Abstract
Angiopoietin-like protein 2 (ANGPTL2), a member of the glycoprotein family, is mainly secreted by adipose tissues under normal conditions. Recently, ANGPTL2 has been found to be upregulated in some types of cancers and is considered to be a tumor promoter. However, the functional significance of ANGPTL2 in glioma has not yet been elucidated. In this study, we investigated the specific role of ANGPTL2 in glioma. The results showed that ANGPTL2 was highly expressed in glioma tissues and cell lines. Knockdown of ANGPTL2 reduced the proliferative and invasive abilities of glioma cells. Moreover, the tumorigenesis assay showed that ANGPTL2 knockdown inhibited glioma tumor growth in vivo. We also found that ANGPTL2 knockdown decreased the protein levels of p-ERK1/2 in glioma cells and thus blocked the activity of the ERK/MAPK signaling pathway. Taken together, our study provided the first evidence that ANGPTL2 played an oncogenic role in glioma development and might be considered as a new therapeutic target for glioma treatment.
Key words: Angiopoietin-like protein 2 (ANGPTL2), Proliferation, Invasion, Glioma
INTRODUCTION
Glioma, a common type of brain tumor in the central nervous system, is among the top on a list of the leading causes of cancer-associated deaths in the world1,2. According to cell type, glioma can be classified into astrocytomas, oligodendrogliomas, oligoastrocytomas, and so on3. According to malignant degrees, glioma can be classified into four grades, from I to IV4. Among all types of gliomas, high-grade types such as glioblastoma multiforme (GBM) account for a large proportion and are characterized by rapid development5. Unfortunately, most glioma patients are diagnosed with high-grade gliomas and thus have a dismal prognosis6. Despite the improvement in therapeutic approaches, patients with high-grade gliomas only have a median survival of 9 to 12 months because of postsurgery relapse or resistance to radiotherapy and chemotherapy7–10. Therefore, it is crucial to develop more effective therapies via exploring novel molecules associated with glioma progression and revealing their underlying mechanisms.
Angiopoietin-like proteins (ANGPTLs) are usually used for growing stem cells in laboratories11. Some researchers have made a careful study of them and found their necessity for proper development of the vascular system in embryos11. These proteins resemble angiopoietins12–14. They are incapable of binding to either Tie-2 or its homolog Tie-1 despite their structural similarities to angiopoietins and thus have different functions, such as regulation of energy metabolism and inflammation15–17. Recently, a member of the protein family, ANGPTL2, has been reported to be a promising biomarker for the diagnosis of lung cancer18. Subsequently, more and more studies have demonstrated the promoting effect of ANGPTL2 on the progression of different types of cancers, such as breast and colorectal cancers18,19. However, the functional significance of ANGPTL2 in glioma has not yet been elucidated.
In this study, we investigated the specific role of ANGPTL2 in glioma. The results showed that ANGPTL2 was highly expressed in glioma tissues and cell lines. Knockdown of ANGPTL2 inhibited glioma cell proliferation and invasion. In addition, the xenograft tumor assay indicated that ANGPTL2 knockdown suppressed glioma tumor growth in vivo. We also observed that ANGPTL2 knockdown decreased the protein levels of p-ERK1/2 in glioma cells. In combination with our findings, we suggest ANGPTL2 as a potential therapeutic target for glioma.
MATERIALS AND METHODS
Patients and Tissue Specimens
In the study, glioma tissues and normal brain tissues were obtained from 32 patients. These patients were from Peking University People’s Hospital (P.R. China) and underwent no adjuvant therapies before surgery. All patients provided written consent. After collection, tissue samples were frozen in liquid nitrogen and stored at −80°C for future experiments. The study was carried out with the approval of the ethics committee of Peking University People’s Hospital.
Cell Lines and Cell Culture
Human glioma cell lines (LN18 and T98G) and normal human astrocytes (NHA) were purchased from the cell bank of Shanghai Biology Institute, Chinese Academy of Science (Shanghai, P.R. China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 mg/ml penicillin, and 100 μg/ml streptomycin, before incubation at 37°C in a humidified atmosphere of 5% CO2.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from tissue samples or cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA was generated using the SuperScript II First-Strand Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). qRT-PCR was performed in the ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). Reactions were conducted under the following conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 45 s. The following primers were used: ANGPTL2, 5′-CCACCCTGGACAGAGATCAT-3′ (forward) and 5′-CTCGGAACTCAGCCCAGTAG-3′ (reverse); GAPDH, 5′-AACGGATTTGGTCGTATTG-3′ (forward) and 5′-GGAAGATGGTGATGGGATT-3′ (reverse). The relative expression was normalized to GAPDH and calculated using the 2−ΔΔCt method.
Western Blot
Tissues or cells were lysed in RIPA buffer for protein extraction. Total protein was separated on 10% SDS-PAGE before transferring to PVDF membranes. The protein concentration was determined with a BCA Protein Assay Kit (Thermo Fisher Scientific). The membranes were blocked with 5% skim milk and probed overnight at 4°C with primary antibodies against ANGPTL2, p-ERK1/2, ERK1/2, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing three times with PBS–Tween 20 for 10 min, the membranes were incubated at 4°C with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). Immunoreactive bands were visualized using enhanced chemiluminescence (Thermo Fisher Scientific) and quantified with the Gel Documentation System (Alpha Innotech, San Leandro, CA, USA).
Small Interfering RNA (siRNA) and Cell Transfection
ANGPTL2-specific siRNA (siANGPTL2) and a control siRNA (siNC) were purchased from Thermo Fisher Scientific. LN18 and T98G cells were cultured in normal medium and grown to 80% confluency before transfection with siANGPTL2 or siNC using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, knockdown efficiency was assessed by Western blot.
Cell Proliferation Assay
Cell proliferation was measured by MTT assay. Briefly, cells were plated into 96-well culture plates at a density of 2 × 103 cells/well, and 20 μl of MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well at different times. Four hours later, the culture medium was removed, and 200 μl of DMSO (Sigma-Aldrich) was added. The absorbance was measured at 490 nm.
Cell Invasion Assay
Transwell chambers with Matrigel-coated membranes (8-μm pore) were used to perform the cell invasion assay. Cells were cultured in serum-free medium and plated into the upper chamber at a density of 5 × 104 cells/well. Culture medium containing 10% FBS was added to the lower chamber as a chemoattractant. After incubation for 24 h, cells remaining on the upper side of membranes were removed with cotton swabs while cells invading to the lower side of membranes were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of invading cells from five independent fields was counted under a microscope.
Animal Experiments
Female BALB/c nude mice (SLAC, Shanghai, P.R. China) at an age of 4 to 6 weeks were used for animal experiments. All mice were kept under specific pathogen-free conditions and handled according to the protocols approved by the Institutional Animal Care and Use Committee of Peking University People’s Hospital. LN18 cells (2 × 106 in 200 μl of PBS) transfected with siANGPTL2 or siNC were subcutaneously injected into the right flank of nude mice (n = 8). Tumors were measured every week using a vernier caliper. The tumor volume was calculated according to the following formula: volume (mm3) = 1/2 × width2 × length. Five weeks after injection, mice were sacrificed for weighing of tumor tissues.
Statistical Analysis
All values were shown as means ± standard deviation (SD). The Student’s t-test was used for comparison between different groups. The SPSS 19.0 software (IBM Corporation, Armonk, NY, USA) was used to make a statistical analysis. A value of p < 0.05 was considered statistically significant.
RESULTS
Expression of ANGPTL2 Is Elevated in Glioma Tissues and Cell Lines
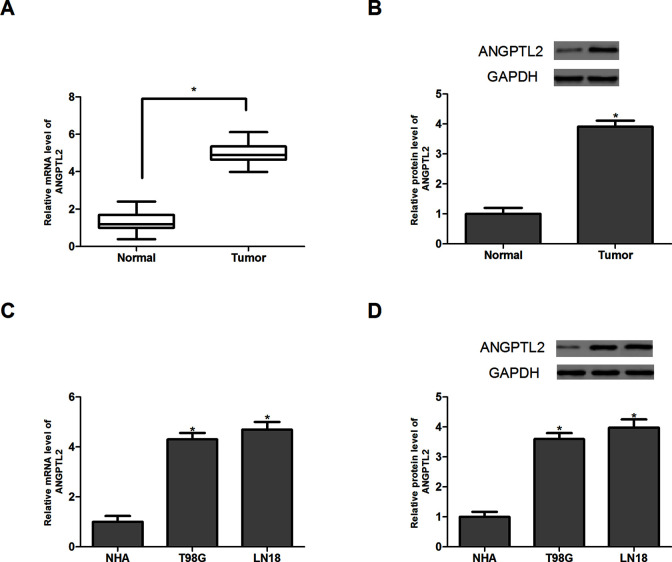
We first analyzed the expression pattern of ANGPTL2 in glioma tissues (n = 32) and normal brain tissues (n = 32) by RT-PCR and Western blot. Our data indicated that ANGPTL2 expression in glioma tissues was markedly higher than in normal brain tissues at both mRNA and protein levels (Fig. 1A and B). Furthermore, we evaluated ANGPTL2 expression in glioma cell lines (LN18 and T98G) and normal astrocytes (NHA). Similarly, the results showed a significant increase in ANGPTL2 expression in glioma cell lines compared with normal astrocytes (Fig. 1C and D).
Figure 1.
Expression of angiopoietin-like protein 2 (ANGPTL2) is elevated in glioma tissues and cell lines. (A, B) Relative mRNA and protein expression levels of ANGPTL2 in glioma tissues and normal brain tissues (n = 32). (C, D) Relative mRNA and protein expression levels of ANGPTL2 in glioma cell lines (LN18 and T98G) and normal astrocytes (NHA). *p < 0.05.
Knockdown of ANGPTL2 Inhibits the Proliferation and Invasion of Glioma Cells
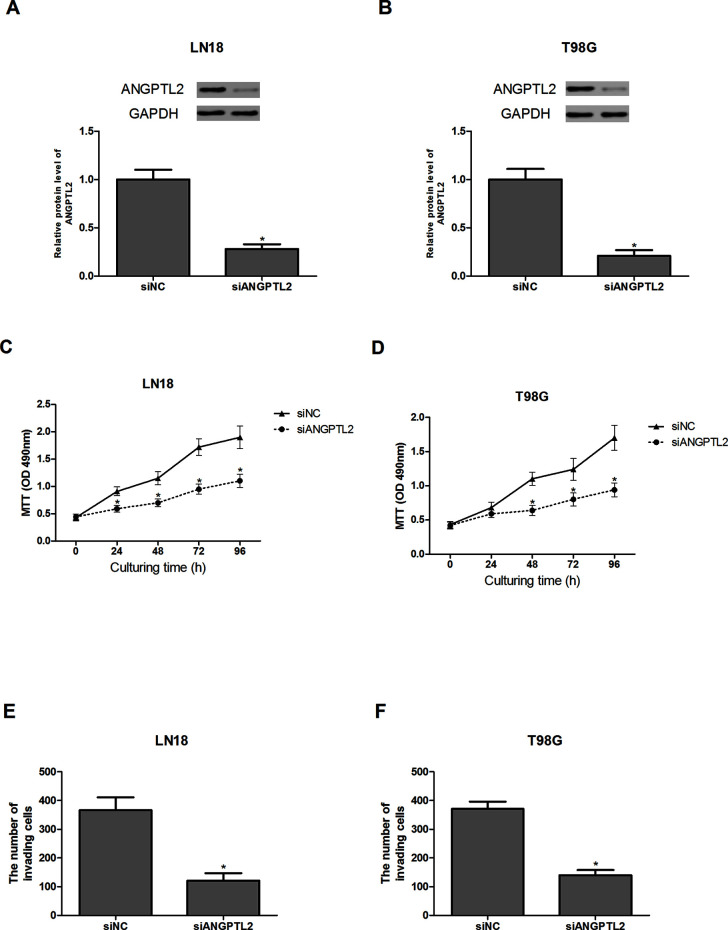
To investigate the function of ANGPTL2 in glioma, we decreased its expression in glioma cells by siRNA transfection. The knockdown effect was evaluated by Western blot analysis. siANGPTL2 remarkably reduced ANGPTL2 expression in LN18 and T98G cells compared with control cells (Fig. 2A and B).
Figure 2.
Knockdown of ANGPTL2 inhibits the proliferation and invasion of glioma cells. (A, B) Western blot analysis was used to detect the expression of ANGPTL2 in LN18 and T98G cells after transfection with siANGPTL2 or siNC. (C, D) The effect of ANGPTL2 knockdown on the proliferation of LN18 and T98G cells was detected via the MTT assay. (E, F) The effect of ANGPTL2 knockdown on the invasion of LN18 and T98G cells was detected via the Transwell assay. *p < 0.05.
We examined the effect of ANGPTL2 knockdown on cell proliferation through the MTT assay. The MTT assay revealed that cell growth was greatly decreased in siANGPTL2-transfected LN18 and T98G cells compared with the corresponding control cells (Fig. 2C and D).
The Transwell assay was performed to determine the effect of ANGPTL2 knockdown on cell invasion. The invasive ability of siANGPTL2-transfected LN18 and T98G cells was strongly inhibited in comparison with the control cells (Fig. 2E and F).
Knockdown of ANGPTL2 Inhibits Glioma Tumor Growth In Vivo
To further confirm the effect of ANGPTL2 knockdown on glioma tumor growth in vivo, LN18 cells transfected with siANGPTL2 or siNC were subcutaneously inoculated into nude mice. Tumor volume was measured every week. The growth curve of tumors indicated that ANGPTL2 knockdown drastically slowed down the growth rate of tumors (Fig. 3A). Five weeks after injection, tumors were resected and weighed. The weight of tumors formed by siANGPTL2-transfected LN18 cells was lower than that of tumors formed by siNC-transfected LN18 cells (Fig. 3B).
Figure 3.
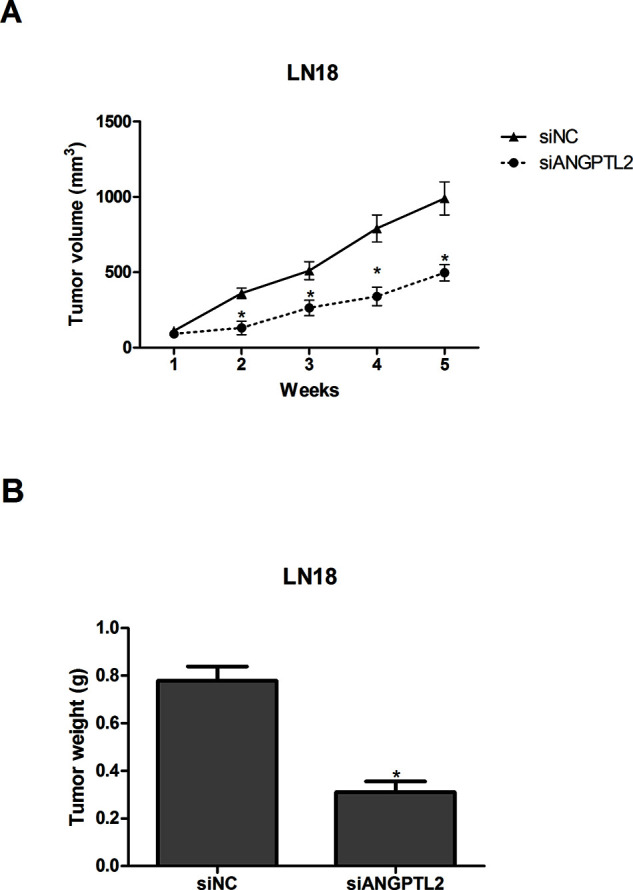
Knockdown of ANGPTL2 inhibits glioma tumor growth in vivo. (A) The growth curve of tumors was established by measuring tumor volume every week after injection. (B) Tumors were weighed 5 weeks after injection. *p < 0.05.
Knockdown of ANGPTL2 Inhibits the Activity of the ERK/MAPK Signaling Pathway
To determine the possible mechanism by which ANGPTL2 knockdown regulated glioma cell proliferation and invasion, Western blot analysis was carried out to detect the effect of ANGPTL2 knockdown on the ERK/MAPK pathway, which frequently shows aberrant activation in human cancers and contributes to promoted cell proliferation and invasion20–22. The results indicated that ANGPTL2 knockdown significantly decreased the protein levels of p-ERK1/2 in LN18 cells, while no detectable changes were found in the total protein levels of ERK1/2 (Fig. 4A). We also treated siANGPTL2-transfected LN18 cells with 10 μM U0126 (a specific inhibitor of ERK/MAPK). siANGPTL2-inhibited glioma cell proliferation and invasion were enhanced after treatment with U0126 (Fig. 4B and C).
Figure 4.
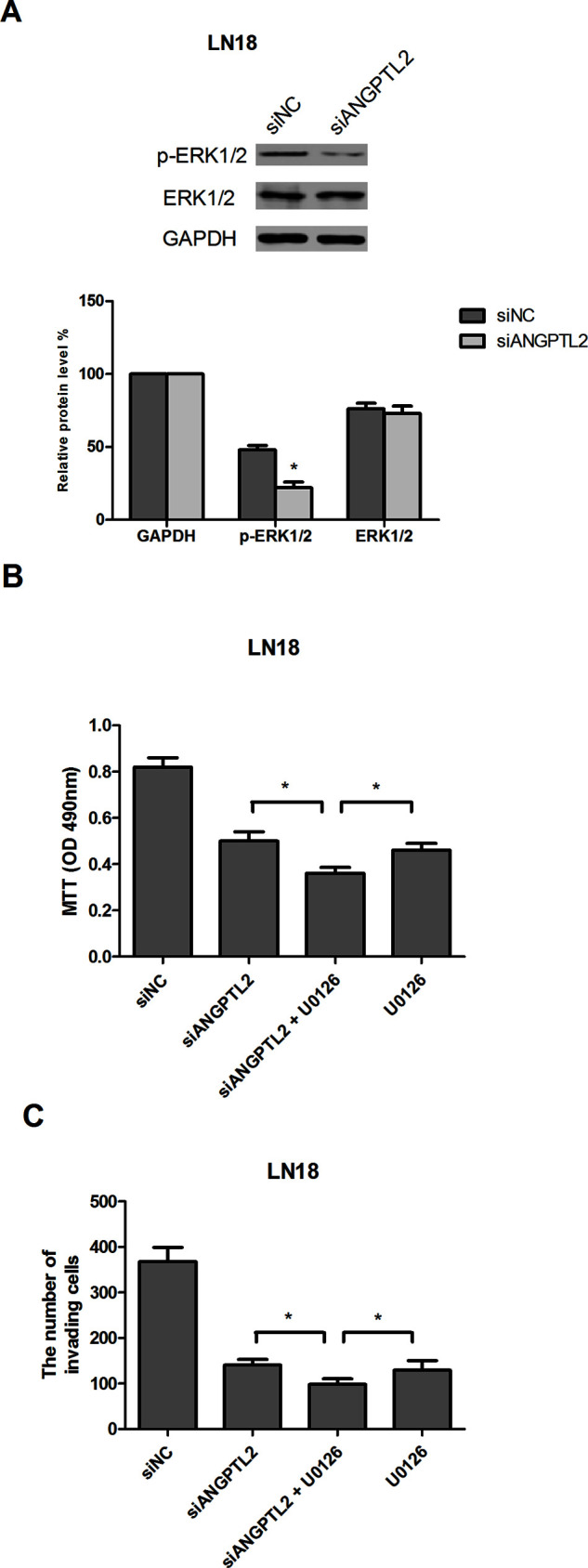
Knockdown of ANGPTL2 inhibits the activity of the ERK/MAPK signaling pathway. (A) Western blot analysis showed that the protein levels of p-ERK1/2 in siANGPTL2-transfected LN18 cells were remarkably reduced, while the total protein levels of ERK1/2 remained unchanged. GAPDH was used as an internal control. (B, C) Transfected LN18 cells were exposed to 10 μM U0126. Cell proliferation and invasion were measured by MTT and Transwell assays, respectively. *p < 0.05.
DISCUSSION
According to investigation statistics, glioma patients have the lowest 5-year survival rate among all cancer patients owing to limited advances in glioma treatment. Therefore, exploring novel molecular targets and elucidating mechanisms underlying glioma progression are essential for the development of effective glioma therapies.
ANGPTL2, a member of the glycoprotein family, is mainly secreted by adipose tissues under normal conditions23. With hypoxia and endoplasmic reticulum stress, the expression of ANGPTL2 will be increased17. In addition, ANGPTL2 is identified as an essential mediator of chronic inflammation and its related diseases including metabolic, autoimmune, and cardiovascular diseases24–27. Recently, ANGPTL2 has been found to be upregulated in some types of cancers and considered as a tumor promoter. For example, Endo et al. reported overexpression of ANGPTL2 in lung cancer tissues and demonstrated its driving function in the metastatic process of lung cancer28. Toiyama et al. found similar results, that ANGPTL2 was highly expressed in colorectal cancer tissues, and suggested ANGPTL2 as a novel diagnostic biomarker for colorectal cancer patients19. In our study, we also observed an elevated expression of ANGPTL2 in glioma tissues and cell lines. Moreover, we found a suppressive effect of ANGPTL2 knockdown on glioma cell proliferation and invasion. Our tumorigenesis experiments showed that ANGPTL2 knockdown inhibited glioma tumor growth in vivo, further verifying our in vitro results. All observations in our study were consistent with the previous studies. However, in a study by Kikuchi et al., ANGPTL2 was found to have a reduced expression in ovarian cancer, and its overexpression inhibited ovarian cancer cell growth29. This result was inconsistent with ours and indicates the tumor-inhibitor role of ANGPTL2 in cancer development. Collectively, we suggest that ANGPTL2 might function as an oncogene or a tumor suppressor, depending on cancer type.
We also investigated the possible mechanism by which ANGPTL2 knockdown inhibited glioma cell proliferation and invasion. The ERK/MAPK signaling pathway is an important signal transduction pathway and is activated by serum, cytokines, growth factors, and osmotic stresses30. In addition, it is capable of phosphorylating and regulating various substrates such as kinases, cytoskeletal proteins, and transcription factors, which lead to changes in gene expression and cellular functions31. More importantly, aberrant activation of the ERK/MAPK signaling pathway is often found in cancers including glioma20–22. Furthermore, ANGPTL2 has been reported to promote metastasis during breast cancer development via regulating the ERK/MAPK pathway32. In this study, we found that ANGPTL2 knockdown significantly reduced the protein levels of p-ERK1/2 in glioma cells, indicating an inhibitory effect of ANGPTL2 knockdown on the activity of the ERK/MAPK signaling pathway. We also used U0126 (a specific inhibitor of ERK/MAPK) to treat siANGPTL2-transfected LN18 cells. The results showed that siANGPTL2-inhibited glioma cell proliferation and invasion were potentiated after treatment with U0126. According to these results, we may infer that other signaling pathways are also involved. Therefore, more studies should be performed for further investigation.
In summary, we found that ANGPTL2 was elevated in glioma tissues and cells. Knockdown of ANGPTL2 reduced the proliferative and invasive abilities of glioma cells. Moreover, the tumorigenesis assay showed that ANGPTL2 knockdown inhibited glioma tumor growth in vivo. We also found that ANGPTL2 knockdown decreased the protein levels of p-ERK1/2 in glioma cells and thus blocked the activity of the ERK/MAPK signaling pathway. Taken together, our study provided the first evidence that ANGPTL2 plays an oncogenic role in glioma development and might be considered to be a new therapeutic target for glioma treatment.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Taylor LP. Diagnosis, treatment, and prognosis of glioma: Five new things. Neurology 2010;75:28–32. [DOI] [PubMed] [Google Scholar]
- 2. Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–104. [DOI] [PubMed] [Google Scholar]
- 3. Fuller GN. The WHO Classification of tumours of the ventral nervous system. Arch Pathol Lab Med. 2008;132:906. [DOI] [PubMed] [Google Scholar]
- 4. Kohsaka S. Epiregulin enhances tumorigenicity by activating the ERK/MAPK pathway in glioblastoma. Neuro Oncol. 2014;16:960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tao T, Shi Y, Han D, Luan W, Qian J, Zhang J, Wang Y, You Y. TPM3, a strong prognosis predictor, is involved in malignant progression through MMP family members and EMT-like activators in gliomas. Tumor Biol. 2014;35:9053–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Y, Liu F, Xu Q, Wang X. Analysis of the expression profile of Dickkopf-1 gene in human glioma and the association with tumor malignancy. J Exp Clin Cancer Res. 2010;29:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, Komotar RJ. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev Anticancer Ther. 2013;13:1453–61. [DOI] [PubMed] [Google Scholar]
- 8. Yin AA, Cai S, Dong Y, Zhang LH, Liu BL, Cheng JX, Zhang X. A meta-analysis of temozolomide versus radiotherapy in elderly glioblastoma patients. J Neurooncol. 2014;116:315–24. [DOI] [PubMed] [Google Scholar]
- 9. Yin A, Zhang L, Cheng J, Dong Y, Liu B, Han N, Zhang X. Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: A meta-analysis. Plos One 2013;8:e74242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Tao J. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–46. [DOI] [PubMed] [Google Scholar]
- 11. Lin MI, Price EN, Boatman S, Hagedorn EJ, Trompouki E, Satishchandran S, Carspecken CW, Uong A, Dibiase A, Yang S. Angiopoietin-like proteins stimulate HSPC development through interaction with notch receptor signaling. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, Kim NG, Koh GY. Molecular cloning, expression, and characterization of angiopoietin-related protein. Angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem. 1999;274:26523–8. [DOI] [PubMed] [Google Scholar]
- 13. Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346(Pt 3):603–10. [PMC free article] [PubMed] [Google Scholar]
- 14. Oike Y, Ito Y, Maekawa H, Morisada T, Kubota Y, Akao M, Urano T, Yasunaga K, Suda T. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood 2004;103:3760–5. [DOI] [PubMed] [Google Scholar]
- 15. Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25:245–54. [DOI] [PubMed] [Google Scholar]
- 16. Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6–14. [DOI] [PubMed] [Google Scholar]
- 17. Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Hui JZ, Tsukano H, Tazume H. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–88. [DOI] [PubMed] [Google Scholar]
- 18. Sasaki H, Suzuki A, Shitara M, Hikosaka Y, Okuda K, Moriyama S, Yano M, Fujii Y. Angiopoietin-like protein ANGPTL2 gene expression is correlated with lymph node metastasis in lung cancer. Oncol Lett. 2012;4:1325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toiyama Y, Tanaka K, Kitajima T, Shimura T, Kawamura M, Kawamoto A, Okugawa Y, Saigusa S, Hiro J, Inoue Y. Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: A serum biomarker for early diagnosis and recurrence. Clin Cancer Res. 2014;20:6175–86. [DOI] [PubMed] [Google Scholar]
- 20. Li B, Sun B, Zhu J, Zhou N, Yang Z, Gu J. Expression of RKIP in chronic myelogenous leukemia K562 cell and inhibits cell proliferation by regulating the ERK/MAPK pathway. Tumor Biol. 2014;35:10057–66. [DOI] [PubMed] [Google Scholar]
- 21. Xu WH, Zhang JB, Dang Z, Li X, Zhou T, Liu J, Wang DS, Song WJ, Dou KF. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10:664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohsaka S. Epiregulin enhances tumorigenicity by activating the ERK/MAPK pathway in glioblastoma. Neuro Oncol. 2014;16:960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tortora G, Melisi D, Ciardiello F. Angiogenesis: A target for cancer therapy. Curr Pharm Des. 2004;10:11–26. [DOI] [PubMed] [Google Scholar]
- 24. Okada T, Tsukano H, Endo M, Tabata M, Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol. 2010;176:2309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogata A, Endo M, Aoi J, Takahashi O, Kadomatsu T, Miyata K, Tian Z, Jinnin M, Fukushima S, Ihn H. The role of angiopoietin-like protein 2 in pathogenesis of dermatomyositis. Biochem Biophys Res Commun. 2012;418:494–9. [DOI] [PubMed] [Google Scholar]
- 26. Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34:790–800. [DOI] [PubMed] [Google Scholar]
- 27. Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T, Nakashima Y. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400–9. [DOI] [PubMed] [Google Scholar]
- 28. Endo M, Nakano M, Kadomatsu T, Fukuhara S, Kuroda H, Mikami S, Tai H, Aoi J, Horiguchi H, Miyata K. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012;72:1784–94. [DOI] [PubMed] [Google Scholar]
- 29. Kikuchi R, Tsuda H, Kozaki K, Kanai Y, Kasamatsu T, Sengoku K, Hirohashi S, Inazawa J, Imoto I. Frequent inactivation of a putative tumor suppressor, angiopoietin-like protein 2, in ovarian cancer. Cancer Res. 2008;68:5067–75. [DOI] [PubMed] [Google Scholar]
- 30. Han Y, Zhou L, Wu T, Huang Y, Cheng Z, Li X, Sun T, Zhou Y, Du Z. Downregulation of lncRNA-MALAT1 affects proliferation and the expression of stemness markers in glioma stem cell line SHG139S. Cell Mol Neurobiol. 2016;36:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Z. Hirudin inhibits cell growth via ERK/MAPK signaling in human glioma. Int J Clin Exp Med. 2015;8:20983–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Masuda T, Endo M, Yamamoto Y, Odagiri H, Kadomatsu T, Nakamura T, Tanoue H, Ito H, Yugami M, Miyata K. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170. [DOI] [PMC free article] [PubMed] [Google Scholar]