Abstract
Gastric cancer (GC) is the fourth most common cancer globally. Recently, microRNAs (miRNAs) have been suggested to be closely associated with tumorigenesis. Aberrant expression of miR-509-3p has been reported in cancer studies. However, the expression and mechanism of its function in GC remain unclear. Here we showed that miR-509-3p was downregulated in GC specimens, which was associated with overall survival. Functional investigations demonstrated that the overexpression of miR-509-3p inhibited the migration and proliferation of the GC cells. Additionally, we identified X-linked inhibitor of apoptosis protein (XIAP) as a direct target of miR-509-3p. Knockdown of XIAP significantly attenuated the ability of proliferation, migration, and invasion of GC cells. The data therefore suggest that miR-509-3p plays an important role in the development and progression of GC, implicating possible applications in the clinic as a biomarker and a potential new target.
Key words: Gastric cancer (GC), MicroRNA-509-3p (miR-509-3p), X-linked inhibitor of apoptosis protein (XIAP)
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer globally1. Though the efficacy of routine chemotherapy for treating GC is not ideal, recently targeted therapies became a hot spot in GC including basic research and their clinical application. The results of the ToGA study, a clinical trial, established the efficacy of trastuzumab (anti-HER2 antibody) plus chemotherapy in patients with HER2/neu+ advanced GC2. Ramucirumab (a VEGFR-2 antibody) has revealed promising results in the treatment of patients with advanced or metastatic GC3,4. However, other trials are assessing the safety and efficacy of targeted therapies such as VEGFR inhibitors (sorafenib and bevacizumab) and EGFR inhibitors (cetuximab and erlotinib) combined with chemotherapy in patients with advanced gastric adenocarcinoma who have not ideally achieved a clinical benefit5–8. To improve the poor prognosis of GC patients, it is important to understand the molecular mechanisms underlying and supporting tumor cell survival and invasion. MicroRNAs (miRNAs) are evolutionally conserved, endogenous, noncoding, small (20–23 nucleotides) RNAs, which regulate gene expression at the posttranscriptional level9. Each miRNA seems to modulate tens to hundreds of target genes to coordinate cellular signaling pathways. miRNAs accelerate the degradation and/or block the translation of their target miRNAs to induce posttranscriptional gene repression and regulate various cellular processes such as proliferation, differentiation, metabolism, and apoptosis10. Lost expression of miRNA genes was first observed in leukemia and demonstrated the critical role of miRNAs in carcinogenesis11. Mounting evidence has shown that microRNAs (miRs; small noncoding RNAs of approximately 18–24 nucleotides in length) are key regulators of various biological processes, including bone homeostasis and bone pathologies, cancer progression, and metastasis, through mediating the posttranscriptional silencing of specifically targeted miRNAs. Recently, it was reported that miR-509-3p functions as a tumor suppressor and plays a prominent role in the development of various types of cancer, including renal cell carcinoma, breast cancer, acute lymphoblastic leukemia, lung cancer, and hepatoma. However, although there are a large number of mechanistic reports on the molecular mechanism of GC, the functions of miR-509-3p and its target gene in regulating GC progression need further study.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human GC cell lines HGC27 and MKN45 were obtained from the American Type Culture Collection (Manassas, VA, USA). Cell lines were cultivated in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplied with 10% fetal bovine serum (FBS; Gibco) at 37°C in a humidified atmosphere containing 5% CO2. miR-509-3p mimics and miR-509-3p inhibitor were from GenePharma (Shanghai, P.R. China). Human X-linked inhibitor of apoptosis protein (XIAP) siRNA and scramble siRNA were purchased from GenePharma. The GC cell line cells were transfected using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Scramble siRNA was used as the control. After 48 h, the cells were harvested for further analysis.
Tissue Samples and Patient Data
Tumor specimens and nontumorous specimens from 100 patients with GC were obtained from The First Affiliated Hospital of Zhengzhou University between 2010 and 2014. Follow-up data were obtained from a review of the patients’ medical records. None of the patients had received radiotherapy or chemotherapy before surgical resection. This study was conducted under the regulations of the Institutional Review Board of Zhengzhou Medical University. Informed consent was obtained prior to surgery from all enrolled patients.
RNA Extraction, Reverse Transcription (RT), and Quantitative Real-Time PCR
Total RNA, including the miRNA of cell lines and tissue specimens, was isolated using the TRIzol reagent (Invitrogen) following the manufacturer’s instructions. TaqMan microRNA assays (Applied Biosystems, Foster City, CA, USA) were used to determine the expression levels of miR-509-3p after reverse transcribing by sequence-specific primers (Applied Biosystems), and U6 small nuclear RNA was used as an internal control. The relative expression level (defined as fold change) of target gene (2−ΔΔCt) was normalized to the endogenous U6 reference (ΔCt) and related to the amount of target gene in the control sample, which was defined as the calibrator at 1.0. Three independent experiments were carried out to analyze the relative gene expression, and each sample was tested in triplicate.
Cell Proliferation and Colony Formation Assay
Cells (2 × 103 cells per well) were incubated in 96-well culture plates in 100 μl of medium. CCK-8 (Dojindo Laboratories, Japan) was used according to the manufacturer’s instructions. Absorbance was measured at 450 nm 2 h after the addition of 10 μl of CCK-8 reagent per well to calculate the number of viable cells using a microplate. Cell viability was shown as the percentage of viable cells with vehicle-treated cells arbitrarily set as 100% viability.
Apoptosis Assay
Cells were harvested and stained with annexin VI and suspended in 500 μl of annexin V-binding buffer. Then the cells were incubated with 5 μl of annexin V–APC for 15 min in the dark and at room temperature. The samples were analyzed by PI flow cytometry utilizing a FACScan flow cytometer (BD FACSCanto II; BD Biosciences, San Jose, CA, USA). Triplicate independent experiments were carried out.
Migration and Invasion Assay
Cells were seeded into the upper chamber of the Transwell insert (Corning, Corning, NY, USA) in 200 μl of RMPI-1640 medium, and 500 μl of RMPI-1640 medium containing 10% FBS was added into the lower wells as the chemoattractant. Twenty-four hours later, the filters were stained with crystal violet. For the invasion assay, the chambers were coated with Matrigel (BD Biosciences). Cell migration and invasion were assessed by counting the number of cells that had penetrated through the filter. The experiments were repeated at least three times.
Polycarbonate filters (8-um pore size) were coated with Matrigel (BD Biosciences, Canada) at a concentration of 1 μg/ml and placed in a 24-well cell culture plate. Hereby 1 × 105 cells per well in 100 μl of FBS-free media were seeded onto the upper part of the chamber, whereas its lower compartment was filled with media containing 10% FBS as a control. After 36 h at 37°C, cells that had invaded the Matrigel-coated membrane were fixed with 4% formaldehyde and stained using H&E reagent. Nonmigrated cells at the upper side of the filter were swiped off. A minimum of 10 fields per filter were counted.
Statistical Analysis
All statistical analyses were performed using the SPSS 17.0 (SPSS Statistics, Chicago, IL, USA) software. Data were expressed as mean ± SD and analyzed using the Student’s t-test. Paired t-test was used for paired samples. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-509-3p Was Frequently Downregulated in Gastric Cancer
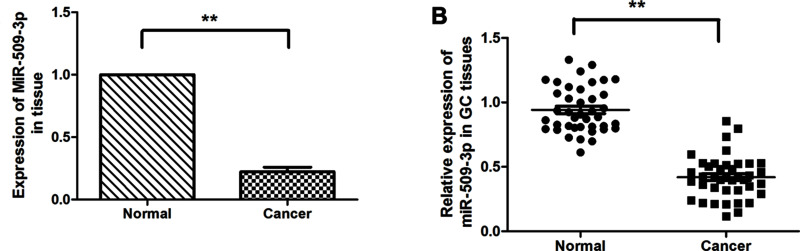
In order to investigate the role of miR-509-3p in GC, real-time PCR was done in 100 tumor tissues and 64 corresponding nontumorous tissues. Real-time PCR analysis revealed frequent downregulation of miR-509-3p expression in tumor tissues compared with neighboring noncancerous tissues (p < 0.01) (Fig. 1A). In addition, 40 paired tumor tissues and corresponding neighboring noncancerous tissues were analyzed, showing significant downregulation of miR-509-3p in tumor tissues (p < 0.001) (Fig. 1B). These data suggested that miR-509-3p might act as a tumor suppressor in human GC.
Figure 1.
miR-509-3p was significantly downregulated in gastric cancer. (A) The expression of miR-509-3p in nontumorous tissues (Normal, n = 64) and gastric cancer tissues (Cancer, n = 100) was determined by real-time PCR. (B) Expression of miR-509-3p in 64 representative gastric cancer tissues and their corresponding nontumorous tissues from the same patients were analyzed side by side for comparison.
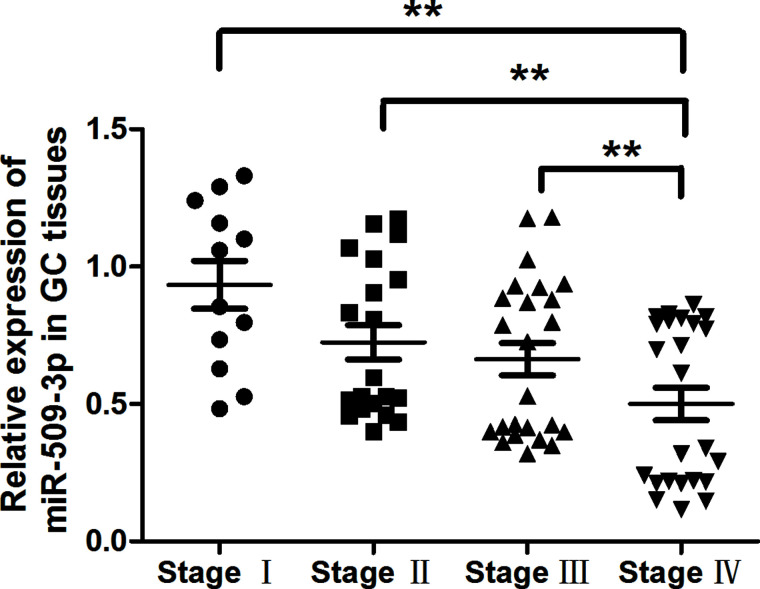
miR-509-3p Expression Related to Disease Progression
To investigate the prognostic role of miR-509-3p downregulation in GC, patients were divided into high and low miR-509-3p groups according to the median value of miR-509-3p expression in all 100 cases. Kaplan–Meier analysis revealed that the downregulation of miR-509-3p was significantly correlated with poorer outcome of GC (Fig. 2). These results suggested that miR-509-3p might play an important role in GC progression.
Figure 2.
The low expression of miR-509-3p was correlated with poor prognosis. The Kaplan–Meier curve for overall survival rate of gastric cancer patients according to expression of miR-509-3p.
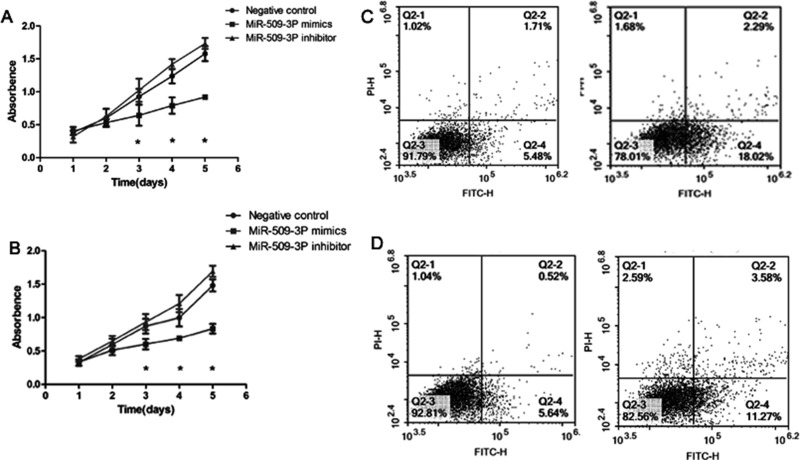
miR-509-3p Inhibited Cell Proliferation Through Inducing Cell Apoptosis In Vitro
To further assess the tumor-suppressive role of miR-509-3p in GC, miR-509-3p mimics and miR-509-3p inhibitor were used. CCK-8 results indicated that miR-509-3p overexpression could inhibit HGC27 and MKN45 cell proliferation (Fig. 3A and B). Moreover, we found that upregulation of miR-509-3p could promote cell apoptosis compared with control cells (Fig. 3C and D). These data suggested that miR-509-3p might inhibit cell proliferation by promoting apoptosis in GC cells.
Figure 3.
miR-509-3p overexpression inhibited cell proliferation by inducing cell apoptosis. Cell proliferation was detected by the CCK-8 assay at 24, 48, 72, and 96 h after transfection with NC or miR-509-3p mimics in HGC27 (A) and MKN45 (B) cells. Apoptosis analysis of HGC27 (C) and MKN45 (D) in response to overexpression of miR-509-3p was performed.
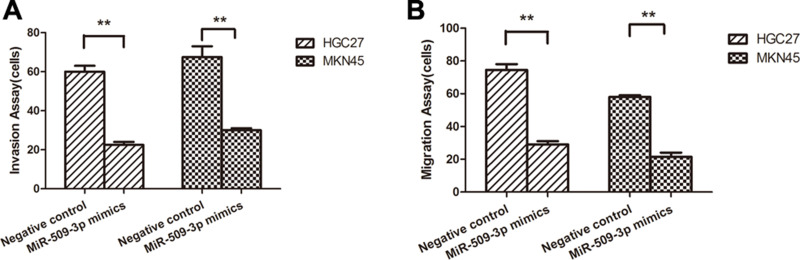
Overexpression of miR-509-3p Inhibited Cell Motility and Invasiveness In Vitro
Invasion assay was utilized to evaluate the effect of miR-509-3p on the metastatic potential of human GC. HGC27 and MKN45 were transfected with miR-509-3p mimics and then analyzed for their metastatic potential using Transwell assays. Our results showed that the migration and invasion abilities of the miR-509-3p overexpression group were significantly reduced compared with those of the control group (Fig. 4A and B). These results demonstrated that upregulation of miR-509-3p could inhibit the metastatic potential of human GC.
Figure 4.
miR-509-3p overexpression suppressed cell motility and invasiveness in vitro. Migration and invasion assays were performed using Transwell chambers after transfection with NC or miR-509-3p mimics in HGC27 (A) and MKN45 (B) cells.
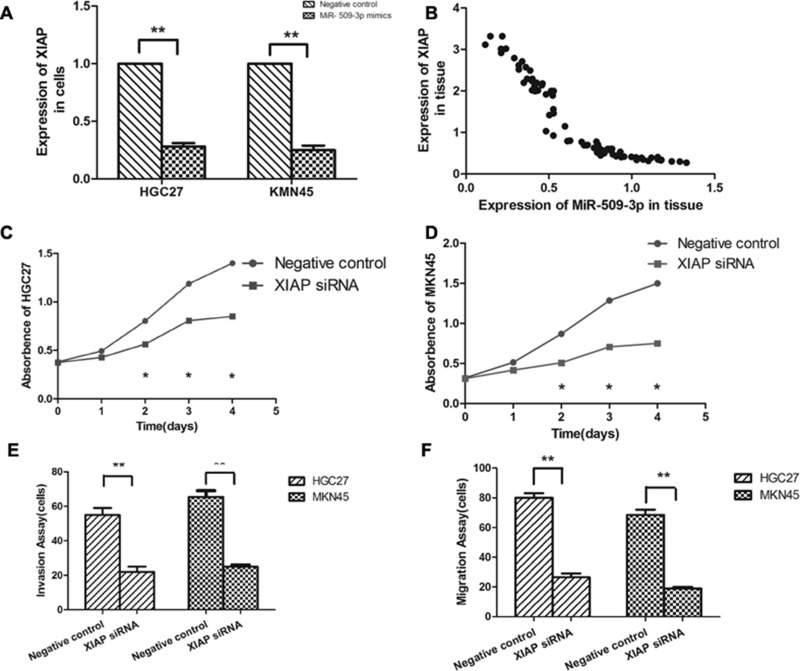
XIAP Was a Functional Target of miR-509-3p in Human Gastric Cancer
We further confirmed whether the XIAP could have been regulated by mir-509-3p in human GC cells. The results showed that miRNA expression of XIAP was significantly inhibited by the overexpression of miR-509-3p (Fig. 5A). Further analysis revealed that expression of miR-509-3p is inversely correlated with the XIAP miRNA level in human GC (Fig. 5B). We found that XIAP silencing could inhibit cell proliferation, cell motility, and invasiveness in vitro (Fig. 5C–F). These results showed that XIAP has an opposite effect on cell proliferation and migration, in comparison with miR-509-3p, indicating that it may be a functional target of miR-509-3p in human GC cells.
Figure 5.
XIAP was a functional target of miR-509-3p in human gastric cancer. (A) Transfection of miR-509-3p mimics in gastric cancer cells significantly inhibited XIAP miRNA expression levels, as assessed by qRT-PCR analysis. (B) The correlation between the relative miR-509-3p expression and the relative XIAP expression in gastric cancer specimens was analyzed. Cell proliferation was detected by the CCK-8 assay at 0, 24, 48, 72, and 96 h after transfection with NC or XIAP siRNA in HGC27 (C) and MKN45 (D) cells. Transwell chambers were used to analyze the migration (F) and invasion (E) capabilities after transfection with NC or XIAP siRNA in HGC27 and MKN45 cells.
DISCUSSION
While cancer treatment and survival have improved worldwide, the need for further understanding of the underlying tumor biology remains. In recent years, there has been a significant shift in scientific focus toward the role of miRNAs on the development, growth, and metastatic spread of malignancies. miRNAs are a class of evolutionally conserved, small (18–25 nucleotides), noncoding RNAs that have an important function in posttranscriptional gene regulation12. More recent identifications reveal that hundreds of miRNAs are aberrantly expressed in cancerous tissues through high-throughput biochemical screens13,14. For example, miR-320a has been reported to be deregulated in multiple types of cancers, including intrahepatic cholangiocarcinoma15. Ning et al. demonstrated that miR-7-5p suppresses the growth and metastasis of several tumor types, including hepatocellular carcinoma, breast cancer, glioma, and GC, by inhibiting the expression of specific target genes, including EGFR and its signaling pathways16. Raffel et al. demonstrated miR-126 as a regulator of PI3K–AKT–mTOR and CDK3 signaling driving leukemic stem cells to self-renewal and chemotherapy resistance17. In addition, >50% of human miRNA genes are located in cancer-associated genomic regions or at fragile sites.
The current study revealed that miR-509-3p was significantly downregulated in GC tissue specimens when compared with normal tissues. Moreover, our results showed that overexpression of miR-509-3p inhibited the cell proliferation and cell motility of GC cells. These functional effects correlate with the in vitro data reported by Yoon et al.18, where they showed that overexpression of miR-509-3p induced G1 cell cycle arrest and inhibited colony formation and migration through downregulation of CDK2, Rac1, and PIK3C2A. We also found that low levels of miR-509-3p expression significantly correlated with poor GC outcomes. This evidence suggested that the decreased expression of miR-509-3p might be associated with GC tumorigenesis. XIAP is the most potent inhibitor of apoptosis-919. XIAP binds and inactivates effector caspases 3 and 7 and initiator caspase 920. XIAP also functions as an E3 ligase, inducing the degradation of caspases via the proteasome system. It has been reported that XIAP expression is elevated in many cancers21. Thus, downregulation of XIAP is recognized as an efficient anticancer approach22. Recently, it was reported that miR-509-3p may function as a tumor suppressor in renal cancer23. The expression level of miR-509-3p is lower in renal cancer than in the adjacent normal tissues, and ectopic expression of miR-509-3p inhibits renal cell growth and migration. In epithelial ovarian cancer, miR-509-3p (a downregulated miRNA) can directly target the XIAP via its 3′-UTR24.
Generally, miRNAs are believed to function by binding to the 3′-UTRs of target genes. We found that miR-509-3p negatively regulated XIAP expression. We further showed that miR-509-3p and XIAP were inversely correlated in human GC specimens. These results further confirmed that XIAP was a target gene of miR-509-3p in GC cells. Downregulation of XIAP could significantly attenuate the ability for the proliferation, migration, and invasion of GC cells.
In conclusion, our study demonstrated that miR-509-3p was frequently downregulated in GC compared with normal tissues and that low levels of miR-509-3p expression correlated with poor GC outcomes. XIAP was a target of miR-509-3p, and miR-509-3p functioned as a negative regulator of XIAP. Together, miR-509-3p, considered as a tumor-suppressor gene, inhibits cell proliferation and invasion by targeting XIAP in GC, providing new insights and the basis for the development of miRNA-targeted therapies for GC.
ACKNOWLEDGMENTS
This study was supported by the Department of Gastrointestinal Surgery and Institute of Clinical Medicine, The First Affiliated Hospital, Zhengzhou University. Author responsibilities: Jihong Sun and Jingjing Li made a significant contribution to the study and to the writing of the manuscript. Daguo Wan contributed to the study and to the statistical analysis. Juan Zhang obtained the specimens. Shenjie Sun, Guiqi Li, and Hengliang Song contributed to the study.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. [DOI] [PubMed] [Google Scholar]
- 2. Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–9. [DOI] [PubMed] [Google Scholar]
- 3. Wagner AD, Moehler M. Development of targeted therapies in advanced gastric cancer: Promising exploratory steps in a new era. Curr Opin Oncol. 2009;21:381–5. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van-Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 5. Pinto C, Di-Fabio F, Siena S, Cascinu S, Rojas-Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C, Berardi R, Longobardi C, Piana E, Martoni AA. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol. 2007;18:510–7. [DOI] [PubMed] [Google Scholar]
- 6. Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD, Abbruzzese JL. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–7. [DOI] [PubMed] [Google Scholar]
- 7. Katoh M. Genetic alterations of FGF receptors: An emerging field in clinical cancer diagnostics and therapeutics. Expert Rev Anticancer Ther. 2010;10:1375–9. [DOI] [PubMed] [Google Scholar]
- 8. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. [DOI] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, Dell-Aquila ML, Alder H, Rassenti L, Kipps TJ, Bullrich F, Negrini M, Croce CM. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA 2004;101:11755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, Perez-Ordonez B, Jurisica I, O’Sullivan B, Waldron J, Gullane P, Cummings B, Liu FF. Comprehensive microRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res. 2010;16:1129–39. [DOI] [PubMed] [Google Scholar]
- 14. Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, Van-Kouwenhove M, Kreike B, Sie D, Hovestadt V, Wessels LF, Vande-Vijver MJ, Tuschl T. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L, Yan HX, Yang W, Hu L, Yu LX, Liu Q, Li L, Huang DD, Ding J, Shen F, Zhou WP, Wu MC, Wang HY. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50:358–69. [DOI] [PubMed] [Google Scholar]
- 16. Ning BF, Ding J, Liu J, Yin C, Xu WP, Cong WM, Zhang Q, Chen F, Han T, Deng X, Wang PQ, Jiang CF, Zhang JP, Zhang X, Wang HY, Xie WF. Hepatocyte nuclear factor 4α-nuclear factor-κB feedback circuit modulates liver cancer progression. Hepatology 2014;60:1607–19. [DOI] [PubMed] [Google Scholar]
- 17. Raffel S, Trumpp A. miR-126 drives quiescence and self-renewal in leukemic stem cells. Cancer Cell 2016;29:133–5. [DOI] [PubMed] [Google Scholar]
- 18. Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein—A critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreno-Martínez D, Nomdedeu M, Lara-Castillo MC, Etxabe A, Pratcorona M, Tesi N, Díaz-Beyá M, Rozman M, Montserrat E, Urbano-Ispizua A, Esteve J, Risueño RM. XIAP inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells. Oncotarget 2014;5:4337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li G, Chang H, Zhai YP, Xu W. Targeted silencing of inhibitors of apoptosis proteins with siRNAs: A potential anti-cancer strategy for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:4943–52. [DOI] [PubMed] [Google Scholar]
- 22. Zhai Q, Zhou L, Zhao C, Wan J, Yu Z, Guo X, Qin J, Chen J, Lu R. Identification of miR-508-3p and miR-509-3p that are associated with cell invasion and migration and involved in the apoptosis of renal cell carcinoma. Biochem Biophys Res Commun. 2012;419:621–6. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Zeng W, Li X, Xiong W, Zhang M, Huang Y, Zhou L, Jiang S. MicroRNA-509-3p increases the sensitivity of epithelial ovarian cancer cells to cisplatin-induced apoptosis. Pharmacogenomics 2016;17:187–97. [DOI] [PubMed] [Google Scholar]