFSF10, proapoptotic proteins, are the two most prominent genes upregulated by E2F3a. In this study, we found four genes associated with cell apoptosis progression, including Bim, Bax, caspase 3, and caspase 9, that were upregulated by E2F3a knockdown. Bim may act by directly binding and activating Bax and Bak28. Also, previous data have shown that caspase 9 is a highly specific protease that only cleaves a few proteins, whereas caspase 3 and caspase 7 contribute to the majority of the cleavage that takes place during apoptosis29. Thus, E2F3a may inhibit the apoptosis of glioma cells through the Bim, Bax, caspase 3, and caspase 9 pathways. Moreover, four genes associated with cell motility, including MMP-2, MMP-3, MMP-9, and MMP-13, were downregulated by E2F3a knockdown. It has been shown that the enhanced expression of MMPs might favor adhesion and migration not only of glioma-infiltrating microglia but also of glioma cells. Expression of the MMP-2 in human glioma tissues is upregulated and directly correlated to glioma invasiveness and the survival rate of tumor-bearing mice30. A recent study showed that MMP-9 may play a special role in mediating glioma stem-like cells as its expression is induced by TGF-β secreted by glioma-infiltrating macrophages31. One of the main MMPs implicated in tumor invasion is the membrane-bound MMP, MMP-14, which is important for the glioma-infiltrating macrophage stimulation of GBM invasion32. MMP-19 is highly expressed in glioma tumors and enhances their invasive character in vitro33.
In summary, we demonstrated that E2F3a functions as an inhibitor of apoptosis and motility in glioma cell lines. Western blot identified 10 target genes regulated by E2F3a, which are involved in cell apoptosis, migration, and invasion. These results provide significant insights into the functions of the E2F3a and the underlying molecular mechanisms. Future studies will focus on the elucidation of the specific E2F3a target genes and their exact roles in mediating apoptosis and motility in glioma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Gladson CL, Prayson RA, Liu WM. The pathobiology of glioma tumors. Ann Rev Pathol. 2010;5:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. [DOI] [PubMed] [Google Scholar]
- 3. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. [DOI] [PubMed] [Google Scholar]
- 5. Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kalin R, van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA 2009;106:12530–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia 2012;60:502–14. [DOI] [PubMed] [Google Scholar]
- 8. Gokhman D, Livyatan I, Sailaja BS, Melcer S, Meshorer E. Multilayered chromatin analysis reveals E2f, Smad and Zfx as transcriptional regulators of histones. Nat Struct Mol Biol. 2013;20:119–26. [DOI] [PubMed] [Google Scholar]
- 9. Paulson QX, McArthur MJ, Johnson DG. E2F3a stimulates proliferation, p53-independent apoptosis and carcinogenesis in a transgenic mouse model. Cell Cycle 2006;5:184–90. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Ni GX, Zhang P, Zhang ZX, Li W, Wu Q. Characterization of E2F3a function in HepG2 liver cancer cells. J Cell Biochem. 2010;111:1244–51. [DOI] [PubMed] [Google Scholar]
- 11. Chong JL, Tsai SY, Sharma N, Opavsky R, Price R, Wu L, Fernandez SA, Leone G. E2f3a and E2f3b contribute to the control of cell proliferation and mouse development. Mol Cell Biol. 2009;29:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson AY, Feber A, Edwards S, Te Poele R, Giddings I, Merson S, Cooper CS. Role of E2F3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene 2007;26:1028–37. [DOI] [PubMed] [Google Scholar]
- 13. Reimer D, Hubalek M, Kiefel H, Riedle S, Skvortsov S, Erdel M, Hofstetter G, Concin N, Fiegl H, Muller-Holzner E, Marth C, Altevogt P, Zeimet AG. Regulation of transcription factor E2F3a and its clinical relevance in ovarian cancer. Oncogene 2011;30:4038–49. [DOI] [PubMed] [Google Scholar]
- 14. Wang KL, Mei YY, Cui L, Zhao XX, Li WJ, Gao C, Liu SG, Jiao Y, Liu FF, Wu MY, Ding W, Li ZG. E2F3a gene expression has prognostic significance in childhood acute lymphoblastic leukemia. Eur J Haematol. 2014;93:281–9. [DOI] [PubMed] [Google Scholar]
- 15. Paulson QX, Pusapati RV, Hong S, Weaks RL, Conti CJ, Johnson DG. Transgenic expression of E2F3a causes DNA damage leading to ATM-dependent apoptosis. Oncogene 2008;27:4954–61. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan X, Gong Y, Yin B, Liu W, Qiang B, Zhao J, Yuan J, Peng X. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med. 2009;87:43–51. [DOI] [PubMed] [Google Scholar]
- 17. Ziebold U, Reza T, Caron A, Lees JA. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 2001;15:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parisi T, Yuan TL, Faust AM, Caron AM, Bronson R, Lees JA. Selective requirements for E2f3 in the development and tumorigenicity of Rb-deficient chimeric tissues. Mol Cell Biol. 2007;27:2283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziebold U, Lee EY, Bronson RT, Lees JA. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol. 2003;23:6542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo Z, Dong W, Huang J, Lin T. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int J Cancer 2011;128:1758–69. [DOI] [PubMed] [Google Scholar]
- 21. Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen L, Chen H, Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J Transl Med. 2014;12:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez LA, Goluszko E, Chen HZ, Leone G, Post S, Lozano G, Chen Z, Chauchereau A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol Cell Biol. 2010;30:524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao T, Li H, Hu Y, Ma D, Cai X. miR-144 suppresses the proliferation and metastasis of hepatocellular carcinoma by targeting E2F3. Tumor Biol. 2014;35:10759–64. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Zhang Z, Li Z, Gong D, Zhan B, Man X, Kong C. MicroRNA-497 inhibits the proliferation, migration and invasion of human bladder transitional cell carcinoma cells by targeting E2F3. Oncol Rep. 2016;36:1293–300. [DOI] [PubMed] [Google Scholar]
- 25. Dong D, Gong Y, Zhang D, Bao H, Gu G. miR-874 suppresses the proliferation and metastasis of osteosarcoma by targeting E2F3. Tumor Biol. 2016;37:6447–55. [DOI] [PubMed] [Google Scholar]
- 26. Tang G, Wu J, Xiao G, Huo L. MiR-203 sensitizes glioma cells to temozolomide and inhibits glioma cell invasion by targeting E2F3. Mol Med Rep. 2015;11:2838–44. [DOI] [PubMed] [Google Scholar]
- 27. McClellan KA, Ruzhynsky VA, Douda DN, Vanderluit JL, Ferguson KL, Chen D, Bremner R, Park DS, Leone G, Slack RS. Unique requirement for Rb/E2F3 in neuronal migration: Evidence for cell cycle-independent functions. Mol Cell Biol. 2007;27:4825–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA 2003;100:2432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, Jiang XF, Yang L, Zhang P, Qian C, Cui YH, Zhang X, Bian XW. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189:444–53. [DOI] [PubMed] [Google Scholar]
- 32. Coniglio SJ, Segall JE. Review: Molecular mechanism of microglia stimulated glioblastoma invasion. Matrix Biol. 2013;32:372–80. [DOI] [PubMed] [Google Scholar]
- 33. Lettau I, Hattermann K, Held-Feindt J, Brauer R, Sedlacek R, Mentlein R. Matrix metalloproteinase-19 is highly expressed in astroglial tumors and promotes invasion of glioma cells. J Neuropathol Exp Neurol. 2010;69:215–23. [DOI] [PubMed] [Google Scholar]