Abstract
Latent transforming growth factor-β (TGF-β)-binding protein 2 (LTBP2) is one of four proteins in the LTBP family of proteins (LTBP1–4) and was shown to play a vital role in tumorigenesis. However, little is known regarding the functional role of LTBP2 in thyroid carcinoma. Therefore, the current study aimed to evaluate the effect of LTBP2 expression on the proliferation, invasion, and tumorigenesis in thyroid carcinoma cells and to explore the molecular mechanism of LTBP2 in tumor progression. Our results showed that the expression of LTBP2 is upregulated in human thyroid carcinoma and cell lines. Knockdown of LTBP2 inhibits the proliferation, invasion, and EMT phenotype in thyroid carcinoma cells. Furthermore, knockdown of LTBP2 attenuates thyroid carcinoma growth in nude mice. Finally, knockdown of LTBP2 inhibits activation of the PI3K/Akt pathway in thyroid carcinoma cells. In summary, the present study has provided further evidence that knockdown of LTBP2 inhibits invasion and tumorigenesis in thyroid carcinoma cells. Our findings may help to further elucidate the molecular mechanisms underlying thyroid carcinoma progression and provide candidate targets for the prevention and treatment of thyroid carcinoma.
Key words: Thyroid carcinoma, Latent transforming growth factor-β (TGF-β)-binding protein 2 (LTBP2), Invasion, PI3K/Akt pathway
INTRODUCTION
Thyroid carcinoma is the most common malignant tumor involving the endocrine system. In China, the incidence of thyroid carcinoma has been experiencing a significant rise in recent years1,2. Current treatment approaches for thyroid carcinoma include surgery, chemotherapy, and radiotherapy, but most patients have a poor outcome3–5. Thus, elucidation of the molecular mechanisms underlying thyroid carcinoma tumorigenesis and progression is critical to individual treatment of thyroid carcinoma.
Latent transforming growth factor-β (TGF-β)-binding proteins (LTBPs) belong to the LTBP/fibrillin superfamily, a group of high-molecular weight ECM proteins that contain several eight-cysteine repeats. LTBP2 is one of four in the LTBP family of proteins (LTBP1–4)6. Previous studies have shown that LTBP2 may play an important role in regulating elastogenesis in the aorta and other fibrillin-rich tissues7,8, cell adhesion9, and embryonic development10. In addition, LTBP2 expression has been observed in a variety of malignancies, such as in nasopharyngeal carcinoma, esophageal squamous cell carcinoma, melanoma, and ovarian cancer, and its overexpression is associated with tumor progression and a poor prognosis11–14. Ren et al. reported that the expression of LTBP2 was higher in cervical adenocarcinoma than in normal cervical epithelial tissue, and knockdown of LTBP2 expression can inhibit the proliferation and migration of HeLa cells15. However, little is known regarding the functional role of LTBP2 in thyroid carcinoma. Therefore, the current study aimed to evaluate the effect of LTBP2 expression on the proliferation, invasion, and tumorigenesis in thyroid carcinoma cells and to explore the molecular mechanism of LTBP2 in tumor progression.
MATERIALS AND METHODS
Patients and Tissue Samples
Frozen tumor samples from biopsy specimens were collected from 12 patients with thyroid carcinoma undergoing surgery at the Linyi Tumor Hospital (P.R. China). None of these patients received chemotherapy or radiotherapy before the surgery. The present study was approved by the medical ethics committee of the Linyi Tumor Hospital, and informed consent was obtained from each patient.
Cell Culture
Human thyroid carcinoma cell lines (BCPAP, FTC133, and 8305C) as well as normal thyroid epithelial cell-derived cell line HTori-3 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). They were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 μg/ml penicillin, and 0.1 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere of 5% CO2 at 37°C.
RNA Interference and Transfection
Short hairpin RNA (shRNA) targeting LTBP2 (sh-LTBP2) and nontargeting control (mock) were designed and synthesized by GeneChem (Shanghai, P.R. China). FTC133 cells at a density of 5 × 104 cells/well were transfected with sh-LTBP2 or mock using Lipofectamine 2000 (Invitrogen), respectively, according to the manufacturer’s instructions. The transfection efficiency was confirmed by quantitative real-time polymerase chain reaction (RT-qPCR) and Western blotting.
RNA Extraction and Quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from thyroid carcinoma tissues or cell lines and reverse transcribed to cDNA using the RNeasy Mini Kit (Invitrogen) according to the manufacturer’s instructions. RT-qPCR analysis was performed in triplicate on the ABI PRISM 7000 sequence detection system (Applied Biosystems, Eugene, OR, USA) using the SYBR Green PCR Master Mix (Applied Biosystems). The following primers were used: LTBP2, 5′-TTA CAA GCA GAG ACT CAC T-3′ (sense) and 5′-ACA ACA GAA GAG ACC AGA T-3′ (antisense); β-actin, 5′-CCGTGAAAAGATGACCCAGATC-3′ (sense) and 5′-CACAGCCTGGATGGCTACGT-3′ (antisense). Relative quantities of all tested genes in various samples were calculated by the 2−ΔΔCt method.
Western Blotting
Total protein was extracted from thyroid carcinoma tissues or cell lines using radioimmunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich). Thirty micrograms of total proteins from each sample was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was then blocked with 2.5% nonfat dry milk for 1 h and then incubated with primary antibodies at 4°C overnight. The primary antibodies used were mouse anti-LTBP2 (1:1,000), mouse anti-E-cadherin (1:2,500), mouse anti-N-cadherin (1:1,500), mouse anti-vimentin (1:3,000), mouse anti-PI3K (1:2,000), mouse anti-p-PI3K (1:2,000), mouse anti-Akt (1:3,000), mouse anti-p-Akt (1:3,000), and mouse anti-GAPDH (1:3,000). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). After incubation with the corresponding horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), the protein bands were visualized with enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA).
Cell Proliferation Assay
Cell proliferation was assessed with a cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). In brief, FTC133 cells were plated at a density of 1 × 104 cells/well on 96-well plates. At 24, 48, 72, and 96 h after transfection, the CCK-8 reagents were added and incubated with the cells for 1 h. The absorbance of each well was determined at 490 nm with an enzyme-linked immunosorbent assay plate reader.
Cell Migration and Invasion Assay
In vitro Transwell migration assays were performed in modified Boyden chambers with 8-mm pore filter inserts in 24-well plates (BD Biosciences, Eugene, OR, USA). Briefly, FTC133 cells transfected with sh-LTBP2 or mock suspended in 0.1% FBS medium were seeded in the upper compartment, and the lower chamber was filled with DMEM containing 10% FBS. After 24 h of incubation, cells that migrated to the lower surface of the filters were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min, and the number of cells that had migrated through the pores was quantified by counting six independent visual fields under the microscope (Olympus, Tokyo, Japan) using a 40× objective. The invasion assay was done using the same procedure, except that the filters were precoated with 100 ml of Matrigel at a 1:4 dilution in DMEM to form a genuine reconstituted basement membrane.
In Vivo Xenograft Tumor Assay
This study was performed with approval from the animal ethics committee of Linyi Tumor Hospital. Female BABL/c nude mice (4–6 weeks, 18–22 g) were purchased from the Laboratory Animal Centre of Linyi Tumor Hospital. The mice were maintained under pathogen-free conditions at 22°C, with 40%–50% humidity and a 12-h light/dark cycle, and allowed access to food and water ad libitum. FTC133 cells (1 × 106 cells/0.1 ml) transfected with sh-LEBP2 or mock were injected subcutaneously into the flanks of nude mice (n = 5 per group). Tumor size was measured every 5 days using a caliper and calculated using the formula: volume = length × width2 × π/6. The mice were sacrificed 25 days after inoculation, and tumors were excised, measured, and weighed.
Statistical Analysis
All data were expressed as the mean ± SD of three independent experiments. Statistical analysis was performed using one-way ANOVA. A value of p < 0.05 was considered statistically significant.
RESULTS
LTBP2 Is Highly Expressed in Human Thyroid Carcinoma Tissues and Cell Lines
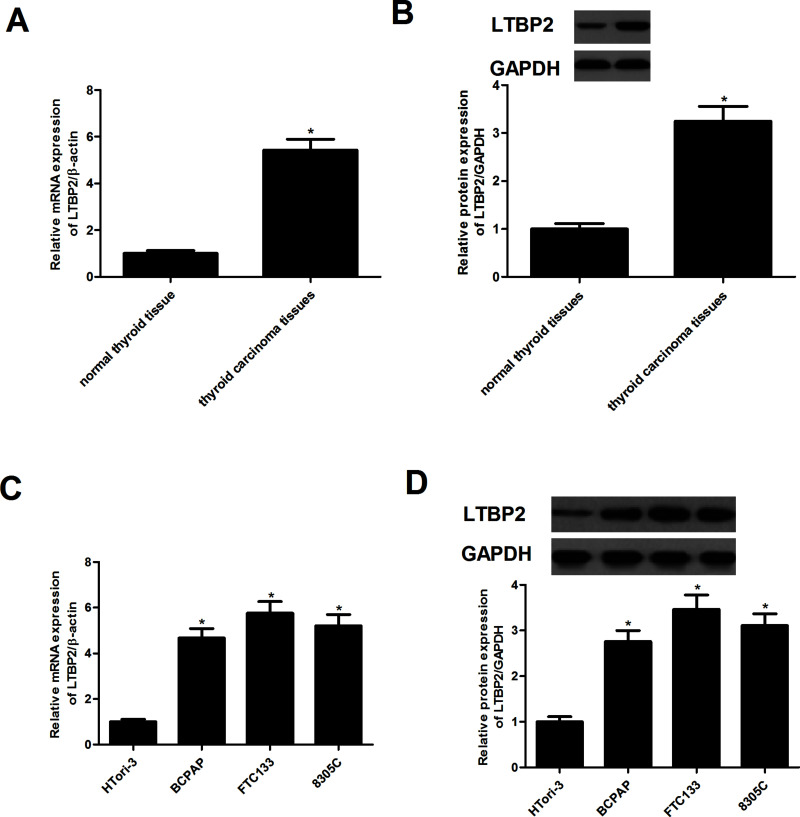
We first assessed the expression of LTBP2 in human thyroid carcinoma tissues by using RT-qPCR and Western blotting. Compared with normal thyroid tissue, the expression of LEBP2 at both mRNA and protein levels was significantly higher in human thyroid carcinoma tissues (Fig. 1A and B). We next examined LTBP2 expression in thyroid carcinoma cell lines. The results demonstrated that the expression of LTBP2 was also significantly increased in thyroid carcinoma cell lines (Fig. 1C and D).
Figure 1.
LTBP2 is highly expressed in human thyroid carcinoma tissues and cell lines. The mRNA expression of LTBP2 in human thyroid carcinoma tissues (A) and cell lines (C) was analyzed by RT-qPCR. The protein expression of LTBP2 in human thyroid carcinoma tissues (B) and cell lines (D) was analyzed by Western blotting. Experiments were performed in triplicate. *p < 0.05 versus control.
Knockdown of LTBP2 Inhibits the Proliferation in Thyroid Carcinoma Cells
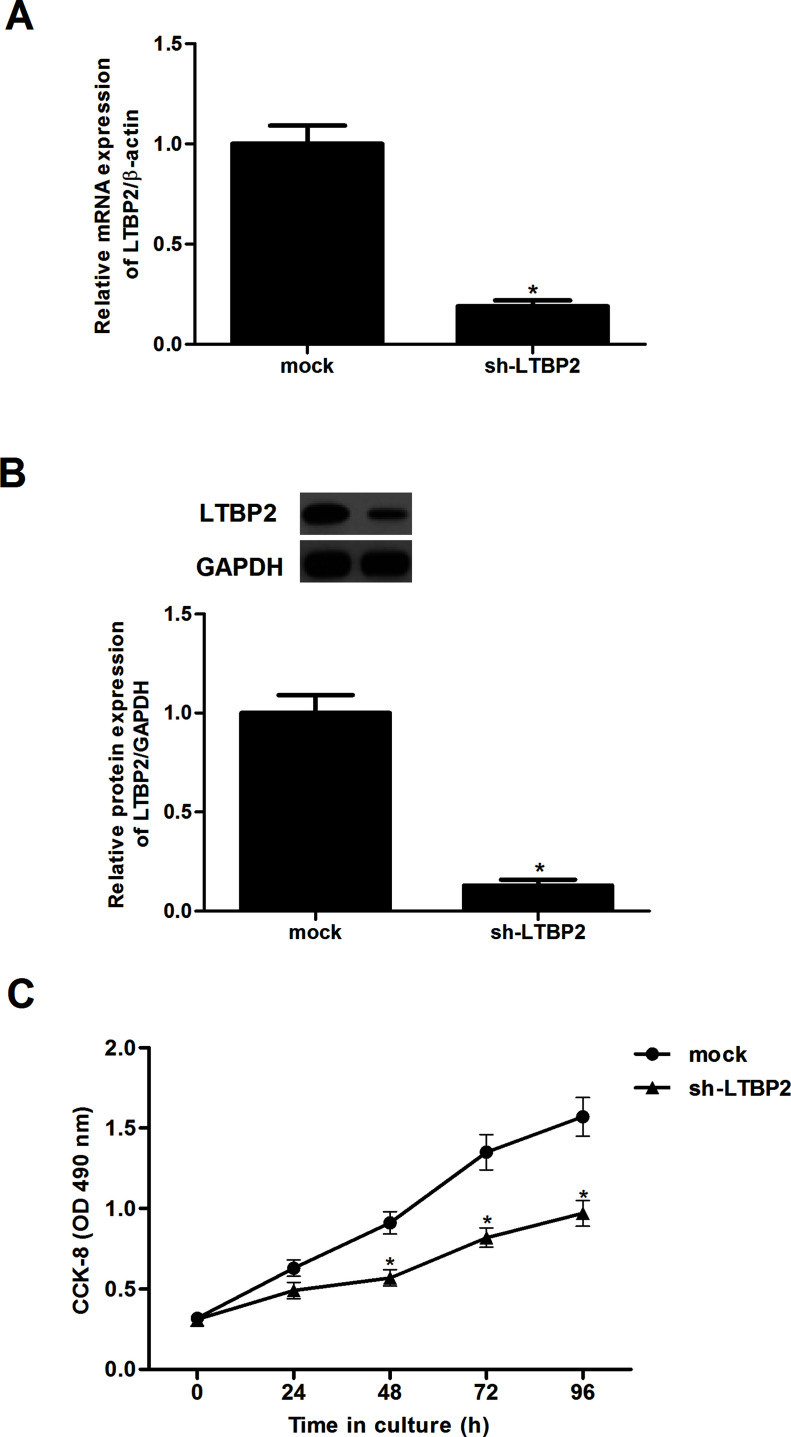
In order to investigate the effect of LTBP2 on thyroid carcinoma cell proliferation, we generated LTBP2 knockdown thyroid carcinoma cell line FTC133. The results indicated that LTBP2 expression was significantly decreased in infected cells, as shown by RT-qPCR (Fig. 2A) and Western blot analysis (Fig. 2B). Furthermore, CCK-8 analysis showed that cell proliferation was significantly suppressed in sh-LTBP2-transfected FTC133 cells compared with the mock-transfected control group (Fig. 2C).
Figure 2.
Knockdown of LTBP2 inhibits the proliferation in thyroid carcinoma cells. FTC133 cells were transfected with sh-LTBP2 or mock for 24 h. The corresponding transfection efficiency was detected by RT-qPCR (A) and Western blotting (B). The effect of LTBP2 on thyroid carcinoma cell proliferation was measured by the CCK-8 assay (C). Experiments were performed in triplicate. *p < 0.05 versus the mock group.
Knockdown of LTBP2 Inhibits the Migration and Invasion in Thyroid Carcinoma Cells
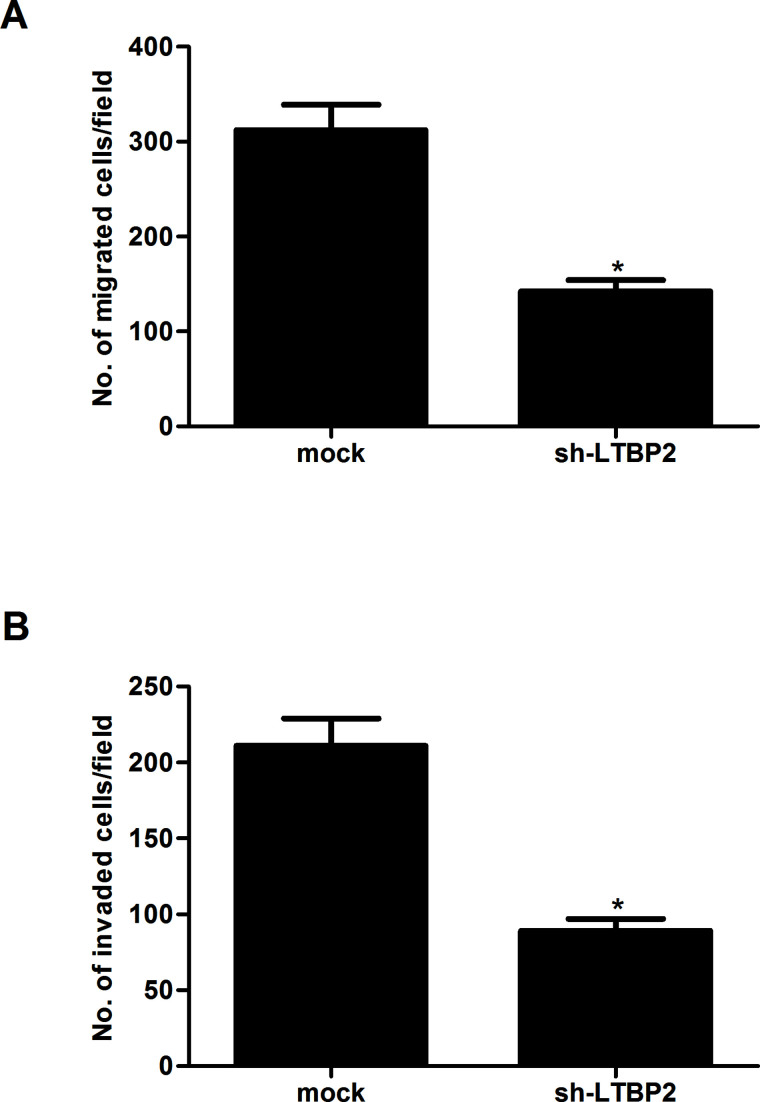
To investigate the potential effect of LTBP2 on motility and invasiveness, Transwell assays were performed in FTC133 cells. The results showed that knockdown of LTBP2 inhibited migration of FTC133 cells, compared with the mock group (Fig. 3A). In addition, knockdown of LTBP2 could also significantly prevent FTC133 cells from invading through Matrigel-coated polycarbonate filter in the Transwell chamber (Fig. 3B).
Figure 3.
Knockdown of LTBP2 inhibits migration and invasion in thyroid carcinoma cells. FTC133 cells were transfected with sh-LTBP2 or mock for 24 h. (A) Transwell assay showing that sh-LTBP2 suppressed the migration in FTC133 cells compared to control cells. (B) Matrigel invasion assay showing that sh-LTBP2 reduced the invasion in FTC133 cells compared to control cells. Experiments were performed in triplicate. *p < 0.05 versus the mock group.
Knockdown of LTBP2 Inhibits the EMT Phenotype in Thyroid Carcinoma Cells
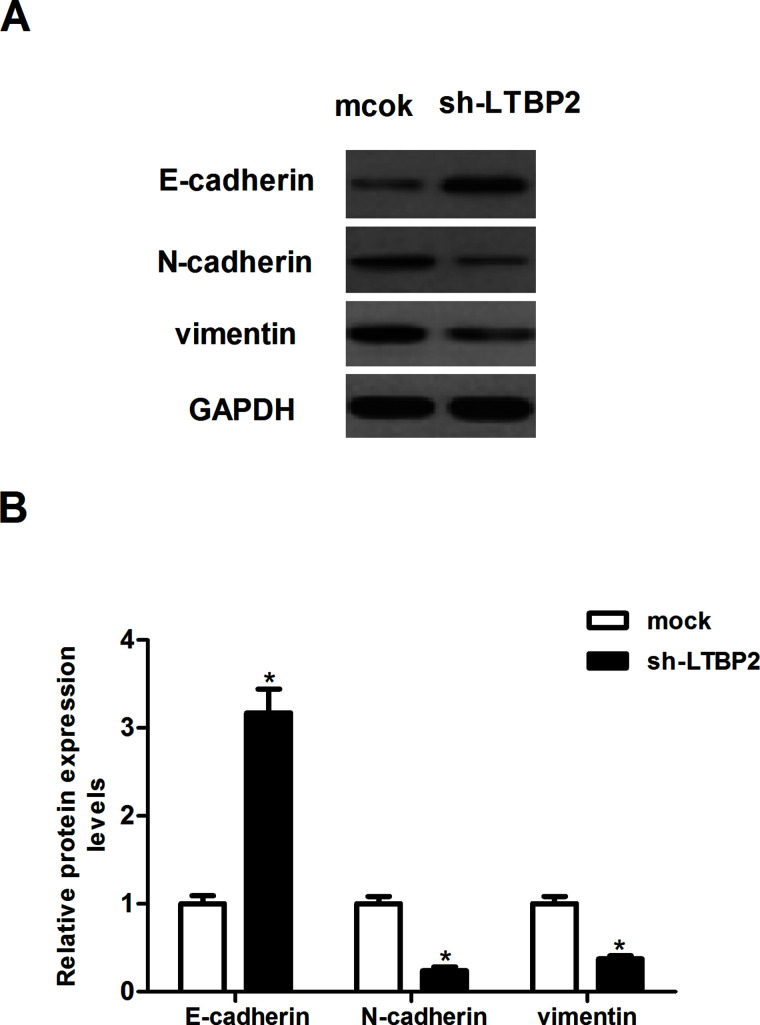
To investigate whether sh-LTBP2 decreased cancer invasion by inhibiting EMT, we analyzed the expression of several EMT markers in LTBP2-silencing and control FTC133 cells using Western blotting. Knockdown of LTBP2 greatly increased the level of the epithelial marker (E-cadherin) and suppressed the levels of the mesenchymal markers (N-cadherin and vimentin) (Fig. 4).
Figure 4.
Knockdown of LTBP2 inhibits the EMT phenotype in thyroid carcinoma cells. FTC133 cells were transfected with sh-LTBP2 or mock for 24 h. (A) The levels of E-cadherin, N-cadherin, and vimentin were detected by Western blot analysis. (B) Quantification of E-cadherin, N-cadherin, and vimentin. Experiments were performed in triplicate. *p < 0.05 versus the mock group.
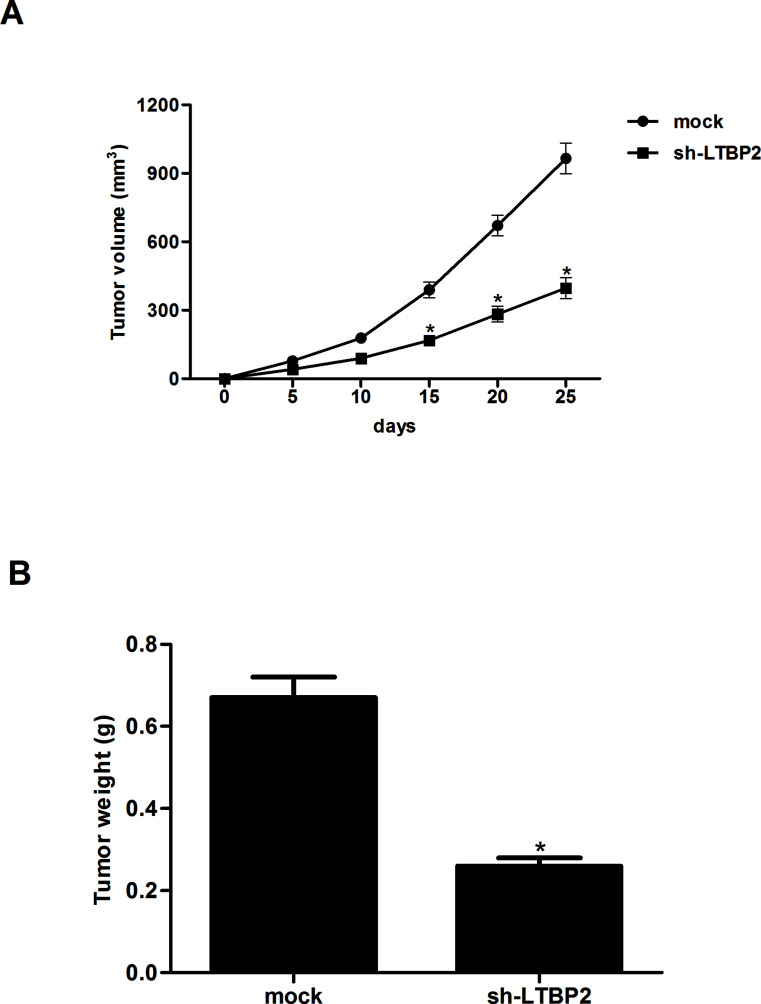
Knockdown of LTBP2 Inhibits the Growth of Thyroid Carcinoma In Vivo
We then investigated whether sh-LTBP2 inhibited tumor growth in vivo. FTC133 cells (1 × 106 cells/0.1 ml) transduced with sh-LTBP2 or mock were injected subcutaneously into the flank of nude mice. The average volume of tumors derived from FTC133 cells with sh-LTBP2 group was smaller than control tumors (Fig. 5A). In addition, an approximately 2.5-fold decrease in tumor weight was observed in sh-LTBP2-transfected nude mice compared to controls (Fig. 5B).
Figure 5.
Knockdown of LTBP2 inhibits the growth of thyroid carcinoma in vivo. FTC133 cells (1 × 106 cells/0.1 ml) transduced with sh-LTBP2 or mock were injected subcutaneously into the flank of nude mice. (A) The volume of tumors was monitored every 5 days. (B) The mice were sacrificed 25 days after inoculation, and tumors were excised, measured, and weighed. Experiments were performed in triplicate. *p < 0.05 versus the mock group.
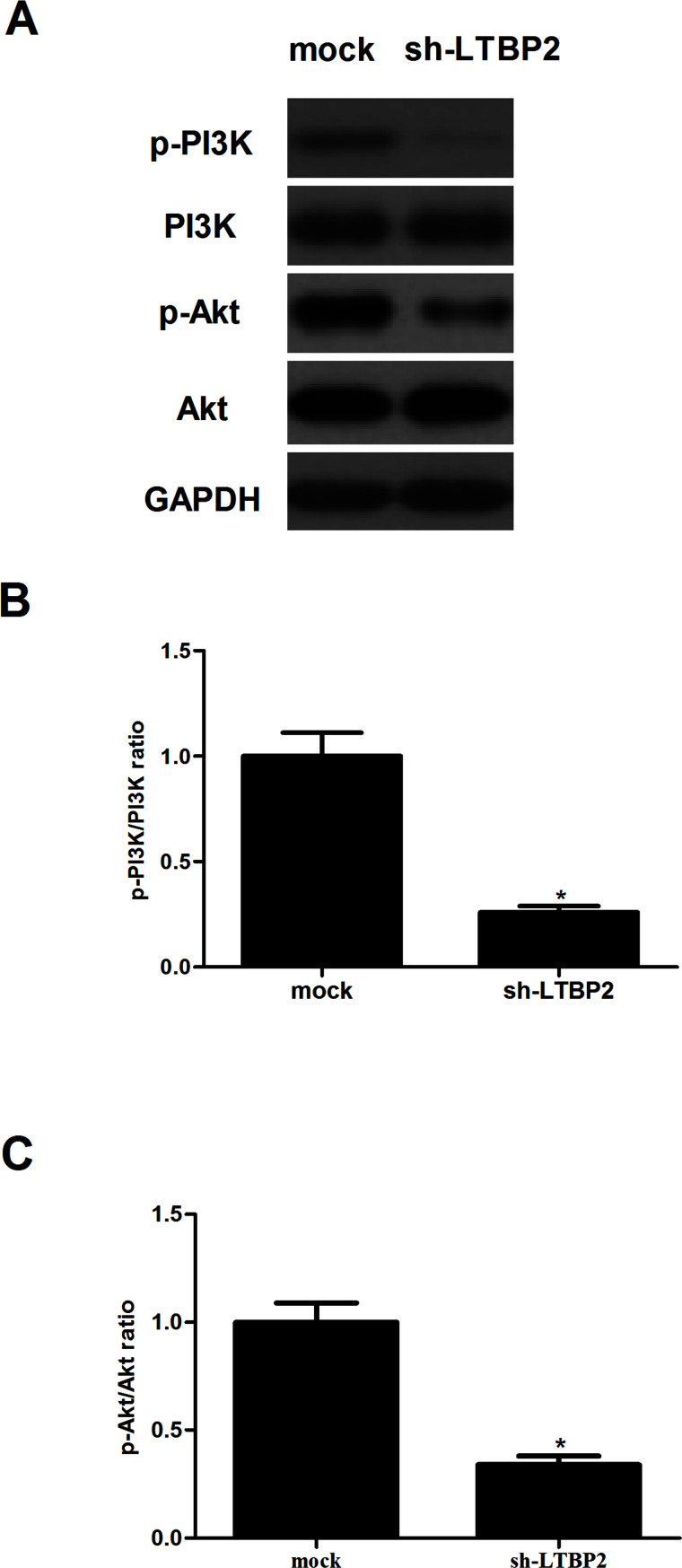
Knockdown of LTBP2 Inhibits the Activation of the PI3K/Akt Pathway in Thyroid Carcinoma Cells
To gain insight into the molecular mechanisms involved in LTBP2-mediated cell migration, we examined the effect of LTBP2 on the expression of phosphorylation levels of PI3K and Akt in FTC133 cells. LTBP2 knockdown significantly decreased the phosphorylation levels of PI3K and Akt in FTC133 cells with sh-LTBP2 compared with the control group by Western blot analysis (Fig. 6).
Figure 6.
Knockdown of LTBP2 inhibits the activation of the PI3K/Akt pathway in thyroid carcinoma cells. FTC133 cells were transfected with sh-LTBP2 or mock for 24 h. (A) The levels of phosphorylated PI3K, total PI3K, phosphorylated Akt, and total Akt were detected using Western blot analysis. Quantification of (B) p-PI3K/PI3K and (C) p-Akt/Akt. Experiments were performed in triplicate. *p < 0.05 versus the mock group.
DISCUSSION
This study is the first to demonstrate the critical role of LTBP2 in thyroid carcinoma. First, the expression of LTBP2 is upregulated in human thyroid carcinoma and cell lines. Second, knockdown of LTBP2 inhibits the proliferation, invasion, and EMT phenotype in thyroid carcinoma cells. Third, knockdown of LTBP2 attenuates thyroid carcinoma growth in nude mice. Finally, knockdown of LTBP2 inhibits activation of the PI3K/Akt pathway in thyroid carcinoma cells. These findings suggest that LTBP2 may play an important role in the development and progression of thyroid carcinoma.
LTBP2 was shown to play a vital role in tumorigenesis. Han et al. reported that both LTBP2 mRNA and protein levels were significantly higher in head and neck squamous cell carcinoma (HNSCC) tissues, and its expression level was related to lymph node metastasis and higher pTNM stages16. However, the study by Chan et al. showed that LTBP2 expression was downregulated in esophageal squamous cell carcinoma (ESCC) cell lines and tumor tissues, and restoration of LTBP2 suppresses tumor growth in vivo in nude mice12. These findings suggest that LTBP2 performs an oncogenic or a tumor suppressing function depending on cell type or context. In this study, we found that LTBP2 is highly expressed in human thyroid carcinoma tissues and cell lines. In addition, knockdown of LTBP2 inhibits thyroid carcinoma cell proliferation in vitro and tumor growth in nude mice. These data strongly suggest that LTBP2 acts as an oncogene in the development and progression of thyroid carcinoma.
EMT plays an important role in the metastasis of many types of carcinomas, including thyroid carcinoma17–19. In general, increased motility and invasion are positively correlated with EMT, which is characterized by repression of epithelial markers and induction of mesenchymal markers20. In the present study, we found that knockdown of LTBP2 greatly inhibited the migration and invasion of FTC133 cells, as well as upregulated the expression of E-cadherin and downregulated the expression of N-cadherin and vimentin. These data suggest that knockdown of LTBP2 inhibits thyroid carcinoma cell migration and invasion through suppression of the EMT process.
Several studies have suggested that the PI3K/Akt signaling pathway is implicated in the pathogenesis and progression of thyroid carcinoma. This signaling pathway regulates cell proliferation, invasion, and apoptosis and, when deranged, promotes tumorigenesis21,22. Akt is a Ser/Thr kinase, and the activation of Akt was shown to increase the expression of N-cadherin and vimentin and decrease E-cadherin expression in several cancer cells23,24. Targeting the PI3K/Akt pathway therefore may represent an attractive potential therapeutic approach for the treatment of thyroid carcinoma25–27. For example, Miao and Zhao confirmed that targeting the PI3K/Akt pathway by its specific inhibitor LY294002 or by Akt small interfering RNA (siRNA) resulted in reduced capacity in invasion of thyroid carcinoma cells28. In this study, we found that LTBP2 knockdown significantly decreased the phosphorylation levels of PI3K and Akt in FTC133 cells. These data suggest that knockdown of LTBP2 inhibits the proliferation, invasion, and tumorigenesis in thyroid carcinoma cells through suppression of the PI3K/Akt signaling pathway.
In summary, the present study has provided further evidence that knockdown of LTBP2 inhibits the invasion and tumorigenesis in thyroid carcinoma cells. Our findings may help to further elucidate the molecular mechanisms underlying thyroid carcinoma progression and provide candidate targets for the prevention and treatment of thyroid carcinoma.
REFERENCES
- 1. Schlumberger MJ, Torlantano M. Papillary and follicular thyroid carcinoma. New Engl J Med. 2009;338:297–306. [DOI] [PubMed] [Google Scholar]
- 2. Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, Liu Y. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit. 2013;19:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haddad R, Sherman SI, Shah JP, Wirth LJ. New frontiers and treatment paradigms for thyroid carcinoma. Clin Advances Hematol Oncol. 2014;12:3–5. [PubMed] [Google Scholar]
- 4. Peng A, Li Y, Yang X, Xiao Z, Tang Q, Qin W. A review of the management and prognosis of thyroid carcinoma with tracheal invasion. J EUFOS 2015;272:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Kałużna M, Gołąb M, Czepczyński R, Dworacki G, Bręborowicz D, Orłowski M, Katulska K, Klimowicz A, Gryczyńska M, Ruchała M. Diagnosis, treatment and prognosis in patients with liver metastases from follicular thyroid carcinoma (FTC). Endokrynol Pol. 2016;67:332–47. [DOI] [PubMed] [Google Scholar]
- 6. Gibson MA, Hatzinikolas G, Davis EC, Baker E, Sutherland GR, Mecham RP. Bovine latent transforming growth factor beta 1-binding protein 2: Molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils. Mol Cell Biol. 1995;15:6932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–23. [DOI] [PubMed] [Google Scholar]
- 8. Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007;26:3283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyytiäinen M, Keski-Oja J. Latent TGF-β binding protein LTBP-2 decreases fibroblast adhesion to fibronectin. J Cell Biol. 2003;163:1363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shipley JM, Mecham RP, Maus E, Bonadio J, Rosenbloom J, Mccarthy RT, Baumann ML, Frankfater C, Segade F, Shapiro SD. Developmental expression of latent transforming growth factor β binding protein 2 and its requirement early in mouse development. Mol Cell Biol. 2000;20:4879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H, Ko JM, Wong VC, Hyytiainen M, Keski-Oja J, Chua D, Nicholls JM, Cheung FM, Lee AW, Kwong DL. LTBP-2 confers pleiotropic suppression and promotes dormancy in a growth factor permissive microenvironment in nasopharyngeal carcinoma. Cancer Lett. 2012;325:89–98. [DOI] [PubMed] [Google Scholar]
- 12. Chan SHK, Ko JMY, Chan KW, Chan YP, Qian T, Hyytiainen M, Keski-Oja J, Law S, Srivastava G, Tang J. The ECM protein LTBP-2 is a suppressor of esophageal squamous cell carcinoma tumor formation but higher tumor expression associates with poor patient outcome. Int J Cancer 2011;129:565–73. [DOI] [PubMed] [Google Scholar]
- 13. Vehviläinen P, Hyytiäinen M, Keski-Oja J. Latent transforming growth factor-beta-binding protein 2 is an adhesion protein for melanoma cells. J Biol Chem. 2003;278:24705–13. [DOI] [PubMed] [Google Scholar]
- 14. Prodoehl MJ, Hatzirodos N, Irvingrodgers HF, Zhao ZZ, Painter JN, Hickey TE, Gibso MA, Rainey WE, Carr BR, Mason HD. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod. 2009;15:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren Y, Lu H, Zhao D, Ou Y, Yu K, Gu J, Wang L, Jiang S, Chen M, Wang J. LTBP2 acts as a prognostic factor and promotes progression of cervical adenocarcinoma. Am J Transl Res. 2015;7:1095–105. [PMC free article] [PubMed] [Google Scholar]
- 16. Han L, Tang MM, Xu X, Jiang B, Huang J, Feng X, Qiang J. LTBP2 is a prognostic marker in head and neck squamous cell carcinoma. Oncotarget 2016;7(29):45052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bencivenga CT. Role of TWIST1 transcription factor in thyroid tumor progression. 2011. Available from http://www.fedoa.unina.it/id/eprint/8747
- 18. Montemayor-Garcia C, Hardin H, Guo Z, Larrain C, Buehler D, Asioli S, Chen H, Lloyd RV. The role of epithelial mesenchymal transition markers in thyroid carcinoma progression. Endocr Pathol. 2013;24:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buehler D, Hardin H, Shan W, Montemayorgarcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Modern Pathol. 2013;26(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De CB, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- 21. Campos M, Kool MMJ, Daminet S, Ducatelle R, Rutteman G, Kooistra HS, Galac S, Mol JA. Upregulation of the PI3K/Akt pathway in the tumorigenesis of canine thyroid carcinoma. J Vet Intern Med. 2014;28:1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glassmann A, Winter J, Kraus D, Veit N, Probstmeier R. Pharmacological suppression of the Ras/MAPK pathway in thyroid carcinoma cells can provoke opposite effects on cell migration and proliferation: The appearance of yin-yang effects and the need of combinatorial treatments. Int J Oncol. 2014;45:2587–95. [DOI] [PubMed] [Google Scholar]
- 23. Wallerand H, Ying C, Wainberg ZA, Garraway I, Lascombe I, Nicolle G, Thiery JP, Bittard H, Radvanyi F, Reiter RR. Phospho-Akt pathway activation and inhibition depends on N-cadherin or phospho-EGFR expression in invasive human bladder cancer cell lines. Urol Oncol. 2010;28:180–8. [DOI] [PubMed] [Google Scholar]
- 24. Grille SJ, Bellacosa A, Upson J, Kleinszanto AJ, Roy FV, Leekwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–78. [PubMed] [Google Scholar]
- 25. Wang SC, Chai DS, Chen CB, Wang ZY, Wang L. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/AKT signaling pathway. Biomed Pharmacother. 2015;75:33–9. [DOI] [PubMed] [Google Scholar]
- 26. Erfani N, Fattahi MJ, Mehr M, Monabati A, Hafizi S, Ghaderi A. Dysregulated expression of tensin2 and components of the pi3 kinase/akt signaling pathway in human thyroid carcinoma. Middle East J Cancer. 2015;7:1–8. [Google Scholar]
- 27. Furuya F, Lu C, Willingham MC, Cheng S. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 2007;28:2451–8. [DOI] [PubMed] [Google Scholar]
- 28. Miao X, Zhao Y. ST6GalNAcII mediates tumor invasion through PI3K/Akt/NF-kB signaling pathway in follicular thyroid carcinoma. Oncol Rep. 2016;35:2131–40. [DOI] [PubMed] [Google Scholar]