Abstract
A Wnt agonist, 2-amino-4-[3,4-(methylenedioxy)benzylamino]-6-(3-methoxyphenyl) pyrimidine, is a cell-permeable pyrimidine compound that has been shown to mimic the effect of Wnt. In this study, leukemic mouse cell lines, RAW 264.7 and J774.1, were incubated with the Wnt agonist. The Wnt agonist showed cell death in the concentration of 1–10 μM. The Wnt agonist did not show inhibition of GSK-3β activity but induced β-catenin accumulation in the nucleus. The Wnt agonist showed caspase-independent cell death, but no further involvement in cell death ER stress signaling. Here we discuss the possible mechanism of Wnt agonist-induced apoptotic cell death in RAW 264.7 cells.
Key words: RAW 264.7 cells, Wnt agonist, Cytotoxicity, β-Catenin, Endoplasmic reticulum (ER) stress
INTRODUCTION
Wnt signaling is known to be involved in embryonic development and in the pathogenesis of cancer1–4. Furthermore, gene mutations of Wnt signaling molecules, which lead to β-catenin accumulation in the cytoplasm or nucleus of cells, have been reported in many kinds of cancer2,3. In this study, we found that the binding of the Wnt receptor and its ligand leads to dephosphorylation of GSK-3β in the Wnt signal pathway, and then β-catenin is stabilized to be transferred to the nucleus2. We examined the cell growth effect of an artificial Wnt mimic reagent in mouse leukemic cell lines RAW 264.7 cells and J774.1. It was found that this Wnt activator reagent led to cell death dose dependently, not to cell growth in RAW 264.7 cells and J774.1. This Wnt activator reagent leads to β-catenin accumulation in the nucleus as physiological Wnt agonists do. Although physiological a Wnt agonist affects GSK-3β activity1, this Wnt activation reagent does not affect GSK-3β. Cell death by this Wnt activation reagent was independent of caspase and endoplasmic reticulum (ER) stress. This suggests that the Wnt/β-catenin signal caused apoptotic cell death rather than cell growth, which depends on the cell type or dose of the Wnt agonist.
MATERIALS AND METHODS
Reagents
Lipopolysaccharide (LPS) from Escherichia coli O55:B5 was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to β-catenin, caspase 3, cleaved caspase 3, PARP [poly(ADP-ribose) polymerase], cleaved PARP, phosphor-PERK (protein kinase R-like endoplasmic reticulum kinase), CHOP (CCAAT-enhancer-binding protein homologous protein), eIF-2α (eukaryotic translation initiation factor), phospho-eIF-2α, CREB, and anti-rabbit IgG antibody as a second antibody were purchased from Cell Signaling Technology (Beverly, MA, USA). Wnt agonist was obtained from Calbiochem (Cat. No. 681665; San Diego, CA, USA). The antibody to β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Caspase inhibitor (Z-VAD-FMK) and caspase 3 inhibitor (DEVD-FMK and IETD-FMK) were obtained from MBL (Nagoya, Japan).
The appropriate concentrations of inhibitors and antibodies were determined in preliminary experiments.
Cell Culture
RAW 264.7 and J774.1 murine leukemic monocytes were maintained in RPMI-1640 medium (Sigma-Aldrich) containing 5% heat-inactivated fetal calf serum and antibiotics. Cells were cultured in 60-mm dishes (1 × 106 cells/ml) or 96-well (5 × 104 cells/100 μl/well) plastic plates (Falcon, San Jose, CA, USA) for 24 h.
Cell Viability and Cell Death
Cell viability was assessed by an MTT assay, and cell death was assessed by a trypan blue dye exclusion test5. RAW 264.7 and J774.1 cells were treated with various concentrations of Wnt agonist in a 96-well plate for 24 h. Cell viability was determined by MTT activity using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (Dojindo, Kumamoto, Japan) according to the instructions. The optical density at 570 nm was determined with a microplate reader. All measurements were corrected for interference of the Wnt agonist at this wavelength. Trypan blue dye exclusion was tested 24 h after Wnt addition, as previously described. Dead cells (%) were counted as the frequency of stained cells in total cells.
Immunoblot
Immunoblot analysis was carried out as described previously6. Briefly, cell pellets were suspended at a concentration of 2 × 107/ml in a lysis buffer. Cell lysates were diluted with an equal volume of sample buffer and boiled for 5 min. Samples were separated under reducing conditions by electrophoresis using 6% or 10% polyacrylamide gel. The membranes where proteins were transferred to were treated with a variety of appropriately diluted antibodies and a horseradish peroxidase-conjugated second antibody. Finally, labeled antigen bands were detected with a chemiluminescence reagent (SuperSignal® West Dura; Pierce, Rockford, IL, USA) and analyzed using an AE6955 light capture system with CS analyzer® (Atto, Tokyo, Japan). Prestained protein markers from Gibco BRL were used to estimate molecular mass.
Preparation of Nuclear Extracts
Nuclear extracts were prepared with nuclear extraction reagents (Active Motif; Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, cells were lysed in a hypotonic buffer. After centrifugation, pellets were lysed in nuclear extraction reagents for the extraction of nuclear proteins. After centrifugation, nuclear proteins (40 μg) were subjected to immunoblot for the detection of β-catenin. Nuclear protein concentration was measured using BCA protein assay reagent and comparative reagents (Pierce, Rockford, IL, USA). Protein concentration was determined by a BCA protein assay reagent kit (Pierce). CREB was used as a loading control of nuclear protein.
Detection of Apoptotic Cells
RAW 264.7 cells were cultured with Wnt agonist for 24 h. The cells were stained with the apoptosis detection kit using FITC-conjugated annexin V and propidium iodide (PI) (MBL). The stained cells were analyzed on a laser flow cytometer (FACSCalibur; Becton Dickinson, Palo Alto, CA, US). The fluorescence intensity has been expressed in a log scale.
Statistical Analysis
Statistical analysis was performed using Student’s t-test, and values of p < 0.01 were considered significant. All experiments were performed independently more than three times. The data represent the mean value of triplicates ± SD.
RESULTS
A Wnt Agonist Leads to Loss of Cell Growth Activity
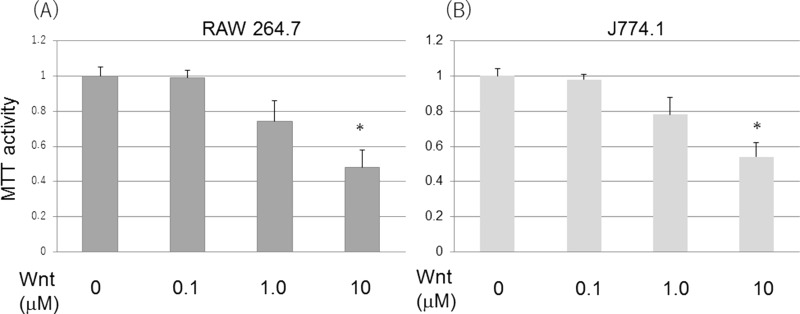
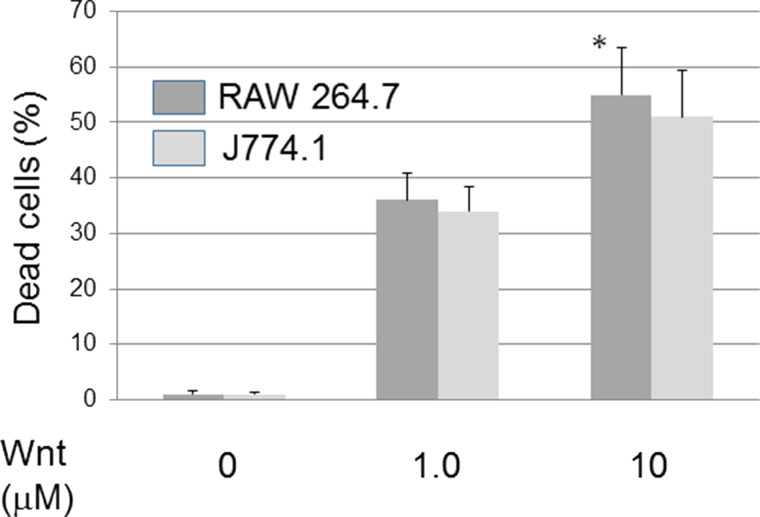
A Wnt agonist, 2-amino-4-[3,4-(methylenedioxy)benzylamino]-6-(3-methoxyphenyl) pyrimidine, was added to mouse leukemic cell lines RAW 264.7 and J774.1 cells. Unexpectedly, both cells showed loss of cell viability by the Wnt agonist dose dependently (Fig. 1). In a concentration of less than 0.1 μM, the Wnt agonist did not show loss of cell viability. Next, the dye exclusion test was done to see if the Wnt agonist caused cell death (Fig. 2). In cooperation with cell viability loss by MTT assay, a dye exclusion test showed that loss of cell viability was dependent on cell death induction by the Wnt agonist.
Figure 1.
Wnt agonist induced loss of cell viability. Wnt induced cell viability loss dose dependently in RAW 264.7 (A) and J774.1 cells (B). Cells were incubated with Wnt agonist for 24 and 48 h. Cell viability was determined by MTT assay. *p < 0.05, significantly different from control.
Figure 2.
Wnt agonist induced cell death in RAW 264.7 cells. Wnt induced cell death dose dependently. Cells were incubated with Wnt agonist. Cell death was determined by a dye exclusion test. *p < 0.05, significantly different from control.
A Wnt Agonist Leads to Accumulation of β-Catenin in Nucleus
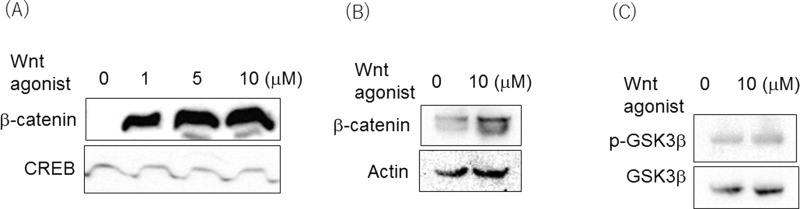
In Wnt signaling, the binding of the Wnt receptor and its ligand leads to loss of GSK-3β activity and β-catenin stabilization. Therefore, we examined whether the Wnt agonist led to β-catenin accumulation in the nucleus. The Wnt agonist induced significant β-catenin accumulation at a concentration of 1–10 μM (Fig. 3A). Even in whole-cell lysates, β-catenin expression was increased in the 10-μM concentration (Fig. 3B). Next, it was examined whether the Wnt agonist changes GSK-3β activity. When the activity of GSK-3β was seen by the phosphorylation status, the Wnt agonist did not change the phosphorylation (Fig. 3C). This suggested that the Wnt agonist induced β-catenin accumulation in the nucleus, independent of GSK-3β activity.
Figure 3.
Wnt induced β-catenin accumulation in the nucleus. (A) Wnt induced β-catenin accumulation in the nucleus dose dependently. (B) β-Catenin expression in whole-cell lysate. Immunoblot was done to see nuclear translocation. CREB was used as a loading control of nuclear protein. (C) Activity of GSK-3β was not changed by Wnt agonist. Phosphorylation of GSK-3β was determined by Immunoblot.
A Wnt Agonist Induced ER Stress and Caspase-Independent Cell Death
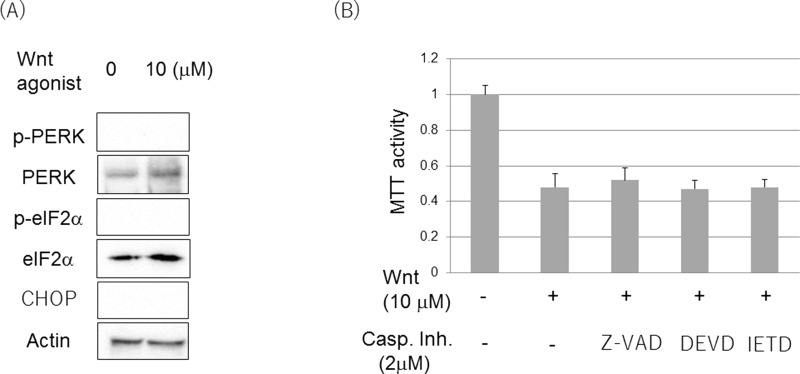
In our previous study7, the involvement of ER stress and β-catenin in cell death of LPS-stimulated RAW 264.7 cells was discussed. As we discovered β-catenin involvement in the Wnt agonist-induced cell death in this study, it was examined whether ER stress was involved in the Wnt agonist-induced cell death. It was determined, however, that the PERK/eIF-2α ER stress pathway was not activated and CHOP was not induced when the Wnt agonist was added (Fig. 4A). Furthermore, caspase inhibitor (Z-VAD-FMK) or caspase 3 inhibitor (DEVD-FMK and IETD-FMK) did not suppress Wnt-induced cell viability loss (Fig. 4B). In addition, cleaved caspase 3 was not detected in whole-cell lysate of Wnt agonist treatment by immunoblot (data not shown). This suggested that cell death by a Wnt agonist was not dependent on ER stress and caspase.
Figure 4.
Wnt agonist induced ER stress- and caspase-independent cell death. (A) Phosphorylation of PERK and eIF2α, and expression of CHOP were determined by immunoblot. DMSO alone was used as control. Cells were incubated with 10 μM of Wnt agonist for 1 h for identifying PERK/eIF2α and 24 h for CHOP. (B) Caspase (Z-VAD-FMK) or caspase 3 inhibitors (DEVD-FMK and IETD-FMK) did not influence Wnt-induced loss of cell viability. Cell viability was determined by MTT assay. *p < 0.05, significantly different from control.
A Wnt Agonist Induced Apoptotic Cell Death
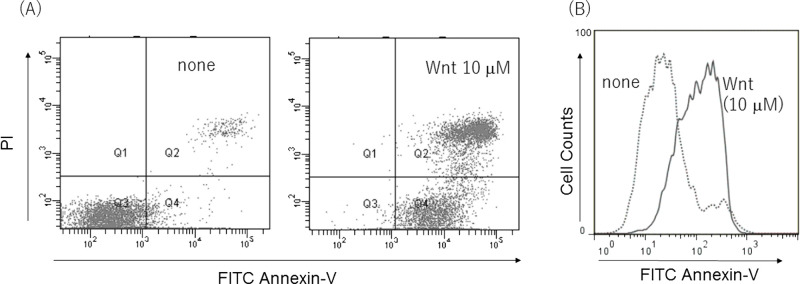
To confirm whether cell death induced by the Wnt agonist was apoptotic cell death, an apoptosis detection kit using annexin V-PI (propidium iodide) was performed. As expected, cell death induced by Wnt agonist was annexin V+ apoptotic cell death (Fig. 5A and B). Considering the previous studies, the Wnt agonist induced ER stress- and caspase-independent apoptotic cell death.
Figure 5.
Wnt agonist induced apoptotic cell death. Wnt agonist induced annexin V+ and/or PI+ cell population. (B) Wnt agonist induced annexin V+ cells in histogram. Wnt-induced apoptotic cell death was detected by annexin V-PI staining kit.
DISCUSSION
In this study, for the first time, a Wnt mimic reagent (Wnt agonist) induced apoptotic cell death through β-catenin accumulation in the nucleus in a murine leukemic cell line, RAW 264.7 cells. In a previous study7, we showed that β-catenin accumulation was involved in LPS-induced cytotoxicity. Furthermore, ER stress was related to cell death. However, different from previous reports, we found that Wnt agonist-induced cell death was not involved in ER stress-related cell death.
In the Wnt signal pathway, the binding of Wnt receptor and its ligand leads to dephosphorylation of GSK-3β, and then β-catenin was dephosphorylated and stabilized to be transferred to the nucleus2,8. The exact mechanism is still unknown, but this Wnt agonist did not change the status of GSK-3β and then transferred β-catenin to the nucleus. This suggested that this Wnt agonist somehow stabilized β-catenin with inhibiting β-catenin degradation in proteasome.
β-Catenin is often related to cell growth and carcinogenesis1,3. On the other hand, it has been reported that augmented β-catenin accumulation induced by PKC inhibitor in a human hematologic cancer causes cell death9. Therefore, whether β-catenin accumulation in the nucleus is cytotoxic might depend on the cell type or the degree of accumulation. Considering that RAW 264.7 and J774.1 cells are mouse leukemic cell lines, hematopoietic cancer cells might be cytotoxic sensitive to β-catenin accumulation.
In conclusion, we show that changing the β-catenin expression by Wnt agonist-influenced cell viability is likely via a non-ER stress and caspase-independent pathway in murine leukemic cell lines like RAW 264.7. This suggests a new aspect of β-catenin, an oncoprotein in murine leukemic cell lines.
Most chemicals can induce apoptosis at high concentrations. Therefore, the possibility that this pyrimidine compound induces apoptosis not via Wnt activation cannot be excluded, since we have not carried out the experiment with protein overexpression. However, this chemical induces apoptosis at the concentrations to increase the expression of β-catenin. Therefore, it is also possible that this chemical induces apoptosis and cell death in RAW264.7 and J774.1 cells.
Cell death induced by the Wnt activator was not reversed by caspase inhibitors. It is likely, therefore, that cell death or apoptosis is independent of caspase activation. However, the apoptosis may be lowered by these inhibitors if we employ lower concentrations of the pyrimidine compound.
ACKNOWLEDGMENTS
All the authors contributed to the work described in the article. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and a grant of MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2011–2015 (S1101027). We are grateful to Akiko Morikawa for technical assistance.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Tarapore RS, Siddiqui IA, Mukhtar H. Modulation of Wnt/β-catenin signaling pathway by bioactive food components. Carcinogenesis 2012;33:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192–205. [DOI] [PubMed] [Google Scholar]
- 3. Yeramian A, Moreno-Bueno G, Dolcet X, Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J, Matias-Guiu X. Endometrial carcinoma: Molecular alterations involved in tumor development and progression. Oncogene 2013;32:403–13. [DOI] [PubMed] [Google Scholar]
- 4. He F, Chen Y. Wnt signaling in lip and palate development. Front Oral Biol. 2012;16:81–90. [DOI] [PubMed] [Google Scholar]
- 5. Koide N, Sugiyama T, Mu MM, Mori I, Yoshida T, Hamano T, Yokochi T. Gamma interferon-induced nitric oxide production in mouse CD5+ B1-like cell line and its association with apoptotic cell death. Microbiol Immunol. 2003;47:669–79. [DOI] [PubMed] [Google Scholar]
- 6. Koide N, Morikawa A, Tumurkhuu G, Dagvadorj J, Hassan F, Islam S, Naiki Y, Mori I, Yoshida T, Yokochi T. Lipopolysaccharide and interferon-gamma enhance Fas-mediated cell death in mouse vascular endothelial cells via augmentation of Fas expression. Clin Exp Immunol. 2007;150:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koide N, Naiki Y, Odkhuu E, Tsolmongyn B, Komatsu T, Ito K, Yoshida T, Yokochi T. Involvement of oncogenic protein β-catenin in LPS-induced cytotoxicity in mouse mononuclear leukemia RAW 264.7 cells. Oncol Res. 2013;21:59–65. [DOI] [PubMed] [Google Scholar]
- 8. Lee H, Bae S, Choi BW, Yoon Y. WNT/β-catenin pathway is modulated in asthma patients and LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol Immunotoxicol. 2012;34:56–65. [DOI] [PubMed] [Google Scholar]
- 9. Raab MS, Breitkreutz I, Tonon G, Zhang J, Hayden PJ, Nguyen T, Fruehauf JH, Lin BK, Chauhan D, Hideshima T, Munshi NC, Anderson KC, Podar K. Targeting PKC: A novel role for beta-catenin in ER stress and apoptotic signaling. Blood 2009;113:1513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]